Introduction
Hepatocellular carcinoma (HCC) is the third most
common cause of cancer-related mortality worldwide, accounting for
approximately 500,000 mortalities annually. The majority of
liver-related cancer cases occur in Asia and Africa (1). HCC has a long latency period, but is
often diagnosed at late stages when the tumors are of high grade
and progress rapidly. At present, only one third of newly diagnosed
HCC patients are eligible for potential curative therapies,
including hepatic resection, liver transplantation, transcatheter
arterial chemoembolization or radiofrequency ablation (2).
Despite the uncertain clinical benefits, patients
with advanced disease are routinely treated with transcatheter
arterial chemoembolization and systemic chemotherapy using
5-fluorouracil (5-FU), doxorubicin, cisplatin or interferon
(3). However, a sizable proportion
of HCC patients do not respond to the cytotoxic effects of 5-FU
treatment, which is mainly due to drug-resistant cancer cells.
Therefore, new strategies for enhancing the sensitivity of cancer
cells to drug-induced apoptosis for cancer therapy have been
intensively explored.
Polycomb group (PcG) proteins are epigenetic
chromatin modifiers involved in cancer development. The
B-cell-specific Moloney murine leukemia virus insertion site 1 gene
(Bmi-1), the first PcG gene to be identified, was originally
discovered as an oncogene that cooperated with c-Myc in the
initiation of lymphoma in murine models (4–6). Bmi-1
is overexpressed in several carcinomas, including liver carcinoma
(7). Overexpression of Bmi-1 was
also observed in a significant number of HCC cases, and was
correlated with advanced invasive stage of tumor progression and
poor prognosis (8). Recently, it
has been reported that the downregulation of Bmi-1 may result in
apoptosis of cancer cells (9).
Therefore, we hypothesize that the abrogation of Bmi-1 expression
may be an effective strategy for sensitizing human cancer cells,
including HCC cells, to cancer chemotherapy.
The present study was designed to demonstrate the
in vitro anticancer effects of the combination of 5-FU
treatment and Bmi-1 depletion on hepatoma cells (SK-HEP-1 and
SMMC-7721), with an emphasis on their in vitro molecular
targets. Our results suggest that the combination of 5-FU treatment
and Bmi-1 depletion may be a potential clinical strategy for cancer
chemotherapy.
Materials and methods
Cell culture
The HCC cell lines SK-HEP-1 and SMMC-7721 were
maintained in Dulbecco’s modified Eagle’s medium (DMEM; Gibco,
Invitrogen Corporation, Carlsbad, CA, USA), supplemented with 10%
fetal bovine serum (Gibco), 100 U/ml penicillin and 100 U/ml
streptomycin (Gibco), and cultured at 37°C in a 5% CO2
humidified incubator.
Gene knockdown
To knockdown Bmi-1, SK-HEP-1 and SMMC-7721 cell
lines were transfected with 50 nmol/l of Bmi-1 targeted small
interfering RNA (siRNA) oligo-nucleotides (siBmi1;
5′-AAAUGGACAUACCUAAUAC-3′) or negative control siRNA
oligonucleotides (siNS; 5′-ACGCATGCATGCTTGCTTT-3′) (Invitrogen Life
Technologies).
Measurement of cytotoxicity
SK-HEP-1 (2×103) and SMMC-7721
(3×103) cells were seeded in 200 μl DMEM into 96-well
plates and cultured overnight. SK-HEP-1 and SMMC-7721 cells were
then treated with various concentrations of 5-FU (Sigma-Aldrich,
St. Louis, MO, USA) for 72 h. At the end of the treatment,
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
was added into each well at a final concentration of 0.5 mg/ml,
which was followed by incubation at 37°C for 3 h in the dark. The
culture medium containing MTT was discarded and the dye crystals
were dissolved in dimethyl sulfoxide (DMSO). The viable cells were
detected by reading the absorbance of the metabolic MTT at
wavelength 570 nm. All experiments were conducted in
triplicate.
