Introduction
Most gastrointestinal stromal tumors (GIST) have
activating mutations in stem cell factor receptor (KIT) or
platelet-derived growth factor receptor-α (PDGFRA) (1). Approximately 85% of GIST cases have
activating mutations in KIT, and 5% have activating mutations in
PDGFRA (1). Although the KIT
gene contains 21 exons, KIT in GIST only has mutations
within exons 8, 9, 11, 13, 14, 17 and 18. Most mutations (70%) in
the KIT gene have been reported in the juxtamembrane domain
(exon 11), and 15% of cases have mutations in the extracellular
domain (exon 9) (2).
Imatinib mesylate (Glivec®; Novartis
Pharma, Basel, Switzerland) selectively inhibits KIT and PDGFRA and
is the first-line treatment in adult patients with KIT-positive
non-resectable malignant GIST. The standard dose of imatinib is 400
mg daily. Recently, it was reported that a high dose of imatinib
(800 mg daily) resulted in a significantly superior
progression-free survival rate in patients with GIST harboring a
KIT gene exon 9 mutation (3). It was suggested that KIT exon 9
mutation caused resistance to imatinib.
Sunitinib malate (Sutent®; Pfizer, New
York, NY, USA) is a multi-target tyrosine kinase inhibitor and the
second-line treatment of GIST following disease progression in
cases intolerant to imatinib. Sunitinib inhibits KIT, PDGFRA,
PDGFRB, vascular endothelial growth factor receptor (VEGFR),
FMS-like tyrosine kinase 3 (FLT3), colony-stimulating factor 1
(CSF-1) and glial cell line-derived neurotrophic factor receptor
rearranged during transfection (RET) (4–5).
Sunitinib is metabolized by cytochrome P450 (CYP) 3A4, and produces
N-desethyl metabolite, SU12662. SU12662 is an active
metabolite that inhibits KIT, PDGFR and VEGFR in a similar manner
to sunitinib. Previous animal studies demonstrated that target
plasma concentrations of sunitinib plus SU12662 for the inhibition
of PDGFRB and fetal liver kinase-1/kinase-insert domain-containing
receptor (Flk-1/KDR)/VEGFR-2 phosphorylation were in the range of
50–100 ng/ml (4). In addition, it
is more crucial to maintain the effective concentration (50–100
ng/ml) than to obtain a high maximum plasma concentration
(Cmax).
The metabolic capability of CYP3A4 varies greatly
among individuals (6). Although
most medications have various metabolic pathways, the plasma
concentration of sunitinib varies greatly among individuals as
sunitinib is metabolized only by CYP3A4.
Here, we describe a patient who developed
thrombocytopenia while taking sunitinib. We assayed the plasma
concentrations of sunitinib and SU12662 to avoid
thrombocytopenia.
Patients and methods
Case report
A 60-year-old Japanese woman took sunitinib 50 mg
once daily after breakfast. The patient's height and weight were
150 cm and 36.45 kg, respectively, resulting in a body surface area
of 1.25 m2. Her medical history consisted of small
intestinal GIST, which was immunohistochemically positive for KIT,
smooth muscle actin, CD34 and vimentin. The tumor metastasized to
the liver. She then took imatinib 400 mg once daily after breakfast
for 3 years. The dose of imatinib was reduced to 300 mg once daily
due to the appearance of adverse effects. However, the dose of
imatinib was increased to 400 mg once daily due to liver
metastasis.
