Introduction
Pulmonary nodular lesions (PNLs) are
intraparenchymal lung lesions smaller than 3 cm in diameter, which
are not associated with atelectasis or lymph node enlargement
(1). According to the 2011
International Association for the Study of Lung Cancer (IASLC),
American Thoracic Society (ATS) and European Respiratory Society
(ERS) international multidisciplinary classification of lung
adenocarcinoma, PNLs are classified as: a) a pure ground-glass
nodule (pGGN) as a focal area of increased lung attenuation, which
the margins of any normal structures, e.g., vessels, remain
outlined; b) a solid nodule as a focal area of increased
attenuation of such density that any normal structures, e.g.,
vessels, are completely obscured; c) part-solid nodule as a focal
nodular opacity containing solid and ground-glass components
(2). With the development of
computed tomography (CT), an increasing number of PNLs are
detected. Since more than 50% of resected PNLs are related to
malignancy, the requirement for rapid and definite histological
diagnoses of PNLs has been stressed (3), in order for the treatment to occur as
soon as possible. A transthoracic or transbronchial fine-needle
biopsy may be considered in selected cases; however, the reported
diagnostic yield for PNLs is rather low. An excisional biopsy of
PNLs may be considered, but this requires invasive access, e.g., a
thoracotomy. With the advent of video-assisted thoracoscopic
surgery (VATS), a minimally invasive procedure has emerged that
allows complete resection of PNLs with minimal morbidity. However,
since the PNLs are small and/or far from the pleural surface this
may limit VATS and render the intraoperative identification of PNLs
difficult (4). Thus, failure to
visualize or palpate PNLs has resulted in a conversion thoracotomy
rate of up to 46% (5). Recently, a
hookwire marking system has been proposed that involves
preoperative CT-guided localization under local anesthesia and
anchorage within the lesion. In this study, we describe the
procedure and assess the value of CT-guided hookwire localization
and VATS in 107 PNLs from 103 patients.
Materials and methods
Patient characteristics
Between January 2010 and December 2011, CT-guided
hookwire localization and VATS were conducted on 107 PNLs from 103
patients who underwent CT examination at the Cancer Hospital of
Fudan University (Shanghai, China). This study was composed of 45
males and 58 females, with a mean age of 54 years (range, 16–78
years). Of the 103 patients, 32 had a history of cancer. Patient
characteristics are shown in Table
I. Patients were informed of the risks associated with the
procedure and written consent was obtained from all patients. The
protocol in our study was approved by the institutional review
board of Fudan University Shanghai Cancer Center, Shanghai,
China.
 | Table I.Characteristics of 103 patients who
underwent CT-guided hookwire localization and VATS. |
Table I.
Characteristics of 103 patients who
underwent CT-guided hookwire localization and VATS.
| Characteristic | No. of patients
(%) |
|---|
| Age (years) | |
| ≤40 | 11 (10.7) |
| >40 | 92 (89.3) |
| Gender | |
| Male | 45 (43.7) |
| Female | 58 (56.3) |
| Cancer history | |
| Yes | 32 (31.1) |
| No | 71 (68.9) |
Selection criterion and lesion
characteristics
The selection criterion was based on at least one of
the following CT findings: lesion diameter ≤10 mm, distance from
pleural surface >5 mm, pGGN or a lesion mostly comprised of
GGNs. Lesion characteristics are shown in Table II. The diameter of the lesions
ranged from 3.8 to 25.3 mm (mean, 13.0 mm). The distance of the
lesion from the pleural surface ranged from 1.6 to 40.3 mm (mean,
12.2 mm). A total of 63 (59%) and 44 (41%) lesions displayed solid
and GGNs, respectively. Among the 103 patients, 4 had 2 lesions to
localize, which were situated in various lobes of the right
lung.
 | Table II.Characteristics of 107 PNLs from 103
patients who underwent CT-guided hookwire localization and
VATS. |
Table II.