Quantification of apoptotic cells
Following the manufacturer’s instructions, flow
cytometry using the Annexin V-Fluorescein Isothiocyanate (FITC)
Apoptosis Detection kit was used to determine 5-FU
treatment-induced apoptosis in HCC cells treated with 5-FU alone
and in combination with Bmi-1 depletion. Briefly, 2×105
cells were treated with 5-FU alone or in combination with Bmi-1
depletion for 72 h. The cells were harvested, washed in
phosphate-buffered saline and then incubated with Annexin V and
propidum iodide for staining in binding buffer at room temperature
for 10 min in the dark. The stained cells were analyzed using a
Becton Dickinson FACSCalibur flow cytometer (BD Biosciences, San
Jose, CA, USA).
Western blot analysis
Western blot analysis was performed (10) and the following antibodies were
used: anti-Bmi-1 (R&D Systems, Minneapolis, MN, USA),
anti-light chain 3 B (LC3) (Novus Biologicals, Littleton, CO, USA),
anti-phospho-AKT, anti-Bcl2, anti-BAX (Santa Cruz Biotechnology,
Inc., Santa Cruz, CA, USA), anti-Beclin-1, anti-β-actin and
secondary antibodies (Proteintech Group, Inc., Chicago, IL,
USA).
Results
Bmi-1 knockdown sensitizes cells to
5-FU
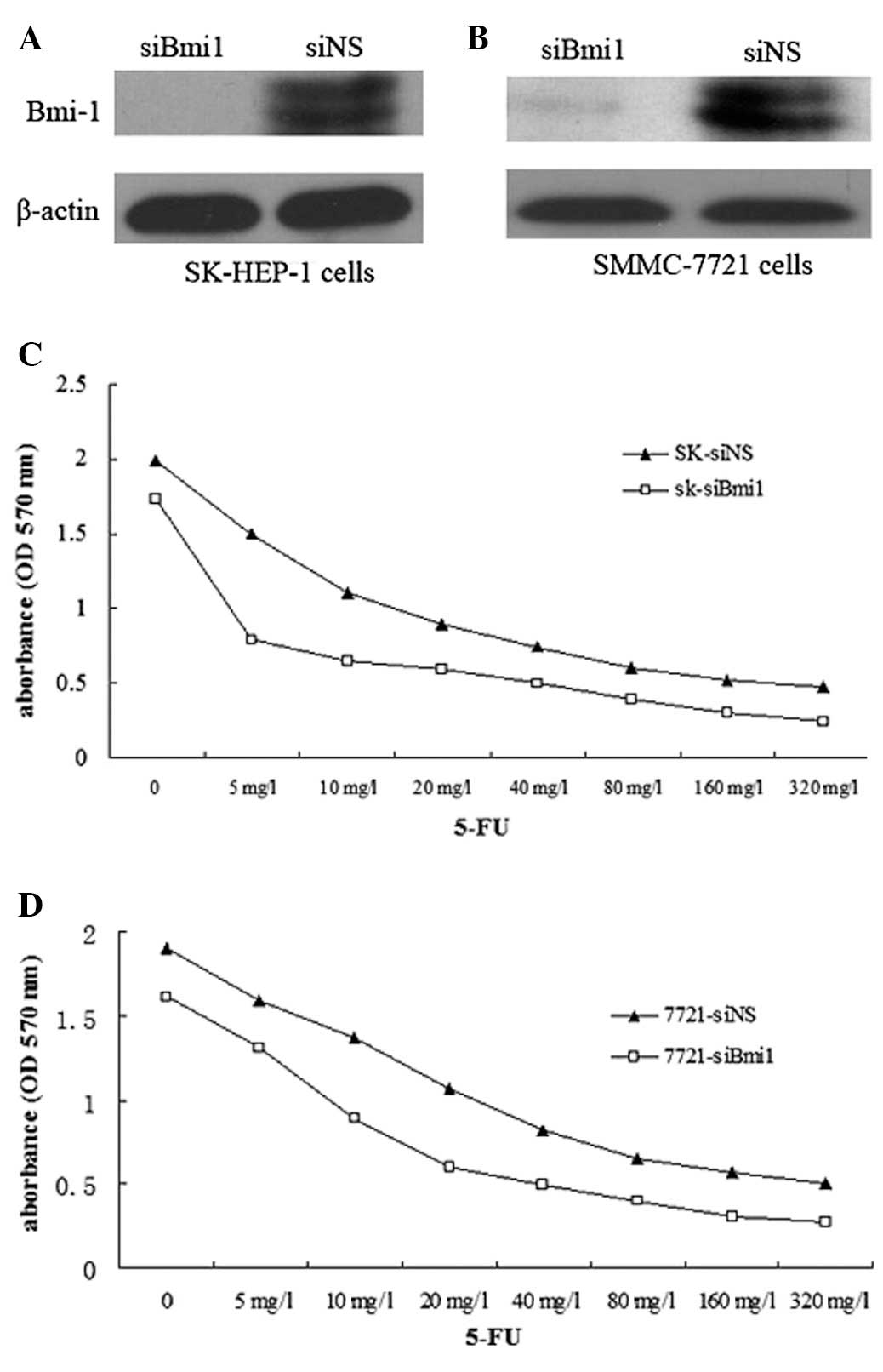
siBmi1 was examined for silencing in SK-HEP-1 and
SMMC-7721 cells. Western blot analysis was used to confirm that
siBmi1 was able to reduce Bmi-1 protein expression in SK-HEP-1
cells and SMMC-7721 cells (Fig. 1A and
B).