She was admitted to Shinshu University Hospital for
one week for the first administration of sunitinib since there was
no decrease in liver metastases following the change to the higher
dose of imatinib. Fourteen days after the first administration of
sunitinib, the patient experienced nosebleeds, stomatitis and
malaise. The platelet (PLT) count was decreased to
1.7×104/μl, which was categorized as grade 4
thrombocytopenia according to the National Cancer Institute
criteria version 4.0. Sunitinib was then discontinued and the
patient was admitted to our hospital. The PLT count was increased
following administration of PLT and adverse effects were eliminated
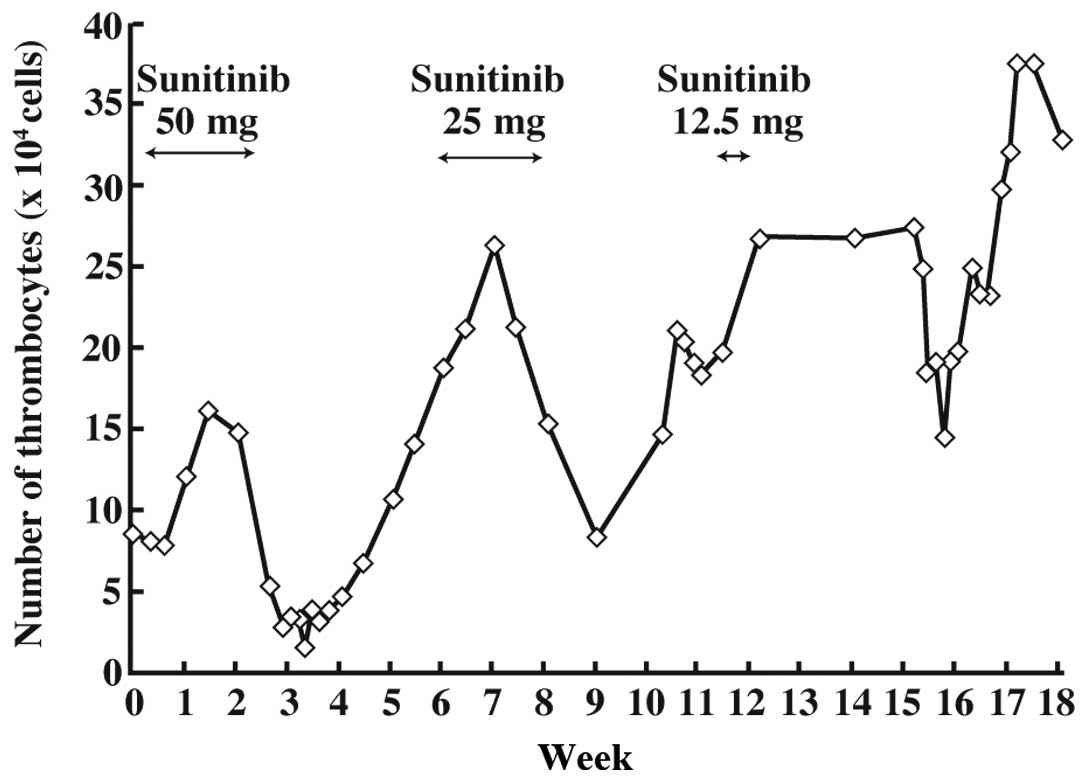
(Fig. 1). The administration of
sunitinib was resumed at 25 mg once daily and continued for 3
weeks. However, sunitinib was discontinued as the PLT count again
decreased.
The PLT count normalized approximately 9 days after
the discontinuation of sunitinib. Sunitinib was resumed at a dose
of 12.5 mg once daily and the plasma concentrations of sunitinib
and its metabolite, SU12662, were analyzed (Fig. 2). However, the patient developed a
dry cough the day after resumption of sunitinib. A computed
tomography scan revealed interstitial pneumonia. Echocardiography
revealed hypokinesis of the left ventricle, which was shown to be
drug-induced heart failure.
Compounds
Sutent 12.5 mg was purchased from Pfizer Global
Research and Development (Japan). Sunitinib malate and
N-desethyl sunitinib (SU12662) were purchased from TRC
(Toronto Research Chemicals, Ontario, Canada). The internal
standard was 4-methyl-mexirethyn.
Pharmacokinetic sampling and assay
Blood sampling (pre-dose, and 2, 4, 6, 8, 10 and 24
h post-dose) was performed on day 7 of course 2 (sunitinib, 25 mg).
