Introduction
Based on clinical and autopsy findings, the stomach
is an unusual site for metastasis and the reported incidence of
metastatic gastric tumors is 0.2–0.7% (1–3).
Metastatic spread to the stomach may occur a number of years after
the initial treatment of the primary malignancy. The breast,
melanoma, lung and esophagus are the most common primary sites of
metastases to the stomach (2–4). The
most common sites of metastasis in renal cell carcinoma (RCC),
which accounts for 2 to 3% of adult malignant neoplasms, are the
lung (75%), lymph nodes (36%), bone (20%) and liver (18%) (5). RCC is known to cause metastatic
recurrences a number of years after its resection and even after
immunotherapy or molecularly targeted therapy. Whereas the
gastrointestinal tract is a rare site for metastases of RCC, rare
sites of metastasis, including the thyroid, pancreas, skeletal
muscles and skin, are characteristic of RCC (5,6).
As gastric metastasis is a rare condition,
information on gastric metastases is generally limited to single
case reports and there are no reported prognostic outcomes. Our
searches of English literature on gastric metastases arising from
RCC in MEDLINE revealed only 22 studies, including a case treated
in our hospital, with clearly presented data. In the present study,
we reviewed the clinical presentation, surgical management and
prognostic factors of metastatic gastric tumors arising from
RCC.
Materials and methods
Literature search
We performed a search of English literature
published between 1970 and 2011 in MEDLINE and PubMed for articles
on metastatic gastric tumors arising from RCC using the keywords
‘gastric metastasis’ and ‘renal cell carcinoma’. The reference
lists of the articles identified in this manner were then manually
searched to identify any additional references and articles
published only in abstract form were excluded. From this search, we
identified 22 cases of gastric metastases arising from RCC
(1,5,7–22).
We reviewed 22 patients who were diagnosed with
gastric metastases arising from RCC, including one patient who was
treated in our hospital. For each patient, we obtained data on age,
gender, tumor location, tumor size, metastases to other organs,
histological type, the time interval between radical excision of
the primary tumor and the diagnosis of gastric metastasis (IGM),
treatment method and outcome. We analyzed these data to identify
possible correlations between clinical variables and survival.
Statistical analysis
We used the Mann-Whitney U test to evaluate
correlations among the continuous variables for each group and
Pearson's Chi-square test to compare the categorical variables. We
used the Kaplan-Meier method to generate cumulative survival rates
and compared them using the log-rank test to evaluate statistically
significant differences (23).
P<0.05 was considered to indicate a statistically significant
result. Statistical analysis was performed using SPSS for Windows
version 13.0 (SPSS, Inc., Chicago, IL, USA).
Results
Clinical characteristics
The clinical features of the 22 reported cases are
listed in Table I. The median age
of the patients was 68 years (range, 48–83) and there was a male
predominance, with a male-to-female ratio of 16:5. Among these
patients, 6 had lesions in the upper third of the stomach, 10 had
lesions in the middle third and 6 had lesions in the lower third.
The tumor size ranged from 1 to 8 cm (median, 3 cm). Based on gross
appearance, we divided the tumors into ulcerated and protruding
types. Examination of the tumors revealed 7 cases with the
ulcerated type and 11 cases with the protruding type. Symptoms
associated with bleeding of the tumors, such including melena,
hematemesis or anemia, were observed in 18 cases (81.8%).
 | Table I.Clinical data for reported cases of
metastatic gastric tumors arising from renal cell carcinoma. |
Table I.