Once the SK-HEP-1-siBmi1 (SK-siBmi1), SK-HEP-1-siNS
(SK-siNS), SMMC-7721-siBmi1 (7721-siBmi1) and SMMC-7721-siNS
(7721-siNS) cells were treated with 5-FU (1–320 mg/l) for 72 h, an
MTT assay was performed to examine the effect of 5-FU on the
survival of Bmi-1 knockdown cells. The IC50 values of
5-FU in the SK-siBmi1 and SK-siNS cells were 9.97 and 20.85 mg/l,
respectively; while the IC50 values of 5-FU in
7721-siBmi1 and 7721-siNS cells were 15.39 and 27.11 mg/l,
respectively (Fig. 1C and D). The
results indicated that Bmi-1 depletion increased the sensitivity of
the cells to 5-FU.
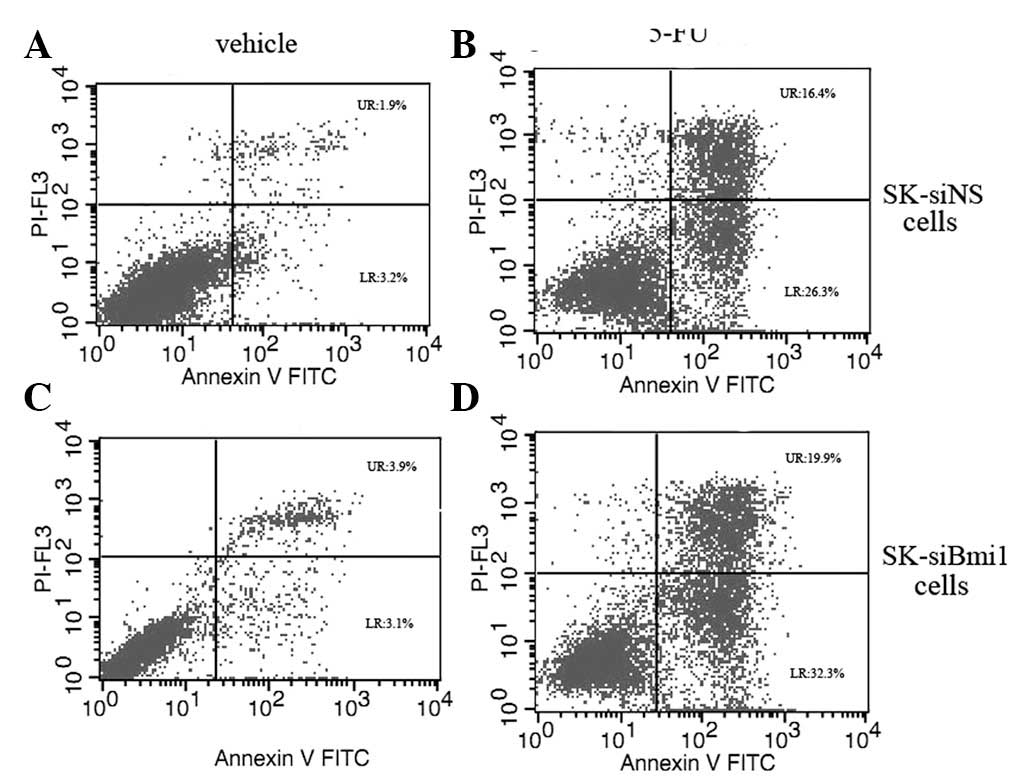
Depletion of Bmi-1 enhances 5-FU-induced
apoptosis
The combination of 5-FU with Bmi-1 knockdown
resulted in a significant reduction in cell viability compared to
the treatment with 5-FU alone in the cells. As demonstrated in
Fig 1, depletion of Bmi-1 increased
the sensitivity of the cells to 5-FU in SK-HEP-1 cells compared to
SMMC-7721; thus, we selected SK-HEP-1 cells for further
investigations. In order to evaluate the effect of Bmi-1 depletion
on the induction of apoptosis, the SK-siBmi1 and SK-siNS cells were
treated with 10 mg/l of 5-FU for 72 h. Flow cytometry data revealed
that the apoptotic rate among the SK-siBmi1 cells was 51% compared
to 42% among the SK-siNS cells (Fig.
2).
Knockdown of Bmi-1 inhibits AKT
activation and regulates expression of apoptosis-related proteins
Bcl-2 and Bax in SK-HEP-1 cells
