Introduction
Breast cancer is a heterogeneous disease
characterized by uncontrolled growth and spread of abnormal cells,
being the most common cause of cancer mortality among women
worldwide (1). Invasive breast
cancer is the most common type of carcinoma in women, accounting
for 22% of all female cancers and comprises a heterogeneous group
of malignant epithelial tumors, characterized by the invasion of
adjacent tissues and a marked tendency to metastasize to distant
sites, differing with regard to their clinicopathological features
and biological potential (2). The
invasive ductal carcinoma of no specific type (IDC-NST) is the most
common invasive breast cancer, accounting for 40–75% of all
invasive tumors and is a histologically diverse group of lesions
which includes all carcinomas that cannot be subclassified into one
specialized type (3).
Carcinogenesis is a multistep process mostly characterized by the
accumulation of genetic alterations that drive normal cells to
malignant transformation (4). The
majority of genes correlated with breast cancer development are
also involved in pathways linked to the regulation of crucial
biological processes such as proliferation, apoptosis,
angiogenesis, invasion and metastasis (5). Angiogenesis is involved in the
development and progression of malignant tumors enabling cancer
cells to acquire an adequate supply of oxygen, metabolites and an
effective way to remove waste products, and also provides a pathway
for metastases. During tumor angiogenesis, the equilibrium between
pro- and anti-angiogenic factors is lost and their relative
contribution may change according to tumor type, tumor
localization, as well as with tumor growth, regression and relapse.
Pro- and anti-angiogenic molecules are released from tumor cells,
endothelial cells (ECs), blood cells and the extracellular matrix.
Among these factors, PAR1 and FGFR1 are key contributors to the
angiogenic process (6–8).
Protease-activated receptors (PARs) are members of
the G-protein coupled receptors (GPCR) superfamily that are
expressed in several tissues by a variety of cells, being activated
by proteolytic cleavage of their extracellular domains (9). PAR1, one of the four members of the
PAR family, is the predominant thrombin receptor in EC and is also
detected in a variety of other cell types (10). PAR1 has been described as a crucial
factor in angiogenesis, promoting numerous biological effects
including coagulation, inflammation, mitogenesis and cell
proliferation (11). The role of
PAR1 in vascular biology and tissue remodelling is further stressed
by the fact that factors activated upon thrombin-induced PAR1
signalling are known to be important during the process of vascular
remodelling. The expression and/or release of several growth
factors, including FGF2, PDGF, VEGF (12,13),
the upregulation of the insulin-growth factor receptor (IGFR)-1
(14), and the activation of
fibroblast growth factor receptor 1 (FGFR1) (15) were shown to be induced in response
to thrombin, metalloproteinase (MMP) 1 and more recently FGF1
(13). Accumulating evidence
suggests that the receptor PAR1 modulates cell proliferation and
motility in physiopathological cell invasion processes, suggesting
a role in initiating cancer growth and metastasis (16). Overexpression of this receptor has
been detected in numerous human cancers, including breast cancer,
where it has been described as being preferentially expressed in
high-grade human breast carcinoma, correlating with the degree of
invasiveness with differential metastatic potential (16). Moreover, there is evidence of the
presence of PAR1 protein and mRNA not only in human malignant tumor
cells, but also on the cell types forming the tumor
microenvironment (17).
FGFR1 is a member of the FGFRs family (FGFR 1–4), a
family of tyrosine kinase receptors, responsible for mediating a
number of actions induced by the members of the fibroblast growth
factor family (FGF 1–24) group of proangiogenic factors playing
critical functions in each step of the angiogenic cycle. Aberrant
regulation of FGF ligands and their receptors has been associated
with prostate and breast tumorigenesis (18). Studies using inducible FGFR1
(iFGFR1) suggest that it is able to directly activate both
proliferation and survival signals (antiapoptotic effects) within
the mammary epithelium to rapidly induce hyperplastic lesions and
regulate MMP secretion. Furthermore, the signalling complexity
related to the iFGFR1-induced lesions, including the loss of
myoepithelium and increased vascular branching suggest that other
indirect effects mediated through stromal interactions also
contribute to the tumor invasive phenotype (19). The aim of this study was to
investigate the expression of PAR1 and FGFR1 in tumor cells and
cells from the tumoral microenvironment in a series of invasive
breast cancers and explore possible significant correlations with
intratumoral microvessel density (iMVD), several breast cancer
clinicopathological parameters and molecular markers.
Materials and methods
Tissue samples
A retrospective series of 224 formalin-fixed
paraffin-embedded samples of ductal invasive breast carcinomas were
used to construct tissue microarrays. The series of ductal invasive
breast carcinomas had previously been characterized regarding
clinicopathological parameters, such as histological grade, lymph
node metastasis and patient survival (20). Samples from each case were collected
from the donor sample with a 1 mm gauge cylinder and transferred to
a receptor block using a manual tissue microarrayer (TMA) (Beecher
Instruments, Sun Prairie, WI, USA). TMA sections (3 μm) were
cut from the receptor block and processed as described in a
previous study (20). Most of the
immunohistochemical characterization was previously reported and
included the evaluation of several molecular markers, including
estrogen receptor (ER), progesterone receptor (PR), epidermal
growth factor receptor (EGFR) 2 (HER2), EGFR, P-cadherin (Pcad),
keratin (CK) 5, CK14, P63, P53 and vimentin (20).
PAR1 and FGFR1 immunohistochemistry
Immunohistochemical staining for PAR1 was carried
out using Dako EnVision polymer (Dako Coorporation, Carpinteria,
CA, USA) and the monoclonal antibody PAR1 (Thrombin R ATAP2:
sc-13503 Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA).
Deparaffinized and rehydrated TMAs were immersed in 1 mM EDTA
buffer solution with 0.05% Tween 20, pH 7.4, and heated at 98°C for
30 min in a bath. Following washing in phosphate-buffered saline
(PBS), slides were incubated with 3% hydrogen peroxide in methanol
for 10 min, washed in PBS again and covered with a blocking serum
for 10 min, prior to incubation with the primary antibody diluted
at 1:100, overnight at room temperature. Sections were sequentially
washed in PBS, incubated with the polymer conjugated with secondary
antibodies for 10 min and finally with 3,3′-diaminobenzidine (DAB
Substrate System; Lab Vision Corporation, Fremont, CA, USA) for 10
min, between washes with PBS. TMAs were then counterstained with
haematoxylin, dehydrated and mounted with the synthetic mounting
medium Entellan (Merck, Darmstadt, Germany).