Samples (0.5 ml) were collected in tubes containing ethylenediamine
tetraacetic acid (EDTA). Samples were centrifuged at 3,500 rpm at
4°C for 10 min. NaOH (0.1 N) was added to the supernatants, and the
compounds were extracted into 3 ml t-butyl methyl ether
(TBME) and agitated for 5 min. The TBME phase was aspirated and
evaporated to dryness (N2). Aliquots were subjected to
high-performance liquid chromatography. The protocol was approved
by the Medical Ethics Committee of Shinshu University and the
patient provided informed consent prior to the study.
High-performance liquid chromatography
conditions
The chromatographic system consisted of a mobile
phase of mixture [0.05 M phosphoric buffer (pH 3), acetonitrile and
B-7 low UV reagent (Waters, Milford, MA, USA) at a ratio of
695:300:5] with an ODS column pumped at a flow rate of 0.3 ml/min
and UV/VIS detection at 431 nm (0–12 min) and 250 nm (12–20 min)
(7). The retention times for
N-desethyl sunitinib, sunitinib and internal control were
5.8, 8.3 and 14.8 min, respectively.
Results
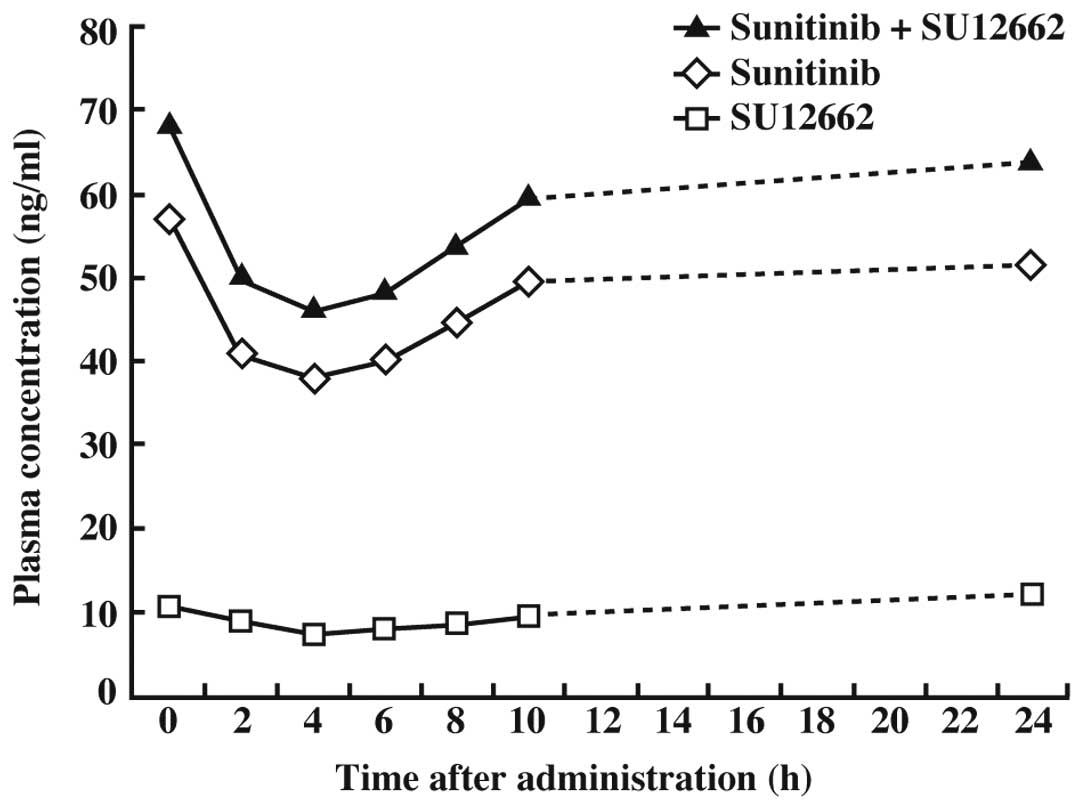
The trough concentrations of sunitinib and SU12662
in plasma after 7 days at 25 mg were 38.0 and 7.4 ng/ml,
respectively, and that of sunitinib plus SU12662 was 46.1 ng/ml.
