Introduction
Preoperative neoadjuvant chemotherapy (NAC) is a
promising method for improving the breast-conserving treatment of
operable breast cancer (1,2). Based on large scale trials that
clearly demonstrate the feasibility and efficacy of NAC, it is now
the treatment of choice for these patients as it effectively
improves prognoses in a practical clinical setting. In 2001,
Wolmark et al demonstrated that NAC, four cycles of
doxorubicin and cyclophosphamide (AC), had a similar effect on
overall survival (OS) and disease-free survival (DFS) to that of
chemotherapy administered post-operatively (1). Van der Hage et al compared the
efficacy of four cycles of combination chemotherapy with
cyclophosphamide, epirubicin and fluorouracil (CEF) that were
administered preoperatively with that of the same chemotherapy
regimen administered postoperatively in patients with operable
breast cancer. The study identified no difference in terms of OS
and progression-free survival (PFS) between patients treated with
NAC and those treated with postoperative adjuvant chemotherapy
(2).
NAC also has an advantage in that the response to
anti-cancer agents can be precisely monitored by examining surgical
specimens after treatment. In one study (NSABP B-27), preoperative
chemotherapy with AC followed by docetaxel increased the
pathological complete response rate (pCR; the disappearance of all
clinical evidence of disease) compared with preoperative AC alone
(26.1 vs. 13.7%, respectively) (3),
and a significant improvement in prognosis was observed for
patients demonstrating pCR (4).
This suggests that pCR is a significant predictor of prognosis.
Currently, combination chemotherapy with
anthracyclines and taxanes is considered to be the most effective
method for achieving the maximal effect of adjuvant therapy in
patients with operable breast cancer with lymph node metastasis
(3,5–7).
However, the best protocol/schedule for administering these drugs
has not been identified. Similar questions also arise regarding
NAC. Bonneterre et al revealed that the FEC 100 protocol
yielded significant improvements over the FEC 50 protocol in terms
of DFS and OS after 10 years of follow-up when used as adjuvant
chemotherapy for patients with operable breast cancer with lymph
node metastasis. The treatment had an acceptable rate of adverse
events and no toxicity-related mortalities (8). Another trial demonstrated the efficacy
of weekly paclitaxel followed by four cycles of
fluorouracil/doxorubicin/cyclophosphamide in patients with stage
I–III breast cancer. Patients receiving weekly paclitaxel had a
higher pCR rate (28.2%) than those treated with a ‘once every 3
weeks’ protocol (15.7%; P=0.02) (9,10).
Therefore, based on recent evidence, FEC 100 followed by weekly
paclitaxel was selected as the standard NAC regimen most likely to
have a maximal effect on locally advanced operable breast cancer.
In this study, we present the treatment results and discuss the
clinical significance of this NAC protocol for operable breast
cancer patients.
Patients and methods
Patients
A total of 54 females with histologically confirmed
non-inflammatory invasive ductal carcinoma of the breast with stage
IIA to IIIA disease [according to the UICC Classification of Breast
Cancer (6)] were included in the
study. Patients with stage IIA disease without lymph node
involvement were excluded. All patients received NAC as the initial
treatment at the Department of Surgical Oncology, Osaka City
University Graduate School of Medicine, Osaka, Japan, between
December 2005 and May 2009. The histological diagnosis for each
patient was made by core needle biopsy of the tumor. Cancer cell
expression of estrogen receptors (ERs), progesterone receptors
(PRs) and the Her-2/neu protein was also evaluated before
initiating therapy. A proportion of patients with tumors
overexpressing Her-2/neu were analyzed separately as they had
received anti-Her-2/neu therapy in addition to NAC after 2008. To
confirm that they met the inclusion criteria, all patients
underwent a whole body evaluation that included a complete medical
history and physical examination, a complete blood count, a blood
chemistry profile, an enhanced computed tomography scan of the
lungs and liver and a bone scan. Patients who had been treated with
other chemotherapeutic agents, those not suitable for NAC, pregnant
patients and those with evidence of distant metastasis, ischemic
heart disease or hepato-renal dysfunction were excluded from the
study. Individual patient characteristics are shown in Table I. The age of the patients ranged
from 26 to 68 years (mean, 50.2) and the tumor size ranged from 15
to 60 mm (mean, 30.4). Patients were followed-up for 6 to 55 months
(average, 29). Written informed consent was obtained from the
patients prior to the study. This study was conducted with the
approval of the Ethical Committee of Osaka City University Graduate
School of Medicine.
 | Table I.Clinical and pathological responses
to neoadjuvant chemotherapy. |
Table I.