Bcl-2 family proteins play critical roles in the
regulation of apoptosis (11). To
further explore the mechanism underlying the enhancement of
5-FU-induced apoptosis by the silencing of Bmi-1, we examined the
expression levels of phospho-AKT, Bax and Bcl-2 in SK-siBmi-1 and
SK-siNS cells. Western blot analysis demonstrated that knockdown of
endogenous Bmi-1 led to a substantial reduction in the levels of
phospho-AKT. Consistent with this reduction in the phospho-AKT
level, the results identified a significant decreased expression of
Bcl-2 in Bmi-1 knockdown cells exposed to 5-FU and an increase in
the level of Bax (Fig. 3).
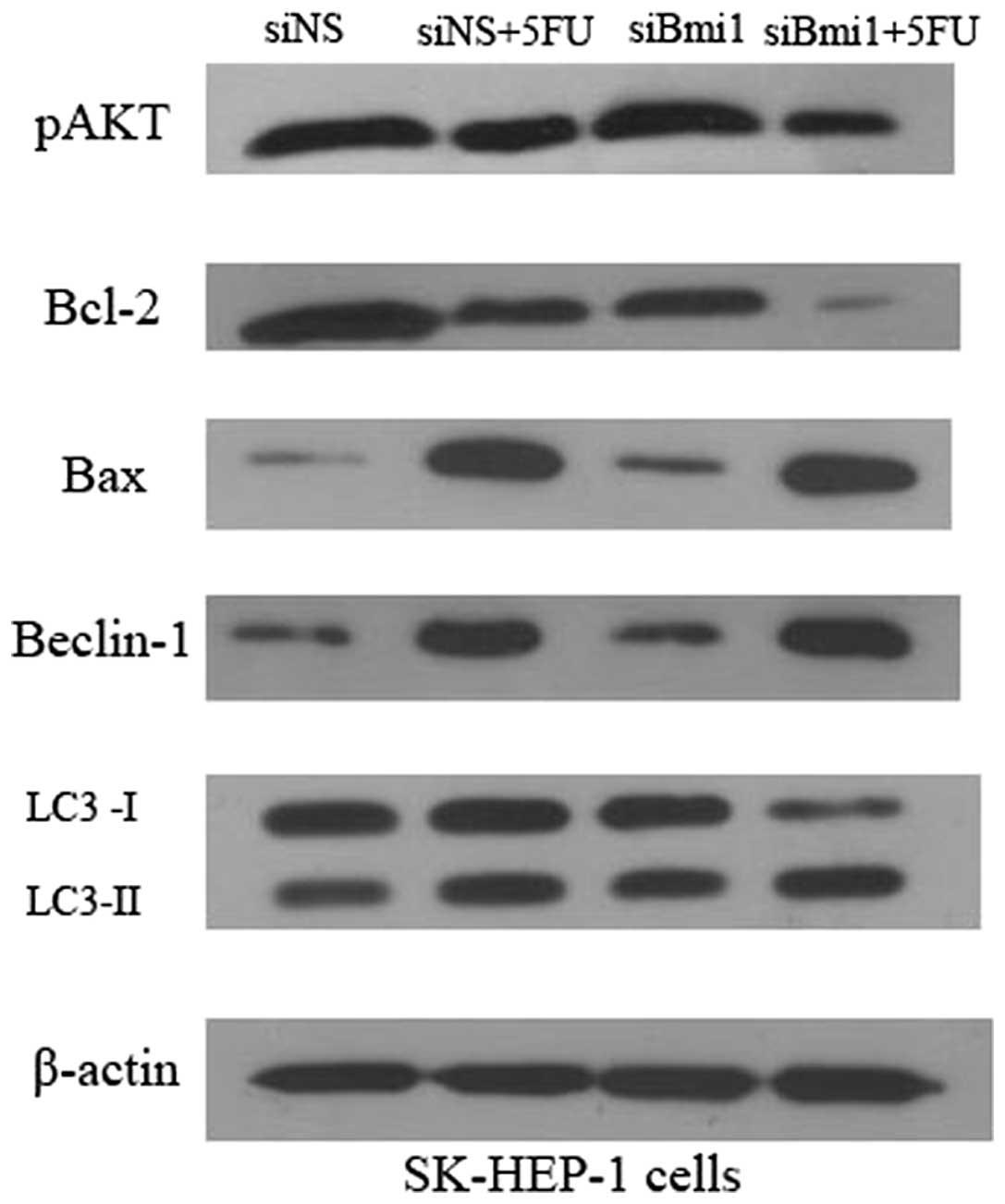 | Figure 3.Effects of depletion of Bmi-1
expression on p-AKT, Bcl-2, Bax, Beclin-1 and LC3 levels. SK-siNS
and SK-siBmi1 cells were incubated for 72 h with or without 5-FU
treatment. The protein expression of p-AKT, Bcl-2, Bax, Beclin-1
and LC3 was detected by western blot analysis. All assays were
conducted in triplicate. siNS, negative control siRNA
oligonucleotides; 5-FU, 5-fluorouracil; siBmi1, Bmi-1 targeted
siRNA oligonucleotide; Bmi-1, B-cell-specific Moloney murine
leukemia virus insertion site 1; LC3, light chain 3. |
5-FU triggers autophagy and depletion of
Bmi-1 enhanced 5-FU-induced autophagy in SK-HEP-1 cells
To investigate whether 5-FU triggers autophagy in
SK-HEP-1 cells, and whether depletion of Bmi-1 enhances
5-FU-induced apoptosis with a concomitantly induced autophagy, we