The average concentration was 56.0 ng/ml (Fig. 2). The area under the curve (AUC) of
sunitinib plus SU12662 was 1,393.0 ng·h/l.
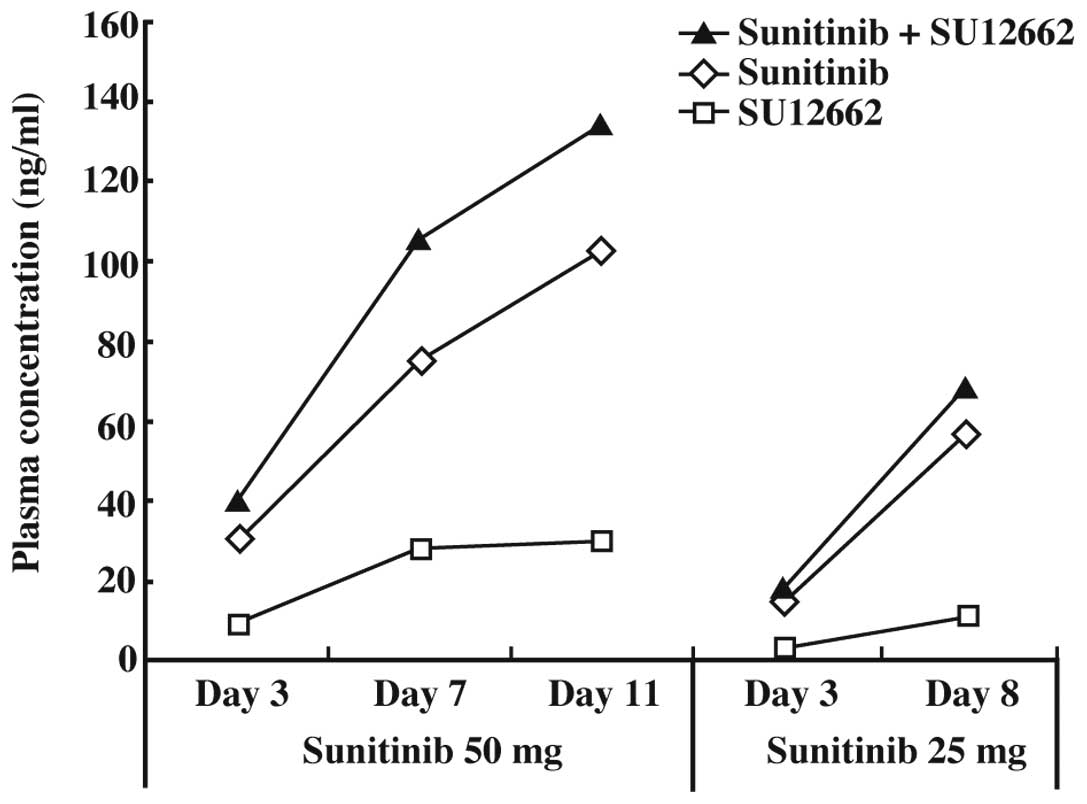
The concentrations of sunitinib, SU12662, and
sunitinib plus SU12662 in plasma at 50 mg were 30.2, 9.2 and 40.1
ng/ml (3 days); 75.2, 28 and 105.3 ng/ml (7 days); and 102.3, 29.5
and 134.0 ng/ml (14 days), respectively (Fig. 3).