Immunohistochemical staining for FGFR1 was performed
using the streptavidin-biotin peroxidase technique (LabVision
Corporation) and the polyclonal antibody anti-FGFR1 (Flg C-15:
sc-121 Santa Cruz Biotechnology, Inc.), raised against the
C-terminus of the human form. TMAs were deparaffinized and
rehydrated and heat-induced antigen retrieval was performed using a
citrate buffer solution (10 mM) with 0.05% of Tween 20, pH 6.0, for
15 min in a microwave at 600 Watts. Following washing with PBS, the
slides were incubated in a 3% hydrogen peroxide solution in
methanol for 10 min to block endogenous peroxidase activity. The
slides were then incubated with a blocking serum for 10 min and
then for 2 h at room temperature with the primary antibody at 1:200
dilution. Slides were incubated with biotinylated goat
anti-polyvalent antibody for 10 min, streptavidin peroxidase for
another 10 min and 3,3′-diaminobenzidine (DAB Substrate System; Lab
Vision Corporation) for a further 10 min, between washes with PBS.
TMAs were counterstained with haematoxylin, dehydrated and mounted
with synthetic mounting medium Entellan (Merck).
Immunohistochemical staining in tumor cells and
tumor stroma was independently assessed by two skilled
pathologists. Discrepancies were resolved by observation through a
double-head microscope. Assessment of the immunohistochemical
results was based on a semiquantitative evaluation as specified in
advance. The stroma of the tumors was classified as negative or
positive, according to the presence or absence of positive
expression for the two receptors in fibroblasts. The classification
of positive immunoreactivity in tumor cells was carried out
according to the percentage of immunopositive cells: <10% was
classified as negative; positive cytoplasmic immunostaining of PAR1
and FGFR1 ranged from 11 to 50% and from 51 to 100%,
respectively.
Intratumoral microvessel density
evaluation
iMVD was evaluated according to the cytoplasmic
staining in blood endothelial cells. Evaluation of positive
reactions was performed by counting positive anti-factor VIII blood
vessels, surrounding a visible lumen clearly separated from
adjacent microvessels and from other connective tissue components.
Packed vessels were considered as one vessel unit. Analysis was
performed at a magnification of ×200 (×20 objective lens and ×10
ocular lens). An average of 10 hot spot fields was defined as iMVD.
Counting of the vonWillebrand factor (anti-factor VIII)
immunohistochemical reactions was performed blindly.
Statistical analysis
For statistical analysis, contingency tables and
Chi-square testing was performed using StatView 5.0 (SAS Institute
Inc., Cary, NC, USA) to estimate the relationship between staining
patterns of the different antibodies used. Survival curves were
generated by the Kaplan-Meier method, using the SPSS 11.5
statistical software for patients with >2 years survival. Two
values were considered significantly different when P<0.05.
Results
PAR1 expression
Among the 224 cases, 211 were eligible for
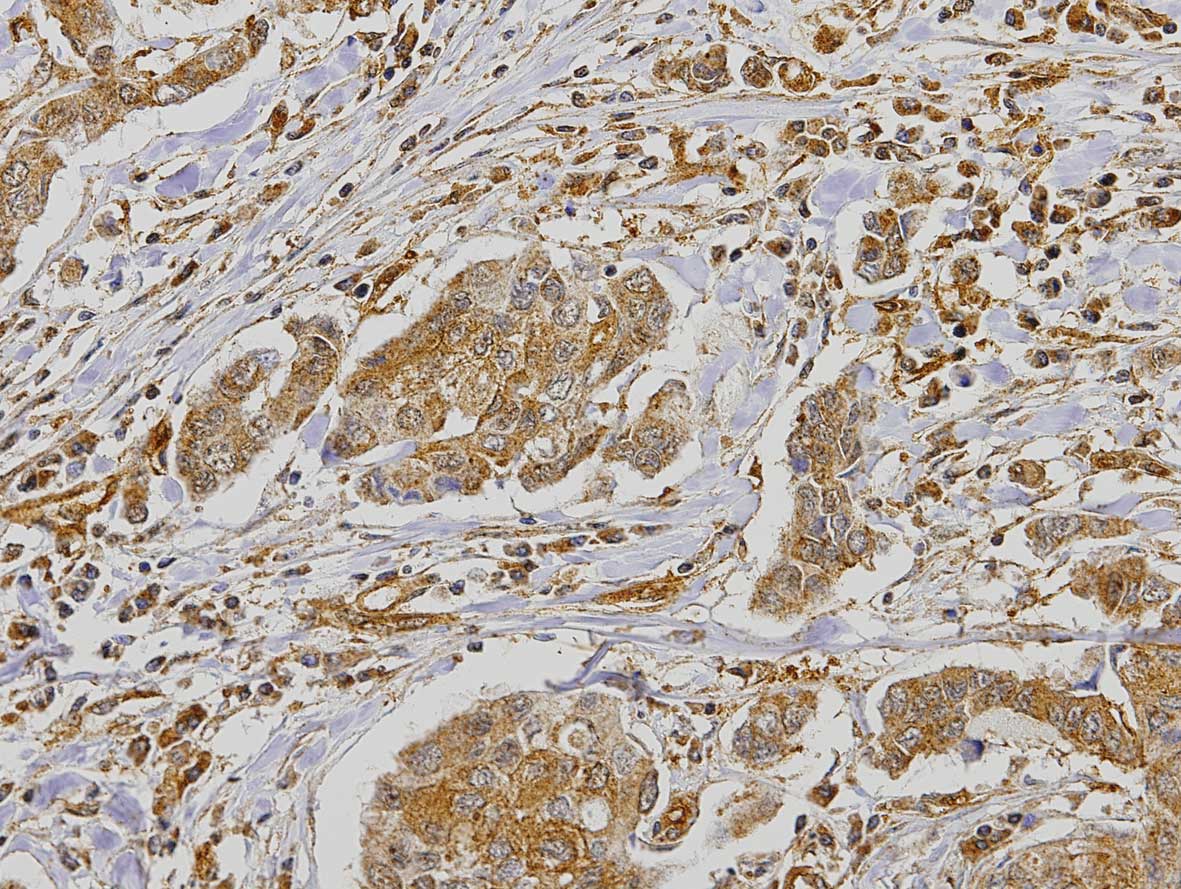
statistical analysis. Expression of PAR1 in the tumor demonstrated
2.4% negative cases, 8% positive <50% and 89.6% positive >50%
(Fig. 1). PAR1 expression was also
analysed in the stroma of the tumor, where 40.8% of the cases were
considered negative and 59.2% positive. The immunohistochemical
staining also revealed a strong expression of PAR1 in the
endothelial cells. PAR1 expression in tumor cells did not exhibit
any significant correlation with any of the clinicopathological
parameters, which included histological grade and lymph node
metastasis, nor with patient survival, molecular subtype and/or
molecular markers, such as ER, PR, HER2, EGFR, Pcad, CK5, CK14,
P63, P53 and vimentin (Table I). By
contrast, statistically significant correlations were observed
between the expression of PAR1 in the stroma and the molecular
subtypes of breast cancer (P=0.0134), ER expression
(P=0.0029), PR
expression (P=0.0263) and adhesion molecule
P-cadherin expression (P=0.0440; Table II). PAR1 expression in the stroma of
the tumor was detected in a high percentage of cases among the
basal and HER2 histological subtypes, as compared to the luminal
subtype (Table III). Statistical
analysis also revealed that a high percentage of ER cases (>70%)
with a negative expression exhibited a positive expression of PAR1
in the stroma, which was not observed with the ER-positive
specimens. Similar results were observed between the expression of
PAR1 in the stroma and the PR expression.
 | Table I.Correlation between PAR1 expression
in the tumor cells and clinicopathological parameters. |
Table I.