examined the expression of Beclin-1 and LC3 in SK-siBmi1 and
SK-siNS cells. The amount of LC3-II correlates with the extent of
autophagosome formation. Thus, the conversion of LC3-I to LC3-II
serves as a marker for the accumulation of autophagic vesicles and
autophagic activity. Following 5-FU treatment, the ratio of
LC3-II/LC3-I and the accumulation of LC3-II was increased. The data
demonstrates that 5-FU triggers autophagy in SK-HEP-1 cells
(Fig. 3). Western blot analysis
revealed that knockdown of endogenous Bmi-1 led to an increase in
the levels of Beclin-1 and accumulation of LC3-II. The results
identified that Bmi-1 depletion enhances 5-FU-induced autophagy in
SK-HEP-1 cells.
Discussion
The overall response rate to systemic chemotherapy
for the treatment of HCC is generally less than 10%, owing to drug
resistance and advanced stage of disease. 5-FU is one of the most
widely used agents in cancer chemotherapy, and enhancing the
sensitization of cancer cells to drug-induced apoptosis has become
an important strategy for chemotherapy. Overexpressed Bmi-1 was
observed in a significant number of HCC cases, which correlated
with advanced invasive stage of tumor progression and poor
prognosis. The deregulation of Bmi-1 expression has been linked
with proliferation and oncogenesis in human cells. Recently, it has
been reported that Bmi-1 is also associated with the protection of
tumor cells from apoptosis.