Clinical and pathological responses
to neoadjuvant chemotherapy.
| Factors | No. of patients
(%) | cCR | P-value | pCR | P-value |
|---|
| Age (years) | | | | | |
| ≤50 | 30 (55.6) | 6 (20) | 0.46 | 13 (43) | 0.45 |
| >51 | 24 (44.4) | 3 (13) | | 8 (33) | |
| Stage | | | | | |
| IIA | 9 (16.7) | 1 (11) | 0.03 | 1 (11) | 0.10 |
| IIB | 31 (57.4) | 3 (10) | | 12 (39) | |
| III | 14 (25.9) | 5 (36) | | 8 (57) | |
| T factor | | | | | |
| T1 | 3 (5.6) | 2 (67) | 0.16 | 2 (67) | 0.31 |
| T2 | 48 (88.9) | 7 (15) | | 18 (38) | |
| T3 | 3 (5.6) | 0 (0) | | 1 (33) | |
| N factor | | | | | |
| N0 | 8 (14.8) | 1 (13) | 0.03 | 1 (13) | 0.10 |
| N1 | 32 (59.3) | 3 (9) | | 12 (38) | |
| N2 | 14 (25.9) | 5 (36) | | 8 (57) | |
| Receptor
status | | | | | |
| ER+/PR+ | 24 (44.4) | 3 (13) | 0.36 | 7 (29) | 0.08 |
| ER+/PR− | 9 (16.7) | 1 (11) | | 4 (44) | |
| ER−/PR+ | 4 (7.4) | 1 (25) | | 1 (25) | |
| ER−/PR− | 17 (31.5) | 4 (24) | | 9 (53) | |
| Her2 status | | | | | |
| Her2+ | 4 (7.4) | 1 (25) | 0.66 | 3 (75) | 0.13 |
| Her2− | 49 (90.7) | 8 (16) | | 18 (36) | |
| Combination | | | | | |
| HR+/HER2+ | 1 (1.9) | 1 (100) | | 1 (100) | |
| HR+/HER2− | 35 (64.8) | 4 (11) | | 10 (29) | |
| HR−/HER2+ | 3 (5.6) | 0 (0) | | 2 (67) | |
| HR−/HER2− | 15 (27.9) | 4 (27) | | 8 (53) | |
| Total | 54 | 9 (17) | | 21 (39) | |
Chemotherapy regimen
Chemotherapy was administered in an outpatient
setting before loco-regional therapy. The FEC 100 regimen consisted
of intravenous administration of fluorouracil (500
mg/m2), cyclophosphamide (500 mg/m2) and
epirubicin (100 mg/m2) on day 1. This regimen was
administered every 21 days for four cycles, followed by a
once-weekly intravenous infusion of paclitaxel (80
mg/m2) for 12 weeks. Patients administered a full dose
of each chemotherapeutic agent over 26 weeks (with no delay greater
than 2 weeks) were classified as the high-dose intensity (high-DI)
group. Common Terminology Criteria for Adverse Events (CTCAE)
version 3.0 was used to assess treatment-related toxicity.
Loco-regional treatment
Surgery (total or partial mastectomy associated with
axillary lymph node dissection) was scheduled 3 weeks following the
termination of paclitaxel administration. The preserved breast
tissue was treated with extra-beam radiation (50 Gy) after
breast-conserving mastectomy.
Clinical and pathological evaluation of
treatment responses
Surgical specimens were fixed in buffered formalin
and cut into 5-mm sections. The tissue was then examined by a
pathologist (K.W.) after conventional H&E staining. Response
Evaluation Criteria in Solid Tumors (RECIST version 1.1) was used
to assess the chemotherapeutic response of each tumor. The size of
the breast tumors and any axillary lymph node metastases were
assessed by ultrasound (US) examination. Clinical complete response
(cCR) was defined as the clinical absence of all cancerous lesions
in the breast and lymph nodes as assessed by US. Pathological
findings were evaluated in accordance with the ‘General Rules for
Clinical and Pathological Recording of Breast Cancer’ 16th edition
(9). For those patients that
underwent breast-conserving surgery, surgical specimens were
treated and evaluated as described above. For patients that
underwent mastectomy, the area immediately surrounding the tumor
(including at least 2 cm of marginal tissue) was examined
pathologically and compared with photographs and images recorded
before NAC. pCR was defined as no histopathological evidence of
viable cancer cells in the breast or dissected axillary lymph nodes
(1). Surgically resected breast and
axillary node specimens were evaluated to assess the pathological
tumor response. Patients with no evidence of invasive breast cancer
were considered to have a pCR. This included patients in whom only
non-invasive or in situ cancer identified in the breast
specimen, as well as those in whom no residual cancer cells were
identified.