Correlation between PAR1 expression
in the tumor cells and clinicopathological parameters.
| Clinicopathological
parameters | n | PAR1 tumor
| P-value |
|---|
| Negative (5 cases)
% (n) | Positive <50%
(17 cases) % (n) | Positive >50%
(189 cases) % (n) |
|---|
| Histological
grade | | | | | |
| I | 98 | 4.1 (4) | 10.2 (10) | 85.7 (84) | 0.3239 |
| II | 78 | 0 (0) | 7.7 (6) | 92.3 (72) | |
| III | 29 | 3.4 (1) | 3.4 (1) | 93.1 (27) | |
| Lymph node
metastasis | | | | | |
| Positive | 93 | 3.2 (3) | 5.4 (5) | 91.4 (85) | 0.6419 |
| Negative | 78 | 2.6 (2) | 9 (7) | 88.4 (69) | |
| Molecular
subtype | | | | | |
| Luminal | 129 | 3.1 (4) | 4.7 (6) | 92.2 (119) | 0.7403 |
| Basal | 35 | 2.8 (1) | 8.6 (3) | 88.6 (31) | |
| HER2 | 20 | 0 (0) | 10 (2) | 90 (18) | |
| ER | | | | | |
| Positive | 126 | 3.2 (4) | 4.8 (6) | 92.0 (116) | 0.0711 |
| Negative | 85 | 1.2 (1) | 12.9 (11) | 85.9 (73) | |
| PR | | | | | |
| Positive | 84 | 1.2 (1) | 3.6 (3) | 95.2 (80) | 0.0905 |
| Negative | 127 | 3.2 (4) | 11 (14) | 85.8 (109) | |
| HER2 | | | | | |
| Positive | 26 | 3.8 (1) | 7.7 (2) | 88.5 (23) | 0.8812 |
| Negative | 180 | 2.2 (4) | 7.8 (14) | 90 (162) | |
| EGFR | | | | | |
| Positive | 13 | 0 (0) | 0 (0) | 100 (13) | 0.4465 |
| Negative | 198 | 2.5 (5) | 8.6 (17) | 88.9 (176) | |
| Pcad | | | | | |
| Positive | 52 | 0 (0) | 9.6 (5) | 90.4 (47) | 0.3969 |
| Negative | 159 | 3.2 (5) | 7.5 (12) | 89.3 (142) | |
| CK5 | | | | | |
| Positive | 51 | 2 (1) | 5.9 (3) | 92.1 (47) | 0.7818 |
| Negative | 160 | 2.5 (4) | 8.8 (14) | 88.7 (142) | |
| CK14 | | | | | |
| Positive | 4 | 0 (0) | 25 (1) | 75 (3) | 0.3696 |
| Negative | 192 | 1 (2) | 6.8 (13) | 92.2 (177) | |
| P63 | | | | | |
| Positive | 4 | 0 (0) | 0 (0) | 100 (4) | 0.7888 |
| Negative | 207 | 2.4 (5) | 8.2 (17) | 89.4 (185) | |
| P53 | | | | | |
| Positive | 48 | 2.1 (1) | 10.4 (5) | 87.5 (42) | 0.7863 |
| Negative | 163 | 2.4 (4) | 7.4 (12) | 90.2 (147) | |
| Vimentin | | | | | |
| Positive | 33 | 3 (1) | 6.1 (2) | 90.9 (30) | 0.7041 |
| Negative | 155 | 1.3 (2) | 8.4 (13) | 90.3 (140) | |
 | Table II.Correlation between PAR1 expression
in the stroma and clinipathological parameters. |
Table II.
Correlation between PAR1 expression
in the stroma and clinipathological parameters.
| Clinicopathological
parameters | n | PAR1 stroma
| P-value |
|---|
| Negative (86 cases)
% (n) | Positive (125
cases) % (n) |
|---|
| Histological
grade | | | | |
| I | 98 | 48 (47) | 52 (51) | 0.1007 |
| II | 78 | 37.2 (29) | 62.8 (49) | |
| III | 29 | 27.6 (8) | 72.4 (21) | |
| Lymph node
metastasis | | | | |
| Positive | 93 | 39.8 (37) | 60.2 (56) | 0.8598 |
| Negative | 78 | 38.5 (30) | 61.5 (48) | |
| Molecular
subtype | | | | |
| Luminal | 129 | 49.6 (64) | 50.4 (65) | 0.0134 |
| Basal | 35 | 22.9 (8) | 77.1 (27) | |
| HER2 | 20 | 35 (7) | 65 (13) | |
| ER | | | | |
| Positive | 126 | 49.2 (62) | 50.8 (64) | 0.0029 |
| Negative | 85 | 28.2 (24) | 71.8 (61) | |
| PR | | | | |
| Positive | 84 | 50 (42) | 50 (42) | 0.0263 |
| Negative | 127 | 34.6 (44) | 65.4 (83) | |
| HER2 | | | | |
| Positive | 26 | 42.3 (11) | 57.7 (15) | 0.8226 |
| Negative | 180 | 40 (72) | 60 (108) | |
| EGFR | | | | |
| Positive | 13 | 38.5 (5) | 61.5 (8) | 0.8619 |
| Negative | 198 | 40.9 (81) | 59.1 (117) | |
| Pcad | | | | |
| Positive | 52 | 28.8 (15) | 71.2 (37) | 0.0440 |
| Negative | 159 | 44.6 (71) | 55.4 (88) | |
| CK5 | | | | |
| Positive | 51 | 47.1 (24) | 52.9 (27) | 0.2930 |
| Negative | 160 | 38.8 (62) | 61.2 (98) | |
| CK14 | | | | |
| Positive | 4 | 50 (2) | 50 (2) | 0.6736 |
| Negative | 192 | 39.6 (76) | 60.4 (116) | |
| P63 | | | | |
| Positive | 4 | 25 (1) | 75 (3) | 0.5173 |
| Negative | 207 | 41.1 (85) | 58.9 (122) | |
| P53 | | | | |
| Positive | 48 | 31.2 (15) | 68.8 (33) | 0.1272 |
| Negative | 163 | 43.6 (71) | 56.4 (92) | |
| Vimentin | | | | |
| Positive | 33 | 33.3 (11) | 66.7 (22) | 0.4350 |
| Negative | 155 | 40.6 (63) | 59.4 (92) | |
 | Table III.Correlation between PAR1 expression
in the tumor cells and stroma related to the expression of FGFR1
positive reaction in the tumor cells and stroma. |
Table III.