Discussion
A plasma concentration above 50 ng/ml of sunitinib
plus SU12662 is required for the inhibition of tyrosine kinase
phosphorylation. Fig. 3 shows that
the plasma concentrations before administration of sunitinib and
sunitinib plus SU12662 11 days after initiation of sunitinib
treatment (50 mg/day) were approximately 100 and 120 ng/ml,
respectively. These concentrations were not troughs since the
trough concentration did not correlate with the plasma
concentration prior to the administration of sunitinib, as shown in
Fig. 2. The results shown in
Fig. 2 indicated that the trough
concentration of sunitinib (50 mg) plus SU12662 at 11 days would
likely be over 50 ng/ml. Sunitinib inhibits Flk-1/KDR and PDGFR
phosphorylation at concentrations over the range of 50–100 ng/ml
(sunitinib plus SU12662) in vivo. It has been reported that
plasma concentrations of sunitinib and SU12662 should be maintained
within the range of 50–100 ng/ml (4). The results shown in Fig. 3 indicate that the plasma
concentration of sunitinib was sufficient to inhibit Flk-1/KDR and
PDGFR in this patient.
Pharmacokinetics in patients with GIST may be
different from those in non-GIST patients. Notably, the
concentration at 4 h after the administration of sunitinib was Cmin
in Fig. 2. This patient underwent
excision of the small intestine, and it was considered that this
excision delayed the absorption of sunitinib. Many patients with
small intestinal GIST undergo excision of the small intestine, and
it is therefore predicted that sunitinib absorption will be delayed
in such cases. Furthermore, the plasma concentrations of SU12662 on
days 7 and 14 at 50 mg sunitinib were the same. This result was due
to the reduced CYP3A4 activity caused by the tumor metastasis in
the left side of the liver.
Reduction of sunitinib may not suppress
thrombocytopenia at the effective concentration. Recently, it was
reported that the adverse effects of sunitinib are correlated with
plasma concentration (8). Diastolic
blood pressure has been shown to be correlated with trough plasma
concentration of total drug (sunitinib plus SU12662). Conversely,
the absolute neutrophil count is correlated with AUCcum28tot
(28-day cumulative AUC of total drug) (8). However, in this patient, the reduction
in plasma concentration did not ameliorate thrombocytopenia.
This case report suggests that sunitinib at a dose
of 50 mg could be an over-dosage in Asian women. Houk et al
reported that the AUC and Cmax of both sunitinib and total drug are
increased in Asians and in females (9). This was suggested to be associated
with a reduction in CYP3A4 activity since both sunitinib and
SU12662 are metabolized only by CYP3A4. Metabolism in the liver is
associated with liver volume (10).
If the liver volume is small, the metabolic capability is also
small. The liver volume is small in Asians and females since the
average weight and height are generally lower than those of
non-Asians and males. Therefore, it is possible to calculate liver
volume by body surface area (11).
The results suggested that the metabolic capability of CYP3A4 is
lower in Asian than non-Asian females.
CYP3A5 is also likely to demonstrate differences in
metabolic ability among individuals. It is difficult to separate
the effects of CYP3A5 and CYP3A4 as they have similar spectra of
substrates. CYP3A5 is the predominant isozyme among human liver
CYP3As (12), and CYP3A5 may be
responsible for the metabolism of sunitinib. However, it has been
reported that there are ethnic and individual differences in the
expression of CYP3A5. The activity of CYP3A5.3 is extremely low and
this CYP3A5*3 genotype was 60% in Japanese or Chinese, 31%
in Indian, 70% in Caucasians, and 35% in African-Americans
(13–15). Thus, it is likely to be involved in
the differences in expression of hepatic CYP3A between ethnicities
and individuals. These differences suggested that plasma
concentration of sunitinib differs between individuals.
We concluded that the monitoring of sunitinib plasma
concentration is essential to determine the appropriate dose in
individual patients. Furthermore, this study showed that the
frequency of thrombocytopenia and hypothyroidism in Asians,
including Japanese, with sunitinib is higher than in Europeans and
Americans. This is because the plasma concentrations in Asians are
higher than those in Europeans and Americans due to the liver
volume being smaller and due to CYP3A5*3 being the major
genotype in Asians.
Acknowledgements
We thank Dr Hajime Ichimura for providing clinical
insights and taking blood samples.