Immunohistochemistry (IHC) and
fluorescence in situ hybridization (FISH)
All tumors were examined for ER and PR expression by
IHC. Patients were classified as having ER- and/or PR-positive
tumors when >10% of cancer cells demonstrated positive staining
by IHC. Her-2/neu expression was also investigated using IHC and/or
FISH. Tumors were classified as Her-2/neu-positive if they were
scored as 3+ by IHC, or if gene amplification (>2.2-fold
increase in fluorescence compared with that in the centromere) was
identified by FISH.
Statistical analysis
The χ2 test was used to compare the
factors and the response rates between the subgroups. P<0.05 was
considered to indicate a statistically significant difference.
Results
cCR and pCR of the patients
The cCR and pCR of the patients are shown in
Table II. Nine (16.7%) of the 54
patients were considered to have no residual tumor upon
preoperative US examination after NAC (i.e., a cCR) and, overall,
49 patients (90.7%) demonstrated a clinical response after NAC.
Histological evaluation of the surgical specimens revealed that
21/54 (38.9%) of patients had a pCR.
 | Table II.Response rates to neoadjuvant
chemotherapy. |
Table II.
Response rates to neoadjuvant
chemotherapy.
| Response | Complete response
(%) | Partial response
(%) | Stable disease
(%) | Progressive disease
(%) |
|---|
| Clinical | 9 (16.7) | 40 (74.1) | 4 (7.4) | 1 (1.9) |
| Pathological | 21 (38.9) | 28 (51.9) | 4 (7.4) | 1 (1.9) |
One patient (1.9%) still displayed evidence of
progressive disease after the 3rd week of paclitaxel
administration, and surgery was performed 33 days after NAC was
terminated. The NAC regimen was amended in five patients due to
adverse events. NAC was terminated in one patient who suffered
drug-induced pneumonia, and the paclitaxel regimen was changed to
‘once every three weeks’ in four patients due to repeated episodes
of grade 3 neutropenia caused by weekly administration. Overall, 48
patients (88.9%) completed the full treatment course. In three
patients, one or more of the administration schedules had to be
postponed for more than 2 weeks due to persistent grade 3
neutropenia. Thus, 45 patients (83.3%) completed the original
regimen within the scheduled period (high-DI group). The pCR rate
of patients administered weekly paclitaxel was 46.7% (21/45). By
contrast, none of the nine patients that did not receive paclitaxel
according to the original schedule demonstrated pCR (P<0.02).
Breast-conserving surgery was performed in 24 patients (44.4%).
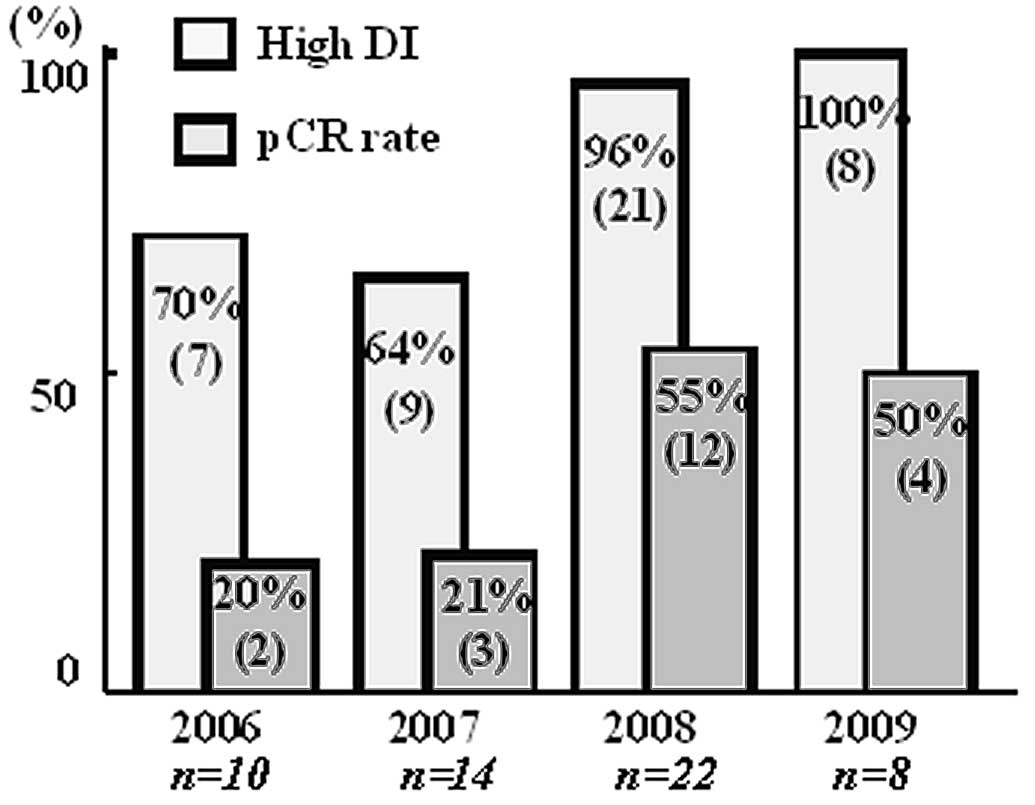
The annual pCR rates increased over the years
(Fig. 1) and were significantly
higher after 2008 compared to before 2007. The chemotherapeutic
schedules were adhered to more completely each year, and the pCR
rate in the high-DI group was significantly higher after 2008 than
before 2007 (Fig. 1). There was no
evidence of recurrence in the patients with pCR, while three