Correlation between PAR1 expression
in the tumor cells and stroma related to the expression of FGFR1
positive reaction in the tumor cells and stroma.
A, PAR1 tumor
cells.
|
|---|
| IpX reaction | n | PAR1 tumor cells
| P-value |
|---|
| Positive <50%
(17 cases) % (n) | Positive >50%
(189 cases) % (n) |
|---|
| FGFR1 tumor | | | | |
| Negative | 6 | 33.3 (2) | 66.7 (4) | 0.0511 |
| Positive
<50% | 48 | 12.5 (6) | 87.5 (42) | |
| Positive
>50% | 139 | 5 (7) | 92.8 (129) | |
| FGFR1 stroma | | | | |
| Positive | 19 | 5.3 (1) | 94.7 (18) | 0.7647 |
| Negative | 174 | 8 (14) | 90.3 (157) | |
B, PAR1 stroma.
|
|---|
| IpX reaction | n | PAR1 stroma
| P-value |
|---|
| Negative (86 cases)
% (n) | Positive (125
cases) % (n) |
|---|
| FGFR1 tumor | | | | |
| Negative | 6 | 50 (3) | 50 (3) | 0.6947 |
| Positive
<50% | 48 | 35.4 (17) | 64.6 (31) | |
| Positive
>50% | 139 | 41 (57) | 59 (82) | |
| FGFR1 stroma | | | | |
| Positive | 19 | 21.1 (4) | 78.9 (15) | 0.0773 |
| Negative | 174 | 42 (73) | 58 (101) | |
A significant statistical correlation was also
observed between the expression of PAR1 in the stroma and the
expression of Pcad, where approximately 70% of the Pcad-positive
cases expressed PAR1. No significant statistical correlation was
found between PAR1 expression in the tumor and patient survival
(data not shown).
FGFR1 expression
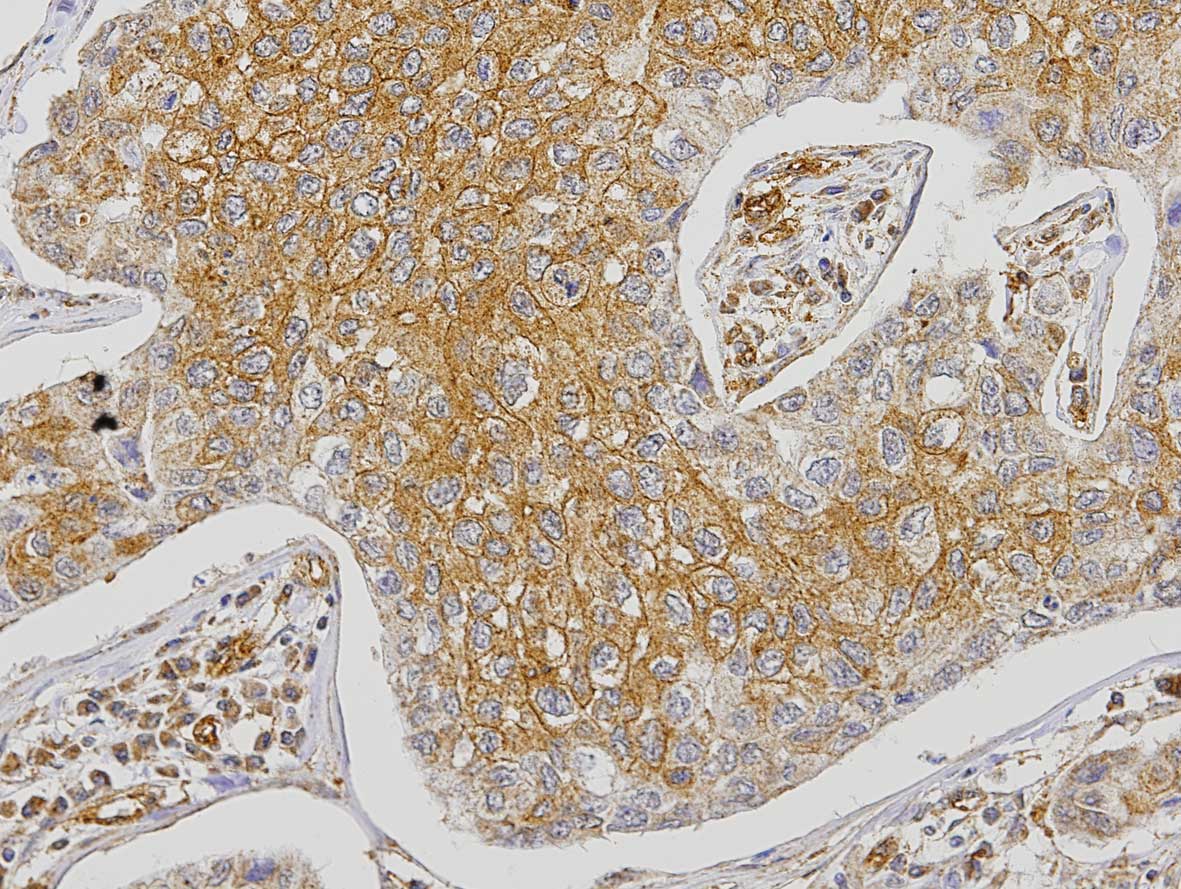
The analysis of FGFR1 expression in the tumor cells
revealed that 3% of the cases were negative, 25.9% positive <50%
and 71.1% positive >50% (Fig.
2). Expression of FGFR1 in tumor cells showed a significant
correlation with tumor grade (P=0.0113) and with P53 expression
(P=0.0126; Table IV). The
statistical analysis also showed an increasing percentage of tumor
cases expressing FGFR1 (positive >50%), from grades I to III.
Results also showed that FGFR1 is highly expressed (positive
>50%) in the tumor cells in 88.6% of the P53-positive cases and
in 66% of the negative ones. A significant correlation with CK14
expression was observed (P=0.0253), although this correlation may
have resulted from the small number of positive cases expressing
CK14. No significant correlation was found between the expression
of FGFR1 in the tumor and patient survival (data not shown).
 | Table IV.Correlation between FGFR1 expression
in the tumor cells and clinicopathological parameters. |
Table IV.