Characteristics of 107 PNLs from 103
patients who underwent CT-guided hookwire localization and
VATS.
| Characteristic | No. of patients
(%) |
|---|
| Location | |
| Upper lobe of
right lung | 35 (32.7) |
| Middle lobe of
right lung | 6 (5.6) |
| Lower lobe of
right lung | 20 (18.7) |
| Upper lobe of
left lung | 24 (22.4) |
| Lower lobe of
left lung | 22 (20.6) |
| Diameter (mm) | |
| ≤5 | 3 (2.8) |
| 5<d≤10 | 24 (22.4) |
|
10<d<30 | 80 (74.8) |
| Distance from
pleural surface (mm) | |
| ≤5 | 30 (28.0) |
| >5 | 77 (72.0) |
| Density | |
| GGN | 44 (41.1) |
| Solid nodule | 63 (58.9) |
CT-guided hookwire localization
A hookwire system is composed of a calibrated
cannula (21-gauge, 10 cm long) and a 20-cm long calibrated wire
with a thorn. A CT scan was conducted to identify the location,
size and shape of the lesion, as well as the correlation with the
surrounding tissues. We then designed an optimal route by measuring
the distance between the skin and the edge of the lesion and
marking a puncture site. Following disinfection of the skin around
the puncture site and local anesthesia, a narrow-ranged CT scan was
conducted to ensure the correct puncture site, using the injection
needle as a marker. The cannula needle housing the hookwire was
inserted gradually through the chest wall and pulmonary parenchyma
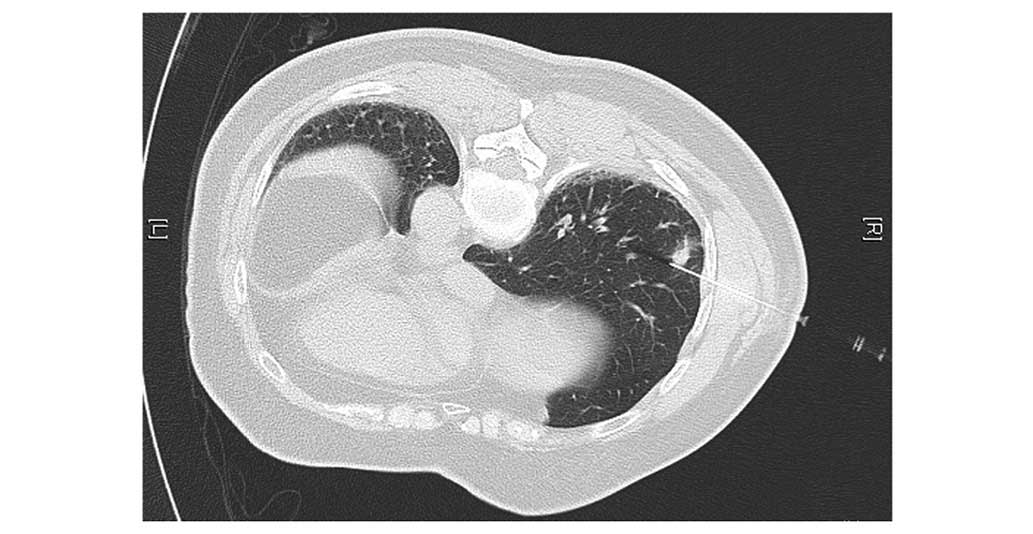
and placed as close as possible to the lesion (Fig. 1). When the outer cannula needle was