patients who did not demonstrate pCR had a recurrence during the 6
to 55 month follow-up period.
The factors influencing the pathological responses
were also analyzed (Table I).
Although not statistically significant, pCR was associated with a
younger age, larger tumors and greater lymph node involvement.
Thus, higher pCR rates were observed in those patients at a more
advanced clinical disease stage (stage IIA, 11%; stage IIB, 39%;
stage IIIA, 57%). Patients with ER- and PR-negative tumors had
significantly higher pCR rates (56%) than those with ER- and
PR-positive tumors (31%).
NAC toxicity
NAC toxicity is shown in Table III. No adverse events of grade ≤2
required treatment or a delay in treatment. Thirteen patients
(24.1%) suffered grade 3 febrile neutropenia (FN) during the FEC
100 protocol. Three of these (23.1%) also suffered FN while
receiving weekly paclitaxel. Nine of the 13 patients (76.9%)
required hospitalization and recovered after treatment with
antibiotics and granulocyte-colony stimulation factor (10). None of these patients required a
postponement of FEC 100 or paclitaxel administration. Toxicities of
grade >3 included neurotoxicity (3 patients; 5.6%), liver
dysfunction (1 patient; 1.9%) and interstitial pneumonia (1
patient; 1.9%). Chemotherapy was terminated after the 11th dose of
paclitaxel in the patient suffering interstitial pneumonia. The
patient recovered immediately following methylpredonisolone
infusion and underwent surgery with no complications 26 days later
after recovery from respiratory symptoms, which included hypoxemia
and coughing. The clinical and pathological responses of the
patient's tumor were classified as cCR and pCR, respectively. In
four patients, paclitaxel administration was delayed for 2 weeks
due to grade 3 neutropenia during the 2nd, 3th, 4th and 9th weeks.
The protocol was then changed to 175 mg/m2 paclitaxel
once every three weeks to minimize further adverse events.
 | Table III.Adverse effects of neo-adjuvant
chemotherapy. |
Table III.
Adverse effects of neo-adjuvant
chemotherapy.
| Adverse event | Grade 2 or less
(%) | Grade 3 (%) | Grade 4 (%) |
|---|
| Febrile
neutropenia | - | 13 (24.1) | 0 (0) |
| Neuropathy | 6 (11.1) | 3 (5.6) | 0 (0) |
| Hepatopathy | 3 (5.6) | 1 (1.9) | 0 (0) |
| Interstitial
pneumonitis | 0 (0) | 1 (1.9) | 0 (0) |
Surgery was performed within 18 to 45 days (average,
23) after the final dose of NAC. None of the patients who completed
the full NAC regimen demonstrated evidence of persistent adverse
events for more than 3 weeks; therefore, none of the scheduled
surgeries required postponing or cancellation.
Discussion
Several studies have examined the pathological
responses of tumors after chemotherapy with NAC. According to
several published prospective randomized case control studies, the
pCR rates after anthracycline- and taxane-based NAC for locally
advanced breast cancer were between 26.1 and 34% in a heterogeneous
group of patients (3,12,13).