Correlation between FGFR1 expression
in the tumor cells and clinicopathological parameters.
| Clinicopathological
parameters | n | FGFR1 tumor
| P-value |
|---|
| Negative (6 cases)
% (n) | Positive <50%
(51 cases) % (n) | Positive >50%
(140 cases) % (n) |
|---|
| Histological
grade | | | | | |
| I | 89 | 1.1 (1) | 37.1 (33) | 61.8 (55) | 0.0113 |
| II | 74 | 5.4 (4) | 16.2 (12) | 78.4 (58) | |
| III | 28 | 3.6 (1) | 14.3 (4) | 82.1 (23) | |
| Lymph node
metastasis | | | | | |
| Positive | 84 | 1.2 (1) | 26.2 (22) | 72.6 (61) | 0.3065 |
| Negative | 75 | 5.3 (4) | 22.7 (17) | 72 (54) | |
| Molecular
subtype | | | | | |
| Luminal | 118 | 1.7 (2) | 27.1 (32) | 71.2 (84) | 0.2303 |
| Basal | 32 | 6.2 (2) | 12.5 (4) | 81.3 (26) | |
| HER2 | 20 | 0 (0) | 20 (4) | 80 (16) | |
| ER | | | | | |
| Positive | 115 | 1.8 (2) | 27.8 (32) | 70.4 (81) | 0.3744 |
| Negative | 82 | 4.9 (4) | 23.2 (19) | 71.9 (59) | |
| PR | | | | | |
| Positive | 75 | 1.4 (1) | 25.3 (19) | 73.3 (55) | 0.5305 |
| Negative | 122 | 4.1 (5) | 26.2 (32) | 69.7 (85) | |
| HER2 | | | | | |
| Positive | 25 | 0 (0) | 16 (4) | 84 (21) | 0.2574 |
| Negative | 167 | 3.6 (6) | 27.5 (46) | 68.9 (115) | |
| EGFR | | | | | |
| Positive | 13 | 0 (0) | 15.4 (2) | 84.6 (11) | 0.5024 |
| Negative | 184 | 3.3 (6) | 26.6 (49) | 70.1 (129) | |
| Pcad | | | | | |
| Positive | 50 | 0 (0) | 20 (10) | 80 (40) | 0.1580 |
| Negative | 147 | 4.1 (6) | 27.9 (41) | 68 (100) | |
| CK5 | | | | | |
| Positive | 50 | 4 (2) | 22 (11) | 74 (37) | 0.7143 |
| Negative | 147 | 2.7 (4) | 27.2 (40) | 70.1 (103) | |
| CK14 | | | | | |
| Positive | 4 | 25 (1) | 0 (0) | 75 (3) | 0.0253 |
| Negative | 188 | 2.7 (5) | 27.1 (51) | 70.2 (132) | |
| P63 | | | | | |
| Positive | 4 | 0 (0) | 25 (1) | 75 (3) | 0.9350 |
| Negative | 193 | 3.1 (6) | 25.9 (50) | 80 (137) | |
| P53 | | | | | |
| Positive | 44 | 2.3 (1) | 9.1 (4) | 88.6 (39) | 0.0126 |
| Negative | 153 | 3.3 (5) | 30.7 (47) | 66 (101) | |
| Vimentin | | | | | |
| Positive | 33 | 6.1 (2) | 18.2 (6) | 75.7 (25) | 0.4315 |
| Negative | 151 | 2.7 (4) | 25.8 (39) | 71.5 (108) | |
Regarding the stromal expression of FGFR1, results
showed that only 9.6% of the cases were positive. Despite the low
percentage of positive cases, significant correlations were
observed between the expression of FGFR1 in the stroma and
histological grade (P=0.0238), breast cancer molecular subtypes
(p=0.0063), ER expression (P=0.0127), Pcad expression (P=0.0041),
P63 expression (0.0057) and P53 expression (P=0.0058; Table V). An increasing percentage of
FGFR1-positive cases were observed, from cases of histological
grades I to III. Additionally, a higher percentage of
FGFR1-positive cases in the basal and HER2 histological subtypes
was observed when compared to the luminal subtype. It was also
possible to observe a significant statistical correlation between
ER expression and FGFR1 expression in the stroma, where the
ER-negative cases exhibited a higher percentage of positive FGFR1
expression when compared to the ER-positive cases. FGFR1-positive
cases (68) were negative for ER expression and 61% of the
FGFR1-negative cases were positive for ER expression, suggesting an
inverse correlation between the expression of the two molecules.
Another statistically significant correlation was found between
FGFR1 and Pcad expression, revealing a higher percentage of
Pcad-positive cases expressing FGFR1 as compared to their negative
counterparts. The results also demonstrated a higher percentage of
P53-positive cases expressing FGFR1 when compared to the
P53-negative cases. A significant correlation between FGFR1 and P63
expression (P=0.0057) was also observed. Nonetheless, this
correlation should be interpreted with caution due to the low
number of positive cases expressing P63. No significant statistical
correlations were found between the expression of FGFR1 in the
stroma and patient survival (data not shown), nor between PAR1 and
FGFR1.
 | Table V.Correlation between FGFR1 expression
in the stroma and clinicopathological parameters. |
Table V.
Correlation between FGFR1 expression
in the stroma and clinicopathological parameters.