Abbreviations:
|
GIST
|
gastrointestinal stromal tumor
|
|
KIT
|
stem cell factor receptor
|
|
Flk-1/KDR
|
fetal liver kinase-1/kinase-insert
domain-containing receptor
|
|
PDGFR
|
platelet-derived growth factor
receptor-α
|
|
VEGFR
|
vascular endothelial growth factor
receptor
|
|
FLT3
|
FMS-like tyrosine kinase 3
|
|
CSF-1
|
colony-stimulating factor 1
|
|
RET
|
glial cell line-derived neurotrophic
factor receptor (rearranged during transfection)
|
|
CYP
|
cytochrome P450
|
|
AUC
|
area under curve
|
References
|
1
|
Rubin BP: Gastrointestinal stromal
tumours: an update. Histopathology. 48:83–96. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Hormick JL and Fletcher CD: The role of
KIT in the management of patients with gastrointestinal stromal
tumours. Hum Pathol. 38:679–687. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Debiec-Rychter M, Sciot R, Le Cesne A, et
al: KIT mutations and dose selection for imatinib in patients with
advanced gastrointestinal stromal tumours. Eur J Cancer.
42:1093–1103. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Mendel DB, Laird AD, Xin X, et al: In vivo
antitumour activity of SU11248, a novel tyrosine kinase inhibitor
targeting vascular endothelial growth factor and platelet-derived
growth factor receptors: Determination of a
pharmacokinetic/pharmacodynamic relationship. Clin Cancer Res.
9:327–337. 2003.
|
|
5
|
Murray LJ, Abrams TJ, Long KR, et al:
SU11248 inhibits tumor growth and CSF-1R-dependent osteolysis in an
experimental breast cancer bone metastasis model. Clin Exp
Metastasis. 20:757–766. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Chen M, Nafziger AN and Bertino JS Jr:
Drug-metabolizing enzyme inhibition by ketoconazole does not reduce
interindividual variability of CYP3A activity as measured by oral
midazolam. Drug Metab Dispos. 34:2079–2082. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Blanchet B, Saboureau C, Benichou AS, et
al: Development and validation of an HPLC-UV-visible method for
sunitinib quantification in human plasma. Clin Chim Acta.
404:134–139. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Houk BE, Bello CL, Poland B, Rosen LS,
Demetri GD and Moltzer RJ: Relationship between exposure to
sunitinib and efficacy and tolerability endpoints in patients with
cancer: results of a pharmacokinetic/pharmacodynamic meta-analysis.
Cancer Chemther Pharmacol. 66:357–371. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Houk BE, Bello CL, Kang D and Amantea M: A
population pharmacokinetic meta-analysis of sunitinib malate
(SU11248) and its primary metabolite (SU12662) in healthy
volunteers and oncology patients. Clin Cancer Res. 15:2497–2506.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Nakazawa Y, Chisuwa H, Ikegami T, et al:
Relationship between in vivo FK506 clearance and in vitro
13-demethylation activity in liver-related liver transplantation.
Transplantation. 66:1089–1093. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Urata K, Kawasaki S, Matsunami H, et al:
Calculation of child and adult standard liver volume for liver
transplantation. Hepatology. 21:1317–1321. 1995.PubMed/NCBI
|
|
12
|
Kuehl P, Zhang J, Lin Y, et al: Sequence
diversity in CYP3A promoters and characterization of the
genetics basis of polymorphic CYP3A5 expression. Nat Genet.
27:383–391. 2001. View
Article : Google Scholar
|
|
13
|
Saeki M, Saito Y, Nakamura T, et al:
Single nucleotide polymorphisms and haplotype frequencies of CYP3A5
in a Japanese population. Hum Mutat. 21:6532003. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Balram C, Zhou Q, Cheung YB and Lee EJ:
CYP3A5*3 and *6 single nucleotide polymorphisms in
three distinct Asian populations. Eur J Clin Pharmacol. 59:123–126.
2003.
|
|
15
|
Fukuen S, Fukuda T, Maune H, et al: Novel
detection assay by PCR-RFLP and frequency of the CYP3A5 SNPs,
CYP3A5*3 and *6, in a Japanese population. Pharmacogenetics.
12:331–334. 2002.PubMed/NCBI
|