withdrawn, the horn of the hookwire was released and a feeling of
resistance emerged when the wire was pulled. A CT scan was repeated
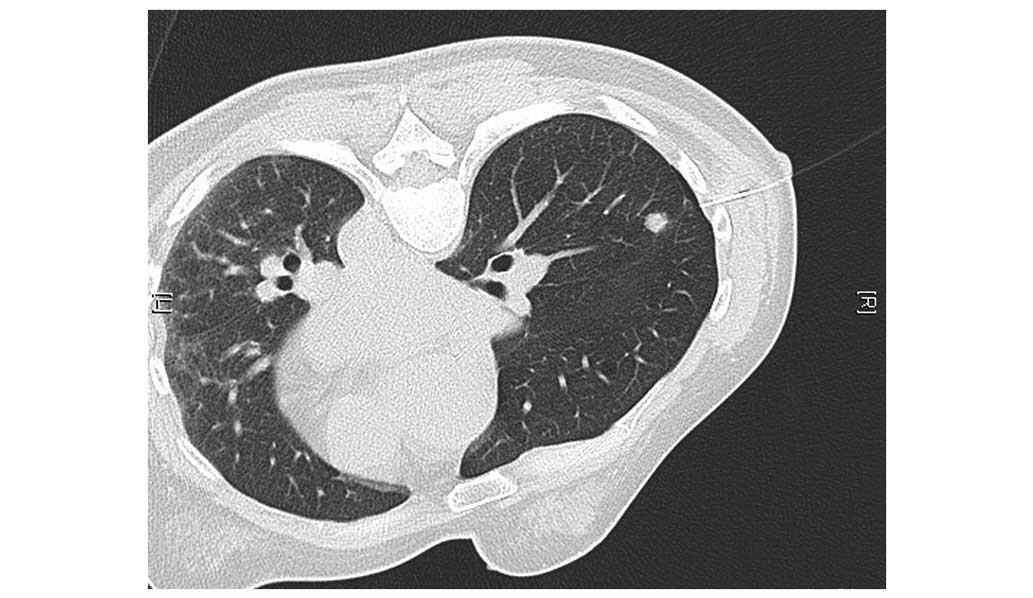
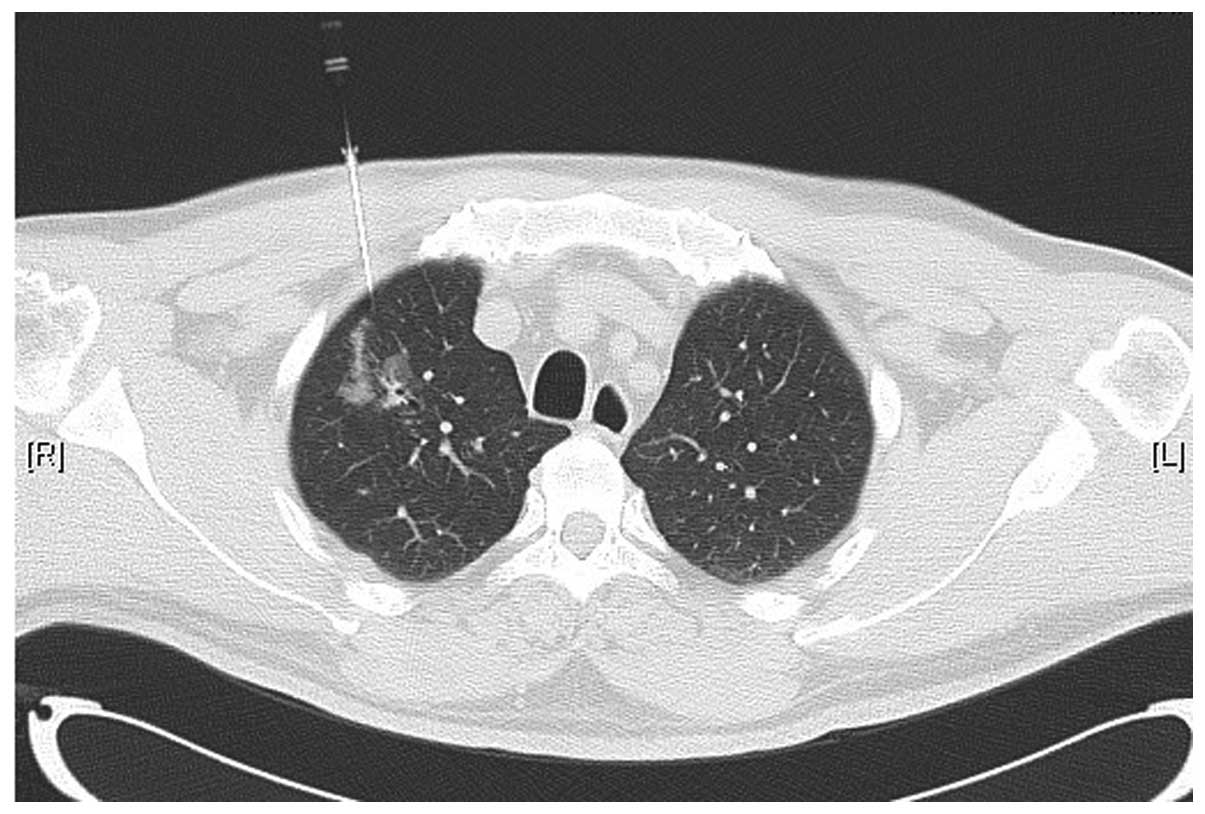
to confirm that the horn anchored the lesion, and to ensure that
the existing complications, including pneumothorax (Fig. 2) and hemorrhage (Fig. 3), were present. The hookwire,
extending outside the chest wall, was positioned carefully on the
skin under gauze dressings. The patient was then transferred to the
operating room for VATS. During this time, the images reconstructed
following localization were uploaded for the thoracic surgeons to
consult.
VATS
Video-assisted thoracoscopic resection of the lesion
was conducted under general anesthesia using single lung
ventilation via a double-lumen endobronchial tube. The procedure
required 2 thoracic ports of 11.5 mm, 1 for the thoracoscope, the
other for the endoscopic stapler, and a 5.5 mm thoracic port for
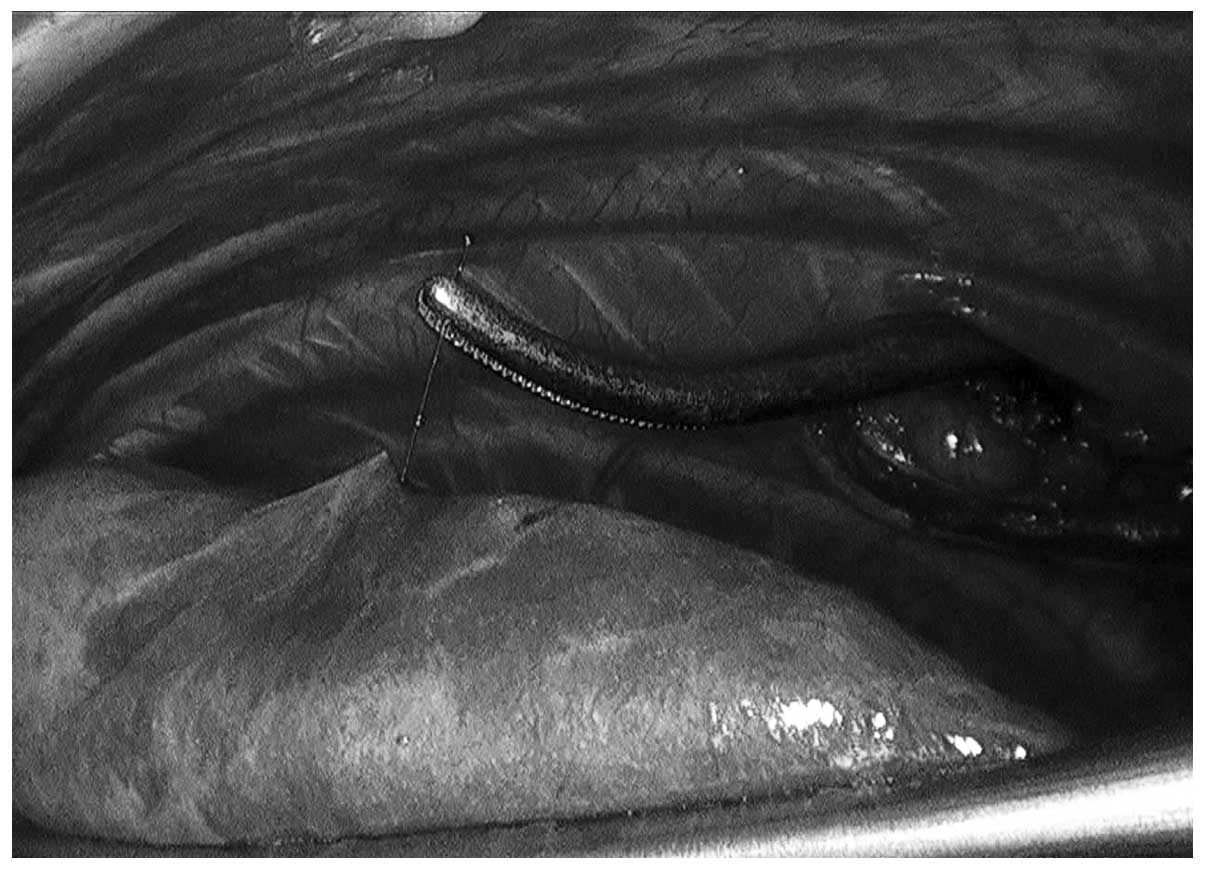
the lung forceps. The hookwire was raised during the procedure, and
the lesion was sequentially resected (Fig. 4). The resected hookwire and lung
tissue was packed into sterile gloves to prevent metastatic
implantation of malignant disease and was withdrawn from the chest
via an intercostal incision. All resected lung specimens were
immediately sent for frozen-section examination. If the