| Clinicopathological
parameters | n | FGFR1 stroma
| P-value |
|---|
| Negative (178
cases) % (n) | Positive (19 cases)
% (n) |
|---|
| Histological
grade | | | | |
| I | 89 | 95.5 (85) | 4.5 (4) | 0.0238 |
| II | 74 | 87.8 (65) | 12.2 (9) | |
| III | 28 | 78.6 (22) | 21.4 (6) | |
| Lymph node
metastasis | | | | |
| Positive | 84 | 88.1 (74) | 11.9 (10) | 0.2593 |
| Negative | 75 | 93.3 (70) | 6.7 (5) | |
| HER2 | | | | |
| Positive | 25 | 88 (22) | 12 (3) | 0.7056 |
| Negative | 167 | 90.4 (151) | 9.6 (6) | |
| Molecular
subtype | | | | |
| Luminal | 118 | 94.1 (111) | 5.9 (7) | 0.0063 |
| Basal | 32 | 75 (24) | 25 (8) | |
| HER2 | 20 | 85 (17) | 15 (3) | |
| ER | | | | |
| Positive | 115 | 94.8 (109) | 5.2 (6) | 0.0127 |
| Negative | 82 | 84.1 (69) | 15.9 (13) | |
| PR | | | | |
| Positive | 75 | 93.3 (70) | 6.7 (5) | 0.2669 |
| Negative | 122 | 88.5 (108) | 11.5 (14) | |
| EGFR | | | | |
| Positive | 13 | 76.9 (10) | 23.1 (3) | 0.0896 |
| Negative | 184 | 91.3 (168) | 8.7 (16) | |
| Pcad | | | | |
| Positive | 50 | 80 (40) | 20 (10) | 0.0041 |
| Negative | 147 | 93.9 (138) | 6.1 (9) | |
| CK5 | | | | |
| Positive | 50 | 90 (45) | 10 (5) | 0.9215 |
| Negative | 147 | 90.5 (133) | 9.5 (14) | |
| CK14 | | | | |
| Positive | 4 | 100 (4) | 0 (0) | 0.5156 |
| Negative | 188 | 90.4 (170) | 9.6 (18) | |
| P63 | | | | |
| Positive | 4 | 50 (2) | 50 (2) | 0.0057 |
| Negative | 193 | 91.2 (176) | 8.8 (17) | |
| P53 | | | | |
| Positive | 44 | 79.5 (35) | 20.5 (9) | 0.0058 |
| Negative | 153 | 93.5 (143) | 6.5 (10) | |
| Vimentin | | | | |
| Positive | 33 | 84.8 (28) | 15.2 (5) | 0.2518 |
| Negative | 151 | 91.4 (138) | 8.6 (13) | |
Intratumoral microvessel density
iMVD evaluation was performed for 200 cases. The
number of intratumoral blood vessels ranged between 0 and 47. The
cases were grouped according to the cut off: One group had an iMVD
of ≤8.5, the median iMVD, and a second group had an iMVD of
>8.5. iMVD was correlated with the expression of PAR1 and FGFR1,
in the tumor cells and stroma, as well as with several
clinicopathological parameters and crucial breast cancer molecular
markers (Table VI). A significant
correlation was found between iMVD and the histological grade
(P=0.022), with an inverse statistically significant correlation
between breast cancer cases of grade III and iMVD. Regarding EGFR
expression and iMVD, the results showed a higher percentage of iMVD
≤8 in positive EGFR cases when compared to iMVD >8.5 cases. A
statistically significant correlation was also observed between
iMVD and patient survival, showing that patients with an iMVD of
>8.5 have better survival (data not shown). No significant
correlations were observed between the iMVD with FGFR1 or PAR1
expression in the tumor cells or stroma.
 | Table VI.Correlation between intratumoral
microvessel density and clinicopathological parameters. |
Table VI.
Correlation between intratumoral
microvessel density and clinicopathological parameters.
| Clinicopathological
parameters | n | iMVD
| P-value |
|---|
| ≤ 8.5 % (100) | >8.5 %
(100) |
|---|
| Histological
grade | | | | |
| I | 94 | 44.7 (42) | 55.3 (52) | 0.022 |
| II | 73 | 46.6 (34) | 53.4 (39) | |
| III | 27 | 74.1 (20) | 25.9 (7) | |
| Lymph node
metastasis | | | | |
| Negative | 77 | 44.2 (34) | 55.8 (43) | 0.609 |
| Positive | 83 | 48.2 (40) | 51.8 (43) | |
| Molecular
subtype | | | | |
| Luminal A | 117 | 55.6 (65) | 44.4 (52) | 0.417 |
| Luminal B | 7 | 28.6 (2) | 71.4 (5) | |
| HER2 | 4 | 50.0 (2) | 50.0 (2) | |
| Basal | 52 | 46.2 (24) | 53.8 (28) | |
| ER | | | | |
| Negative | 79 | 44.3 (35) | 55.7 (44) | 0.193 |
| Positive | 121 | 53.7 (65) | 46.3 (56) | |
| PR | | | | |
| Negative | 123 | 45.5 (56) | 54.5 (67) | 0.110 |
| Positive | 77 | 57.1 (44) | 42.9 (33) | |
| HER2 | | | | |
| Negative | 172 | 50.0 (86) | 50.0 (86) | 0.981 |
| Positive | 27 | 52.2 (14) | 47.8 (13) | |
| EGFR | | | | |
| Negative | 189 | 48.1 (91) | 51.9 (98) | 0.030 |
| Positive | 11 | 81.8 (9) | 18.2 (2) | |
| CK5 | | | | |
| Negative | 150 | 53.3 (80) | 46.7 (70) | 0.102 |
| Positive | 50 | 40.0 (20) | 60.0 (30) | |
| CK14 | | | | |
| Negative | 187 | 49.2 (92) | 50.8 (95) | 0.307 |
| Positive | 4 | 75.0 (3) | 25.0 (1) | |
| P-Cad | | | | |
| Negative | 149 | 47.7 (71) | 52.3 (78) | 0.256 |
| Positive | 51 | 56.9 (29) | 43.1 (22) | |
| P63 | | | | |
| Negative | 196 | 50.0 (98) | 50.0 (98) | 1.000 |
| Positive | 4 | 50.0 (2) | 50.0 (2) | |
| P53 | | | | |
| Negative | 157 | 50.3 (79) | 49.7 (78) | 0.863 |
| Positive | 43 | 48.8 (21) | 51.2 (22) | |
| FGFR1 tumor | | | | |
| Negative | 6 | 50.0 (3) | 50.0 (3) | 0.706 |
| Positive
<50% | 48 | 43.8 (21) | 56.2 (27) | |
| Positive
>50% | 136 | 50.7 (69) | 49.3 (67) | |
| FGFR1 stroma | | | | |
| Negative | 172 | 50.0 (86) | 50.0 (86) | 0.370 |
| Positive | 18 | 38.9 (7) | 61.1 (11) | |
| PAR1 tumor | | | | |
| Negative | 4 | 50.0 (2) | 50.0 (2) | 0.721 |
| Positive
<50% | 17 | 41.2 (7) | 58.8 (10) | |
| Positive
>50% | 173 | 51.4 (89) | 48.6 (84) | |
Discussion
The analysis of PAR1 expression in a retrospective
series of 224 formalin-fixed paraffin-embedded samples of ductal
invasive breast carcinomas demonstrates, as suggested by previous
studies, the expression of PAR1 in human malignant tumor cells as
well as the cell types forming the tumor microenvironment, and no
expression of PAR1 in the surrounding stromal fibroblasts of the
normal and benign breast epithelial cells (19). PAR1 expression in tumor cells did
not exhibit a significant correlation with any of the
clinicopathological parameters analysed, including histological
grade, lymph node metastasis, patient survival, molecular subtype
and the expression of several molecular markers, such as ER, PR,
HER2, EGFR, Pcad, CK5, CK14, P63, P53 and vimentin.
The present study has shown the absence of
statistically significant correlations of PAR1 expressed in the
tumor cells. Specifically, the statistical analysis showed a
significant association between the expression of PAR1 in the
stroma and more aggressive histological subtypes of breast cancer,
more specifically with the basal and HER2 subtypes. Stromal cells,
such as fibroblasts and inflammatory cells recruited to the tumor
microenvironment highly expressed MMPs, and recent studies suggest
that the MMP1 in the stromal-tumor microenvironment is capable of
altering the behaviour of cancer cells through PAR1 to promote cell
migration and invasion (11).