This study represents an investigation into the
possibility of combining 5-FU treatment and Bmi-1 depletion as a
clinical strategy for liver cancer chemotherapy. To examine the
role of Bmi-1-mediated chemotherapy-induced apoptosis, the HCC cell
lines, SK-HEP-1 and SMMC-7721, in which Bmi-1 is highly expressed,
were used in our study. The MTT results demonstrated that depletion
of Bmi-1 increased the sensitivity of the cells to 5-FU in SK-HEP-1
cells compared to SMMC-7721, thus, we selected SK-HEP-1 cells for
further investigation. FACS revealed that silencing Bmi-1
expression may enhance 5-FU-induced apoptosis in SK-HEP-1
cells.
Constitutive activation of the PI3K/AKT signaling
pathway has been firmly established as a major determinant of tumor
cell growth and survival in a multitude of solid tumors (12). PI3K subsequently produces the lipid
second messenger PIP3b, which in turn activates AKT. Activated AKT
phosphorylates the Bcl-2-associated death promoter and the
inactivation of the Bcl-2-associated death promoter decreases
apoptosis and increases cell survival (13). To determine the underlying
mechanisms that are not fully understood, we investigated the
contribution of Bcl-2 family proteins to 5-FU-induced apoptosis and
revealed an increase in the expression of Bax protein and a
decrease in the expression of Bcl-2 in SK-HEP-1 cells. Furthermore,
we demonstrated that knockdown of Bmi-1 inhibits AKT activation,
suggesting an essential role of the PI3K/AKT pathway in the
sensitization of Bmi-1 to 5-FU treatment. The knockdown of
endogenous Bmi-1 expression contributed to sensitizing HCC cells to
the anticancer drug 5-FU by increasing apoptosis.
The connection between autophagy and apoptosis is a
developing area of research. For tumors, autophagy is a
‘doubled-edged sword’. Its protective function helps tumor cells
survive under stress, including certain chemotherapies; however,
autophagy may also have a negative impact on cell growth, and
autophagy-associated cell death has been demonstrated in response
to various anticancer therapies (14). The aim of this study was to
investigate whether 5-FU triggers autophagy and whether depletion
of Bmi-1 enhances 5-FU-induced autophagy in SK-HEP-1 cells. Our
results demonstrate that 5-FU increased the conversion of LC3-I to
LC3-II, suggesting that autophagy was induced in 5-FU-treated
SK-HEP-1 cells. Beclin-1 is a crucial regulator of autophagy that
directly interacts with the anti-apoptotic protein, Bcl-2 (15). Autophagy is induced by the release
of Beclin-1 from Bcl-2. We examined whether Beclin-1 signaling is
involved in 5-FU-induced autophagy. Western blot analysis
demonstrated that treatment of 5-FU led to an increase in the
levels of Beclin-1, decrease in the levels of Bcl-2 and
accumulation of LC3-II. The results suggested that Bcl-2/Beclin-1
signaling is involved in 5-FU-induced autophagy. We examined the
expression of Beclin-1 and LC3 in SK-siBmi1 and SK-siNS cells with
or without 5-FU treatment. Western blot analysis revealed that
knockdown of endogenous Bmi-1 led to an increase in the levels of
Beclin-1 and accumulation of LC3-II. Together, these results
indicated that 5-FU triggers autophagy and depletion of Bmi-1
enhanced 5-FU-induced autophagy.
In conclusion, we report for the first time the
anticancer potential of a combination of 5-FU treatment and Bmi-1
depletion in HCC cell lines. We identified that the knockdown of
Bmi-1 increases the sensitivity of the SK-HEP-1 and SMMC-7721 cells
to 5-FU treatment, and that depletion of Bmi-1 enhances
5-FU-induced apoptosis with a concomitantly induced autophagy in
SK-HEP-1 cells. The PI3K/AKT and Bcl-2/Beclin-1 signaling pathway
was involved in the sensitization of Bmi-1 to 5-FU treatment.