pathological result was benign, a chest tube was inserted and VATS
was conducted following bleeding and air leak exclusion. If the
diagnosis was primary lung cancer, a lobectomy and lymphadenectomy
were conducted, and if necessary, another thoracic incision was
made to facilitate the subsequent thoracoscopic resection. If the
result suggested a metastatic tumor following wedge resection, the
procedure was terminated until a multidisciplinary treatment scheme
was set up. In certain cases, we had to convert to a thoracotomy
due to problems, including adhesion and hookwire dislodgement.
Results
All 107 lesions were localized successfully (100%).
The duration of localization, from the first CT scan to the last,
ranged from 6 to 27 min (mean, 11 min) and the number of needle
insertions or adjustments ranged from 1 to 6 (mean, 2). The
complications of CT-guided hookwire localization were asymptomatic
pneumothorax, asymptomatic hemorrhage and simultaneous pneumothorax
and hemorrhage, which were observed in 26.9, 40.8 and 8.7% of
patients, respectively. VATS was conducted in all 103 patients. A
total of 71 cases underwent wedge resection, including 43 benign
lesions and 28 metastatic lesions. Among the other 32 patients, 28
received a lobectomy and lymphadenectomy and 4 abandoned surgery,
including the two double primary cancer cases, due to their poor
health condition or old age. The mean surgery time (excluding ∼20
min for frozen-section examination) for wedge resection and
lobectomy was 16 min and 95 min, respectively. Conversion
thoracotomy was necessary in 2 patients; 1 PNL demonstrated strong
adherence to the pleural surface, while the other, whose source was
difficult to determine, demonstrated adhesion to the diaphragmatic
muscle. Intraoperative dislodgement occurred in 3 patients,
conversion thoracotomy occurred in 1 case, and the other 2
complications were identified and resected including observed
bleeding from the area upon touching the lesion with a single
finger. A total of 2 patients received a subsequent lobectomy,
according to permanent-section and immunohistochemistry analysis, 1
week later. Postoperative complications of VATS, including 1
alveolar pleural fistula and 1 post-operative acataleptic thoracic
hemorrhage, were both successfully managed by conservative
treatment. The mean hospitalization time following the surgery was
6±3 days; however, the patient with acataleptic hemorrhage was
discharged from hospital after 22 days. The characteristics of
CT-guided hookwire localization and VATS are described in Tables III and IV. All 107 lesions managed to achieve
pathological diagnoses. A total of 40.2% of the lesions were benign
diseases, and detailed results are shown in Table V.
 | Table III.Characteristics of 103 patients who
underwent CT-guided hookwire localization. |
Table III.