The fact that a high percentage of cases negative
for ER and PR expression are positive for PAR1 expression in the
stroma also suggests an association with poor prognosis (21). Furthermore, the finding that a high
percentage of cases positive for Pcad expression are also positive
for PAR1 expression in the stroma suggests an association between
PAR1 and invasive behaviour, since Pcad expression has been
strongly associated with a high histological grade of ductal in
situ breast carcinoma and poor survival, and has also shown a
strong inverse correlation with ER expression in two types of
breast carcinoma (in situ and invasive) (22,23).
These results showed a strong association between the expression of
PAR1 in the stroma and several clinicopathological parameters
associated with more aggressive breast cancers, with worse response
to treatment and consequently poor prognosis, highlighting the
importance of the microenvironment in tumor expression, suggesting
a potential role of PAR1 in autocrine and paracrine mechanisms of
breast cancer progression (24).
Most of our results related to PAR1 expression are
not in agreement with a previous study that demonstrated a
significant correlation between the expression of PAR1 in breast
cancer and tumor grade in the presence of positive axillary lymph
nodes in a series of 75 breast carcinoma specimens (16). Nonetheless, these differences may be
explained by a different classification used for assessment of PAR1
expression in the two studies and the number of cases analyzed in
each study.
PAR1 expression levels are directly correlated with
degree of invasiveness in both primary breast tissue specimens and
established cancer cell lines (25). This finding supports the results
observed for PAR1 expression in the stroma and suggest active
involvement of the former protein in a neoplastic development
scenario. More studies are required to precisely explain the role
of PAR1 in more aggressive tumor behaviour and poor patient
prognosis.
As for FGFR1 expression, significant correlations
were found between FGFR1 expression in tumor cells and tumor grade
and P53 expression, showing an association between FGFR1 and
parameters of cancer aggressiveness (2). These results are coherent due to the
well-known correlation between P53 isoforms and solid malignant
tumor progression (26).
Furthermore, the significant correlations observed between FGFR1
expression in the stroma and the histological grade, molecular
subtypes, ER, Pcad and P53 expression, emphasize the association of
FGFR1 with more aggressive breast cancer phenotypes previously
suggested by in vitro models (27,28).
It was previously demonstrated that when core 2 of
the 8p11.2-p12 is amplified, FGFR1 gene shows increased
levels of mRNA and protein expression, as FGFR1 signalling is
required for the survival of breast cancer cells harbouring
FGFR1 amplification (29).
Furthermore, FGFR1 amplification was observed in up to 10%
of breast carcinomas and patient survival analysis revealed
FGFR1 amplification to be an independent prognostic factor
for survival, more specifically in patients with ER-positive
tumors, where FGFR1 amplification was the strongest
independent predictor of poor outcome, suggesting that this gene is
a useful therapeutic target for a subgroup of breast cancer
patients with FGFR1 gene amplification (30). We were careful to include the
patient’s outcome in the statistical analyses. To the best of our
knowledge, this is the first study to consider the correlation of
PAR1 and FGFR1 expression in a large breast cancer cohort. Patient
survival information was not available for all patients, limiting
the evaluation of survival curves.
The tissue-specific expression of FGFs and FGFRs is
critical factor in regulating FGFR signalling pathways and
malignant transformation (31).
FGF1 and FGFR1 were also demonstrated to be notably expressed in
breast cancer cells. FGFR1 is also expressed in normal breast
tissues but not FGF1, which suggests that breast cancer cells are
able to release FGF1 and express FGFR1 in a neoplastic scenario,
leading to a tumoral mass growing not only by a paracrine, but also
by an autocrine mechanism (30).
FGFR1 is able to directly activate both proliferation and survival
signals (anti-apoptotic effects) within mammary epithelium to
rapidly induce hyperplastic lesions and regulate MMP secretion
(19). This finding suggests a
crucial role for FGFR1 during tumor progression and prognosis and
may explain, in part, the results obtained for FGFR1 in the tumor
and stroma cells, reported in the present study.
Intratumoral angiogenesis, commonly assessed by
determination of iMVD, has been suggested as a prognostic factor in
solid tumors, as it has been shown on numerous occasions that
higher iMVD is associated with poorer prognosis (32). Tissue microarrays are not the
preferred option for determining iMVD; however, we opted for this
method in order to maintain the same pattern of analyses for all
cases. Our results did not show a statistically significant
correlation between iMVD and clinicopathological parameters
associated with cancer aggressiveness. The statistical significant
inverse correlations we observed with histological grade III and
for positive EGFR are debatable. Approximately 75% of grade III
cases showed an iMVD of <8.5. Essentially, a minority of studies
have not demonstrated an association between higher iMVD and poor
prognosis. The apparent paradox between our findings and those of
previous studies may be explained by several factors such as the
lack of a standardized iMVD assessment (vessel counting and
statistical analysis), use of different endothelial markers and
immunostaining techniques with different specificity and
sensitivity, the different size cohorts, different cut off and
inadequate follow up (32).
Regarding breast cancer, the controversies are even more pronounced
since no significant differences in tumor vascularity with
different molecular subtypes have been found (33).
In conclusion, the results have shown that PAR1 and
FGFR1 expression in breast carcinomas are correlated to several
clinicopathological parameters of tumor behaviour, suggesting an
association of PAR1 and FGFR1 with the more aggressive breast
cancers, with possible worse response to treatment and consequently
poor prognosis, emphasizing the importance of the microenvironment
in tumor progression.
Acknowledgements
CICECO is an Associated Laboratory of
the Portuguese Ministry of Science, Technology and Higher
Education, being financed by Pest-C/CTM/LA0011/2011.