The pCR rate in the present retrospective study was as high as
38.9%.
In this study, a considerable difference between cCR
and pCR rate was observed (Table
II). The clinical response to NAC is usually evaluated using US
as the technique is non-invasive and inexpensive to perform
repeatedly. However, it is difficult to distinguish small,
shrinking cancer nests from non-cancerous scar tissue using imaging
studies alone. Therefore, imaging studies tend to under-estimate
response rates. For pathological examination, a method based on the
NSABP B-18 protocol (1) was used,
which is universally accepted. Although more long-term observations
may be necessary to confirm the accuracy of our pCR data for
predicting prognosis, the short-term results suggest a good
prognosis for the pCR group, which indicates that our evaluation
methods were appropriate.
A total of 18 patients (33.3%) developed adverse
events of grade 3 or higher. However, all of these were managed
using standard conservative treatments, and there were no
persisting sequelae after NAC. Nine patients (16.7%) required
hospital admission due to FN. However, all recovered within a week
and continued NAC in the outpatient setting without any need to
reduce the dose. FN accounts for 8–22% of all adverse events during
chemotherapy (3–8,11),
although it is a short-term event when managed appropriately
(10), as demonstrated in the
present study. Grade 3 neurotoxicity occurred in three patients
during paclitaxel administration. One patient suffered from
interstitial pneumonia, which required the termination of
paclitaxel therapy. The patient recovered immediately after an
infusion of methylpredonisolone and underwent surgery without
complications. The incidence of interstitial pneumonia during
paclitaxel administration is reported up be approximately 1%
(14). Our results were in
accordance with these observations. Overall, 83.3% of patients
completed the full schedule in the present study, which
demonstrates the feasibility and safety of the protocol. The high
response and completion rates observed for this protocol are
valuable parameters when considering a standard protocol for
NAC.
The relatively low pCR rates observed for
ER-positive tumors indicate that tumors of this subtype are
resistant to chemotherapy. Similar results have been observed in
other chemotherapeutic studies (15).
Notably, the pCR rate among patients with extensive
lymphatic involvement (N2) was higher than that in patients with N0
or N1 involvement. Thus, the results revealed that patients with a
higher disease stage responded better than those with a lower
disease stage. In practice, adding taxane to anthracycline-based
chemotherapy is only indicated for node-positive cases (6). At present, we have no logical
explanation as to why the node-positive tumors demonstrated the
best response rates in the present study. However, it would be of
use to investigate the drug distribution and characteristics of the
cancer cells that spread to the lymphatic system in these
cases.
In the present study, an increasing pCR rate each
year was observed, along with an increasing number of patients in
the high-DI group. As reported by Citron et al, the
dose-density of chemotherapy is an important factor for improving
clinical outcomes (16). However,
in a practical setting, the prevention of adverse effects should be
the first consideration and, more importantly, the completion of
the treatment protocol. At the same time, being too cautious due to
fear of possible side-effects may impair the achievement of
high-DI. During the early period of this study, the schedule was
often postponed due to the fear of inducing repeated and
life-threatening episodes of FN. This was less evident during the
later period of the study. However, immediate therapy of FN using
recommended treatments (9) resulted
in rapid recovery without any marked delay in subsequent drug
administrations. Moreover, it appears that severe FN does not occur
during weekly administration of paclitaxel. Therefore, this
protocol may be performed safely and without any delay to maintain
high-DI. The results suggest that dose-intensity is the most
significant factor for achieving a high pCR rate in the NAC
setting.
This retrospective study demonstrates that a high
pCR rate was achieved in patients with locally advanced operable
breast cancer using a NAC regimen comprising FEC followed by
paclitaxel. We consider that this will be a useful regimen, which
is both safe and effective. Further prospective studies
incorporating long follow-up periods are required to confirm the
efficacy of this regimen. Moreover, further studies evaluating the
addition of other anti-tumor drugs, including molecular targeting
agents, may lead to enhanced efficacy (17,18).