Characteristics of 103 patients who
underwent CT-guided hookwire localization.
| Characteristic | Value |
|---|
| Posture, n (%) | |
| Supination | 31 (30.1) |
| Pronation | 45 (43.7) |
| Left lateral
decubitus | 15 (14.6) |
| Right lateral
decubitus | 12 (11.6) |
| Complictions, n
(%) | |
| Pneumothorax | 38 (36.9) |
| Hemorrhage | 42 (40.8) |
| Pneumothorax and
hemorrhage | 9 (8.7) |
| Duration (min) | |
| Range | 6–27 |
| Mean | 11 |
| Number of needle
insertions or adjustments | |
| Range | 1–6 |
| Mean | 2 |
 | Table IV.Characteristics of 107 PNLs from 103
patients who underwent VATS. |
Table IV.
Characteristics of 107 PNLs from 103
patients who underwent VATS.
| Characteristic | Value |
|---|
| Complictions, n
(%) | |
| Alveolar pleural
fistula | 1 (0.97) |
| Hemorrhage | 1 (0.97) |
| Conversion
thoracotomy | 2 (1.94) |
| Dislodgement | 3 (2.91) |
| Surgery time (min),
mean ± SD | |
| Wedge
resection | 16±2 |
| Lobectomy | 95±30 |
| Re-lobectomy, n
(%) | 2 (1.94) |
| Hospitalization
time (days), mean ± SD | 6±3 |
 | Table V.Histological diagnosis of PNLs. |
Table V.
Histological diagnosis of PNLs.
| Characteristic | No. of patients
(%) |
|---|
| Benign | 43 (40.2) |
| Atypical
adenomatoid hyperplasia | 9 |
| Hamartoma | 7 |
| Non-caseating
granuloma | 7 |
| Pulmonary
tuberculosis | 2 |
| Inflammatory
pseudotumor | 1 |
| Bronchial
cyst | 1 |
| Lipoma | 1 |
|
Lymphadenopathy | 1 |
| Other benign
disease | 14 |
| Malignant | 64 (59.8) |
|
Adenocarcinoma | 43 |
| Atypical
adenomatoid hyperplasia with local cancerization | 8 |
| Bronchioalveolar
carcinoma | 3 |
|
Leiomyosarcoma | 3 |
| Poorly
differentiated cancer | 3 |
| Squamous cell
carcinoma | 2 |
| Bronchioles clear
cell carcinoma | 1 |
| Mucoepidermoid
carcinoma | 1 |
Discussion
The diagnosis and treatment of PNLs is a problem
affecting radiologists and clinicians. PNLs have no typical imaging
features and various kinds of biopsies, including percutaneous
puncture biopsy and transbronchial needle biopsy, are much less
invasive than surgery, but are less reliable for ruling out
malignancy due to inadequate tissue sampling or biopsy failure
(6). According to statistics, more
than 50% of resected PNLs are related to malignancy; therefore,
they should be considered potentially malignant until proven
otherwise. For patients with PNLs, surgical resection is the ideal
approach as it is diagnostic and therapeutic (7), and with regard to surgery, VATS may be
the best option. With thoracoscopic technology development and
surgical instrument refinement, VATS has been widely accepted for
the resection of PNLs as it minimizes postoperative morbidity and
saves as much lung tissue volume as possible. Furthermore, the
survival rate is no less than open surgery in patients with stage
Ia disease (8–10). However, certain patients require
conversion to thoracotomy due to the change in anatomical position,
the difficulty in distinguishing GGN with normal lung tissue and
the inability to touch lesions far from the pleural surface
following lung deflation. Therefore, it is necessary to conduct
effective preoperative localization. At present, the most commonly
used localization technique is CT-guided hookwire localization.
Hookwire localization was originally applied in mammary glands.
Then, with the advent of VATS, it was gradually used in localizing
pulmonary lesions (11,12), as well as lesions of the abdominal
cavity, retroperitoneum and muscle, with the advantage of stronger
anchorage and less invasion (13).