References
|
1.
|
J FerlayHR ShinF BrayD FormanC MathersDM
ParkinEstimates of worldwide burden of cancer in 2008: GLOBOCAN
2008Int J Cancer12728932917201010.1002/ijc.2551621351269
|
|
2.
|
B WeigeltJS Reis-FilhoHistological and
molecular types of breast cancer: is there a unifying taxonomy?Nat
Rev Clin Oncol6718730200910.1038/nrclinonc.2009.16619942925
|
|
3.
|
B WeigeltHM HorlingsB KreikeRefinement of
breast cancer classification by molecular characterization of
histological special typesJ
Pathol216141150200810.1002/path.240718720457
|
|
4.
|
D HanahanRA WeinbergHallmarks of cancer:
the next
generationCell144646674201110.1016/j.cell.2011.02.01321376230
|
|
5.
|
L PusztaiCurrent status of prognostic
profiling in breast
cancerOncologist13350360200810.1634/theoncologist.2007-021618448548
|
|
6.
|
YJ YinZ SalahM MaozOncogenic
transformation induces tumour angiogenesis: a role for PAR1
activationFASEB J17163174200310.1096/fj.02-0316com12554695
|
|
7.
|
SF WinterVD AcevedoRD GangulaConditional
activation of FGFR1 in the prostate epithelium induces angiogenesis
with concomitant differential regulation of Ang-1 and
Ang-2Oncogene2648974907200710.1038/sj.onc.1210288
|
|
8.
|
A AgarwalL CovicLM SevignyTargeting a
metalloprotease-PAR1 signaling system with cell-penetrating
pepducins inhibits angiogenesis, ascites, and progression of
ovarian cancerMol Cancer
Ther727462757200810.1158/1535-7163.MCT-08-017718790755
|
|
9.
|
TK VuDT HungVI WheatonSR CoughlinMolecular
cloning of a functional thrombin receptor reveals a novel
proteolytic mechanism of receptor
activationCell6410571068199110.1016/0092-8674(91)90261-V1672265
|
|
10.
|
PJ O’BrienN PrevostM MolinoMK HollingerMJ
WoolkalisDS WoulfeLF BrassThrombin responses in human endothelial
cells. Contributions from receptors other than PAR1 include the
transactivation of PAR2 by thrombin-cleaved PAR1J Biol
Chem2751350213509200010788464
|
|
11.
|
A BoireL CovicA AgarwalPAR1 is a matrix
metalloprotease-1 receptor that promotes invasion and
tumourigenesis of breast cancer
cellsCell120303313200510.1016/j.cell.2004.12.01815707890
|
|
12.
|
S BassusO HerkertN KronemannThrombin
causes vascular endothelial growth factor expression in vascular
smooth muscle cells: role of reactive oxygen speciesArterioscler
Thromb Vasc Biol2115501555200110.1161/hq0901.09514811557687
|
|
13.
|
M DuarteV KolevR SoldiThrombin induces
rapid PAR1-mediated non-classical FGF1 releaseBiochem Biophys Res
Commun350604609200610.1016/j.bbrc.2006.09.10717027650
|
|
14.
|
P DelafontaineA AnwarH LouL KuG-protein
coupled and tyrosine kinase receptors: evidence that activation of
the insulin-like growth factor I receptor is required for thrombin
induced mitogenesis of rat aortic smooth muscle cellsJ Clin
Invest97139145199610.1172/JCI118381
|
|
15.
|
BH RauchE MilletteRD KenagyThrombin- and
factor Xa-induced DNA synthesis is mediated by transactivation of
fibroblast growth factor receptor-1 in human vascular smooth muscle
cellsCirc
Res94340345200410.1161/01.RES.0000111805.09592.D814670838
|
|
16.
|
NA HernandezE CorreaEP AvilaPAR1 is
selectively over expressed in high grade breast cancer patients: a
cohort studyJ Transl Med747200910.1186/1479-5876-7-4719538737
|
|
17.
|
MR D’AndreaCK DerianRJ SantulliP
Andrade-GordonDifferential expression of protease activated
receptors-1 and -2 in stromal fibroblasts of normal, benign, and
malignant human tissuesAm J Pathol15820312041200111395381
|
|
18.
|
KL SchwertfegerFibroblast growth factors
in development and cancer: insights from the mammary and prostate
glandsCurr Drug
Targets10632644200910.2174/13894500978868041919601767
|
|
19.
|
BE WelmKW FreemanM ChenInducible
dimerization of FGFR1: development of a mouse model to analyze
progressive transformation of the mammary glandJ Cell
Biol157703714200210.1083/jcb.20010711912011115
|
|
20.
|
A MarinhoR SoaresJ FerroAngiogenesis in
breast cancer is related to age but not to other prognostic
parametersPathol Res
Pract193267273199710.1016/S0344-0338(97)80003-99258952
|
|
21.
|
JD BrentonLA CareyAA AhmedC
CaldasMolecular classification and molecular forecasting of breast
cancer: ready for clinical application?J Clin
Oncol2373507360200510.1200/JCO.2005.03.384516145060
|
|
22.
|
J ParedesAL CorreiaAS RibeiroBreast
carcinomas that co-express E- and P-cadherin are associated with
p120-catenin cytoplasmic localisation and poor patient survivalJ
Clin Pathol61856862200810.1136/jcp.2007.05270418381381
|
|
23.
|
J ParedesF MilaneziL ViegasP-cadherin
expression is associated with high-grade ductal carcinoma in situ
of the breastVirchows
Arch4401621200210.1007/s00428010048711942571
|
|
24.
|
E YangA BoireA AgarwalBlockade of PAR1
signaling with cell-penetrating pepducins inhibits Akt survival
pathways in breast cancer cells and suppresses tumour survival and
metastasisCancer
Res6962236231200910.1158/0008-5472.CAN-09-018719622769
|
|
25.
|
S Even-RamB UzielyP CohenThrombin receptor
over-expression in malignant and physiological invasion
processesNat Med4909914199810.1038/nm0898-9099701242
|
|
26.
|
JC Bourdonp53 and its isoforms in cancerBr
J Cancer97277282200710.1038/sj.bjc.660388617637683
|
|
27.
|
K SuyamaI ShapiroM GuttmanRB HazanA
signaling pathway leading to metastasis is controlled by N-cadherin
and the FGF receptorCancer
Cell2301314200210.1016/S1535-6108(02)00150-212398894
|
|
28.
|
W XianKL SchwertfegerT Vargo-GogolaJM
RosenPleiotropic effects of FGFR1 on cell proliferation, survival,
and migration in a 3D mammary epithelial cell modelJ Cell
Biol171663673200510.1083/jcb.20050509816301332
|
|
29.
|
JS Reis-FilhoPT SimpsonNC TurnerFGFR1
emerges as a potential therapeutic target for lobular breast
carcinomasClin Cancer
Res1266526662200610.1158/1078-0432.CCR-06-116417121884
|
|
30.
|
S Elbauomy ElsheikhAR GreenMB LambrosFGFR1
amplification in breast carcinomas: a chromogenic in situ
hybridisation analysisBreast Cancer Res9R23200717397528
|
|
31.
|
R GroseC DicksonFibroblast growth factor
signaling in tumourigenesisCytokine Growth Factor
Rev16179186200510.1016/j.cytogfr.2005.01.00315863033
|
|
32.
|
J HasanR ByersGC JaysonIntratumoural
microvessel density in human solid tumoursBr J
Cancer8615661577200210.1038/sj.bjc.660031512085206
|
|
33.
|
N LopesB SousaD VieiraVessel density
assessed by endoglin expression in breast carcinomas with different
expression
profilesHistopathology55594599200910.1111/j.1365-2559.2009.03417.x19912365
|