References
|
1.
|
N WolmarkJ WangE MamonasB
FisherPreoperative chemotherapy in patients with operative breast
cancer: nine-year results from National Surgical Adjuvant Breast
and Bowel Project B-18J Natl Cancer Inst
Monogr3096102200111773300
|
|
2.
|
JA van der HageCJ van de VeldeJP JulienM
Tubiana-HulinC VanderveldenL DuchateauPreoperative chemotherapy in
primary operable breast cancer: results from the European
Organization for Research and Treatment of Cancer trial 10902J Clin
Oncol19422442372001
|
|
3.
|
HD BearS AndersonA BrownNational Surgical
Adjuvant Breast and Bowel Project Protocol B-27: The effect on
tumor response of adding sequential preoperative docetaxel to
preoperative doxorubicin and cyclophosphamide: preliminary results
from National Surgical Adjuvant Breast and Bowel Project B-27J Clin
Oncol21416541742003
|
|
4.
|
HD BearS AndersonRE SmithSequential
preoperative or postoperative docetaxel added to preoperative
doxorubicin plus cyclophosphamide for operable breast cancer:
National Surgical Adjuvant Breast and Bowel Project Protocol B-27J
Clin Oncol2420192027200610.1200/JCO.2005.04.1665
|
|
5.
|
IC HerdersonDA BerryGD DemetriImproved
outcomes from adding sequential paclitaxel but not from escalating
doxorubicin dose in an adjuvant chemotherapy regimen for patients
with node-positive primary breast cancerJ Clin
Oncol21976983200310.1200/JCO.2003.02.063
|
|
6.
|
EP MamonasJ BryantBC LemberskyPaclitaxel
after doxorubicin puls cyclophosphamide as adjuvant chemotherapy
for node-positive breast cancer: results from NSABP B-28J Clin
Oncol2336863896200510.1200/JCO.2005.10.51715897552
|
|
7.
|
H RochéP FumoleauM SpielmannSequential
adjuvant epirubicin-based and docetaxel chemotherapy for
node-positive breast cancer patients: the FNCLCC PACS 01 TrialJ
Clin Oncol24566456712006
|
|
8.
|
J BonneterreH RochéP KerbratEpirubicin
increases long-term survival in adjuvant chemotherapy of patients
with poor-prognosis, node-positive, early breast cancer: 10-year
follow-up results of the French Adjuvant Study Group 05 randomized
trialJ Clin Oncol23268629932005
|
|
9.
|
The Japanese Breast Cancer SocietyGeneral
Rules for Clinical and Pathological Recording of Breast Cancer16th
editionKanehara Shuppan602008
|
|
10.
|
VA MorrisonAn overview of the management
of infection and febrile neutropenia in patients with cancerSupport
Cancer Ther28894200510.3816/SCT.2005.n.00218628193
|
|
11.
|
JA SparanoM WangS MartinoWeekly paclitaxel
in the adjuvant treatment of breast cancerN Engl J
Med35816631671200810.1056/NEJMoa070705618420499
|
|
12.
|
MC GreenAU BuzdarT SmithWeekly paclitaxel
improves pathologic complete remission in operable breast cancer
when compared with paclitaxel once every 3 weeksJ Clin
Oncol2359835992200510.1200/JCO.2005.06.23216087943
|
|
13.
|
IC SmithSD HeysAW HutcheonNeoadjuvant
chemotherapy in breast cancer: significantly enhanced response with
docetaxelJ Clin
Oncol2014561466200210.1200/JCO.20.6.145611896092
|
|
14.
|
A KhanD McNallyPJ TutschkaS
BilgramiPaclitaxel-induced acute bilateral pneumonitisAnn
Pharmacother311471147419979416383
|
|
15.
|
DF HayesAD ThorLG DresslerHER2 and
response to paclitaxel in node-positive breast cancerN Engl J
Med35714961506200710.1056/NEJMoa07116717928597
|
|
16.
|
ML CitronDA BerryC CirrincioneRandomized
trial of dose-dense versus conventionally scheduled and sequential
versus concurrent combination chemotherapy as postoperative
adjuvant treatment of node-positive primary breast cancer: first
report of Intergroup Trial C9741/Cancer and Leukemia Group B Trial
9741J Clin Oncol21143114392003
|
|
17.
|
AU BuzdarNK IbrahimD FrancisSignificantly
higher pathologic complete remission rate after neoadjuvant therapy
with trastuzumab, pacritaxel and epirubicin chemotherapy: results
of a randomized trial in human epidermal growth factor receptor
2-positive operable breast cancerJ Clin
Oncol2336763685200510.1200/JCO.2005.07.032
|
|
18.
|
R GrayS BhattacharyaC BowdenK MillerRL
ComisIndependent review of E2100: a phase III trial of bevacizumab
plus paclitaxel versus paclitaxel in women with metastatic breast
cancerJ Clin
Oncol2712151221200910.1200/JCO.2008.21.663019720913
|