In this study, all 107 lesions from 103 patients
were successfully localized. The mean duration of localization was
11±4 min and the mean surgery times for wedge resection and
lobectomy were 16±2 and 95±30 min, respectively. With regard to
surgery time, we did not make a comparison between localization
with and without the CT-guided hookwire system prior to VATS.
However, Ciriaco et al (14)
reported that hookwire localization made VATS resection quicker,
independent of whether it was conducted with preoperative
localization or not. In this study, the average surgery time was
40±7 and 75±12 min (P<0.001), and the average time of CT
hookwire positioning was 20±10 min.
In terms of complications, asymptomatic hemorrhage
and minimal pneumothorax in the procedure of localization did not
require clinical intervention. In the present study, 1 alveolar
pleural fistula and 1 unidentified hemorrhage following VATS were
successfully managed by conservative treatment. Dislodgement
occurred in 3 (2.91%) patients, and 1 case led to thoracotomy. The
dislodgement rate in our study was consistent with a rate of
0.8–20% obtained from previous studies (11,14,15).
With the guidance of hookwire, lesions for frozen-section
examination may be identified more quickly, which will greatly
shorten the intraoperative waiting time. In this study, all 107
lesions achieved pathological diagnoses. Malignant lesions
accounted for 59.8% of cases; this result is similar to 50–70%,
which was demonstrated in certain studies (12,14,16,17),
but significantly higher than 26%, which was demonstrated in others
(6). We suggest that this may be
due to the benign diagnoses of certain patients in this study who
were selected based on imaging results instead of pathological
diagnoses, and the pathological diagnosis of patients lost during
follow-up were not obtained. In our study, the probability of
metastatic tumors in patients with PNLs and with cancer history was
as high as 84.8%.
The first principle of CT-guided localization is to
obtain the shortest needle insertion route. It is agreed that the
insertion of the hookwire should be vertical from the outside chest
into the pleural surface. This not only indicates the definite and
strong anchorage, but it also reduces the damage and complication
incidence. The second principle of CT-guided localization is to
avoid penetration through the lesion, in order for the hookwire to
be placed infinitely near the lesion, but without transfixion.
However, the option of touching or going through the lesion remains
controversial. Certain researchers are concerned that penetrating
through the lesion damages the intact capsula of the lesion and may
prompt potential dissemination of the malignant tumor. However,
Miyoshi et al suggested that no local recurrence of primary
lung cancer was observed when lesions were penetrated (15). In our institute, we prefer the ‘no
penetrating’ principle, which may maintain the integrity of the
lesion. Additionally, penetrating solid nodules increases the
difficulty of localization. The third principle of CT-guided
localization is flexibility and cooperation. For certain lesions
sheltered from bone structure, oblique needling insertion may be
considered. For nodules close to the interlobar pleural, it is
important to avoid damaging 2 lobes of the lung as much as
possible. For lesions in the lower lung, which are easily
influenced by respiration, the cooperation of the patient’s
respiration is of great importance. The final principle of
CT-guided localization is to reduce the dislodgement rate. The
hookwire should not be placed too shallow; it should be placed 2 cm
beyond the edge of the lesion and once released, the free end of
wire is cut off leaving a 2–3 cm tail.
From our experience and previous studies, we
conclude that it is necessary to aggressively treat indeterminate
PNLs. A combination of CT-guided hookwire localization and VATS for
PNLs is a safe and effective procedure for accurate diagnosis and
resection of indeterminate PNLs, with no associated mortality or
significant morbidity.
Acknowledgements
This study was supported by the
Science Technology Commission of Shanghai Municipality
(0952nm03400, 11nm0504000).