Together, the combination of 5-FU and Bmi-1 depletion may be a
potential therapeutic strategy in the treatment of human HCC.
Acknowledgements
This study was supported by the
National Natural Science Foundation of China (Nos. 81041083 and
81172778), the Anhui Provincial Natural Science Foundation (No.
1208085QH162) and AUST Grants (Dong Hu and Jing Wu).
References
|
1.
|
H NaganoTreatment of advanced
hepatocellular carcinoma: intraarterial infusion chemotherapy
combined with interferonOncology78Suppl
1142147201010.1159/000315243
|
|
2.
|
JM LIovetJ BruixMolecular targeted
therapies in hepatocellular
carcinomaHepatology4813121327200810.1002/hep.2250618821591
|
|
3.
|
M BoulinS GuiuB ChauffertS AhoJP CercueilF
GhiringhelliD KrauseP FagnoniP HillonL BedenneB GuiuScreening of
anticancer drugs for chemoembolization of hepatocellular
carcinomaAnticancer
Drugs22741748201110.1097/CAD.0b013e328346a0c521487286
|
|
4.
|
Y HauptWS AlexanderG BarriSP KlinkenJM
AdamsNovel zinc finger gene implicated as myc collaborator by
retrovirally accelerated lymphomagenesis in E mu-myc transgenic
miceCell65753763199110.1016/0092-8674(91)90383-A1904009
|
|
5.
|
M van LohuizenS VerbeekB ScheijenE
WientjensH van der GuldenA BernsIdentification of cooperating
oncogenes in E mu-myc transgenic mice by provirus
taggingCell6573775219911904008
|
|
6.
|
ME Valk-LingbeekSW BruggemanM van
LohuizenStem cells and cancer; the polycomb
connectionCell118409418200410.1016/j.cell.2004.08.00515315754
|
|
7.
|
MJ GunsterFM RaaphorstKM HamerJL den
BlaauwenE FieretCJ MeiierAP OtteDifferential expression of human
polycomb group proteins in various tissues and cell typesJ Cell
Biochem Suppl36129143200110.1002/jcb.109311455578
|
|
8.
|
H WangK PanHK ZhangDS WengJ ZhouJJ LiW
HuangHF SongMS ChenJC XiaIncreased polycomb-group oncogene Bmi-1
expression correlates with poor prognosis in hepatocellular
carcinomaJ Cancer Res Clin
Oncol134535541200810.1007/s00432-007-0316-817917742
|
|
9.
|
L LiuLG AndrewsTO TollefsbolLoss of the
human polycomb group protein BMI1 promotes cancer-specific cell
deathOncogene2543704375200610.1038/sj.onc.120945416501599
|
|
10.
|
J WuD HuG YangJ ZhouC YangY GaoZ
ZhuDown-regulation of Bmi-1 cooperates with artemisinin on growth
inhibition of nasopharyngeal carcinoma cellsJ Cell
Biochem11219381948201110.1002/jcb.2311421445878
|
|
11.
|
JE ChipukDR GreenHow do Bcl-2 proteins
induce mitochondrial outer membrane permeabilization?Trends Cell
Biol18157164200810.1016/j.tcb.2008.01.00718314333
|
|
12.
|
S WhittakerR MaraisAX ZhuThe role of
signaling pathways in the development and treatment of
hepatocellular
carcinomaOncogene2949895005201010.1038/onc.2010.23620639898
|
|
13.
|
YL ChenPY LawHH LohInhibition of PI3K/Akt
signaling: an emerging paradigm for targeted cancer therapyCurr Med
Chem Anticancer
Agents5575589200510.2174/15680110577457464916305480
|
|
14.
|
JM GumpA ThorburnAutophagy and apoptosis:
what is the connection?Trends Cell
Biol21387392201110.1016/j.tcb.2011.03.00721561772
|
|
15.
|
F ZhouY YangD XingBcl-2 and Bcl-xl play
important roles in the crosstalk between autophagy and
apoptosisFEBS
J278403413201110.1111/j.1742-4658.2010.07965.x21182587
|