References
|
1.
|
RG FraserC SandersGT BarnesDigital imaging
of the
chestRadiology171297307198910.1148/radiology.171.2.26499132649913
|
|
2.
|
WD TravisE BrambillaM NoguchiInternational
association for the study of lung cancer/american thoracic
society/european respiratory society international
multidisciplinary classification of lung adenocarcinomaJ Thorac
Oncol6244285201110.1097/JTO.0b013e318206a221
|
|
3.
|
PL ShahS SinghM BowerThe role of
transbronchial fine needle aspiration in an integrated care pathway
for the assessment of patients with suspected lung cancerJ Thorac
Oncol1324327200610.1097/01243894-200605000-0001017409878
|
|
4.
|
MC AmbrogiF MelfiC ZirafaRadio-guided
thoracoscopic surgery (RGTS) of small pulmonary nodulesSurg
Endosc26914919201210.1007/s00464-011-1967-822011947
|
|
5.
|
K SuzukiK NagaiJ YoshidaVideo-assisted
thoracoscopic surgery for small indeterminate pulmonary nodules:
indications for preoperative
markingChest115563568199910.1378/chest.115.2.56310027460
|
|
6.
|
K AlzahouriM VeltenP ArveuxManagement of
SPN in France. Pathways for definitive diagnosis of solitary
pulmonary nodule: a multicentre study in 18 French districtsBMC
Cancer893200810.1186/1471-2407-8-9318402653
|
|
7.
|
BB TanKR FlahertyEA KazerooniThe solitary
pulmonary
noduleChest12389S96S200310.1378/chest.123.1_suppl.89S12527568
|
|
8.
|
WS WalkerM CodispotiSY SoonLong-term
outcomes following VATS lobectomy for non-small cell bronchogenic
carcinomaEur J Cardiothorac
Surg23397402200310.1016/s1010-7940(02)00814-x12614813
|
|
9.
|
M OdaI MatsumotoM TamuraVideoassisted
thoracic surgery for clinical stage I lung cancerKyobu
Geka622812842009(In Japanese).
|
|
10.
|
TD YanD BlackPG BannonBC
McCaughanSystematic review and meta-analysis of randomized and
nonrandomized trials on safety and efficacy of video-assisted
thoracic surgery lobectomy for early-stage non-small-cell lung
cancerJ Clin
Oncol2725532562200910.1200/JCO.2008.18.273319289625
|
|
11.
|
YR ChenKM YeowJY LeeCT-guided hook wire
localization of subpleural lung lesions for video-assisted
thoracoscopic surgery (VATS)J Formos Med
Assoc106911918200710.1016/S0929-6646(08)60061-318063512
|
|
12.
|
O PittetM ChristodoulouE
PezzettaVideo-assisted thoracoscopic resection of a small pulmonary
nodule after computed tomography-guided localization with a
hook-wire system. Experience in 45 consecutive paientsWorld J
Surg31575578200710.1007/s00268-006-0343-7
|
|
13.
|
WB MorrisenTG SandersTW ParsonsBJ
PenrodPreoperative CT-guided hookwire needle localization of
musculoskeletal lesionsAJR Am J
Roentgenol17615311533200110.2214/ajr.176.6.176153111373227
|
|
14.
|
P CiriacoG NegriA PuglisiVideo-assisted
thoracoscopic surgery for pulmonary nodules: rationale for
preoperative computed tomography-guided hookwire localizationEur J
Cardiothorac Surg25429433200410.1016/j.ejcts.2003.11.036
|
|
15.
|
K MiyoshiS ToyookaH GobaraClinical
outcomes of short hook wire and suture marking system in
thoracoscopic resection for pulmonary nodulesEur J Cardiothorac
Surg36378382200910.1016/j.ejcts.2009.03.03919414272
|
|
16.
|
S ChenJ ZhouJ ZhangVideo-assisted
thoracoscopic solitary pulmonary nodule resection after CT-guided
hookwire localization: 43 cases report and literature reviewSurg
Endosc2517231729201110.1007/s00464-010-1502-3
|
|
17.
|
S HiraiY HamanakaN MitsuiRole of
video-assisted thoracic surgery for the diagnosis of indeterminate
pulmonary noduleAnn Thorac Cardiovasc Surg12388392200617228275
|