Clinical data for reported cases of
metastatic gastric tumors arising from renal cell carcinoma.
| Author (Ref.) | Age (years) | Gender | Tumor location | Tumor size
(cm) | Gross appearance
type | Bleeding | Additional
metastases | IGM (years) | Therapy | Outcome |
|---|
| Sullivan WG
(7) | 69 | Male | L | ND | Protruding | Yes | Solitary | 1.0 | Gastrectomy | ND |
| Boruchowicz A
(8) | 48 | Male | U | ND | Protruding | No | Lung, liver,
esophagus | 1.3 | Chemotherapy | Died 4 months after
therapy |
| Blake MA (9) | 63 | Male | L | ND | Ulcerated | Yes | Duodenum | 6.0 | Arterial
embolization | 5 months
survival |
| Odori T (10) | 59 | Male | M | 1.5 | Ulcerated | ND | Solitary | 4.4 | Total
gastrectomy | 17 months
survival |
| Picchio M (11) | 58 | Female | M | 2 | Protruding | Yes | Solitary | 14.0 | Subtotal
gastrectomy | 6 months
survival |
| Mascarenhas B
(12) | 66 | Male | M | 2 | Ulcerated | Yes | Lung | 7.0 | Partial
gastrectomy | 3 years
survival |
| Kok Wee L (13) | 60 | Male | U, M | 10, 3.5 | Protruding,
ulcerated | Yes | ND | 20.0 | ND | ND |
| Kobayashi O
(1) | 78 | Male | L | 5 | Protruding | Yes | Solitary | 6.2 | Resection | Died 5 months after
therapy |
| Lamb GW (14) | 69 | Female | M | ND | ND | Yes | Lung | 18.0 | Arterial
embolization, octreotide | Died 23 months
after therapy |
| Riviello C
(15) | 68 | Male | U | 5 | Ulcerated | Yes | Pancreas, spleen,
liver, PAN | 10.0 | Total
gastrectomy | Died 24 months
after therapy |
| Pezzoli A (16) | 78 | Male | M | 2.5 | Protruding | Yes | Dissemination | 5.0 | Endoscopic
polypectomy | Died 6 months after
therapy |
| Saidi RF (17) | ND | ND | M | 1 | Protruding | Yes | Solitary | 10.0 | Wedge
resection | 18 months
survival |
| Pollheimer MJ
(5) | 69 | Male | M | 7.5 | Ulcerated | No | Lung, bone,
adrenal | 4.2 | Chemotherapy | Died 19 months
after therapy |
| 77 | Male | L | 3 | Ulcerated | No | Lung, bone | 6.3 | Interferon | Died 4 months after
therapy |
| 83 | Female | L | 4.5 | ND | Yes | Lung, liver,
pancreas | 1.7 | Ablative,
interferon | Died 5 months after
therapy |
| 65 | Female | ND | 4 | ND | Yes | Lung, brain | 13.1 | Ablative | Died 3 months after
therapy |
| 69 | Male | M | 5.4 | ND | Yes | Lung, bone | 9.3 | Ablative,
sunitinib | 2 years
survival |
| Yamamoto D
(18) | 74 | Male | M | 8 | Protruding | Yes | Brain | 5.0 | Wedge
resection | Died 1 month after
therapy |
| Kibria R (19) | 53 | Male | U | 1.5 | Protruding | Yes | Lung, bone | 0.0 | Palliative
therapy | Died 2 months after
therapy |
| Sugasawa H
(20) | 69 | Male | U | 2 | Ulcerated | Yes | Solitary | 19.0 | Wedge
resection | 12 months
survival |
| Tiwari P (21) | 58 | Female | L | 4 | Protruding | Yes | Lung | 0.0 | Subtotal
gastrectomy | Died 2 months after
therapy |
| Namikawa T
(22) | 65 | Male | U | 2.5 | Protruding | Yes | Solitary | 23.0 | Wedge
resection | 2 months
survival |
The median IGM was 6.3 years (range, 1–23). The case
with the maximal interval of 23 years was the patient treated in
our hospital. The RCCs were clear-cell carcinomas in all cases. A
total of 7 cases had solitary gastric metastasis and 14 cases had
metastases in other locations. Among the 14 cases with metastases
in other locations, tumors were found in the lung in 8 cases, bone
in 4 cases, liver in 3 cases, pancreas in 2 cases and in the brain,
esophagus, duodenum, spleen, peritoneum and paraaortic lymph nodes
in 1 case each. Treatment included wedge resection, gastrectomy and
endoscopic resection in 12 cases and palliative therapy in 9 cases
due to the general condition of the patients and the presence of
additional metastases.
Survival analysis
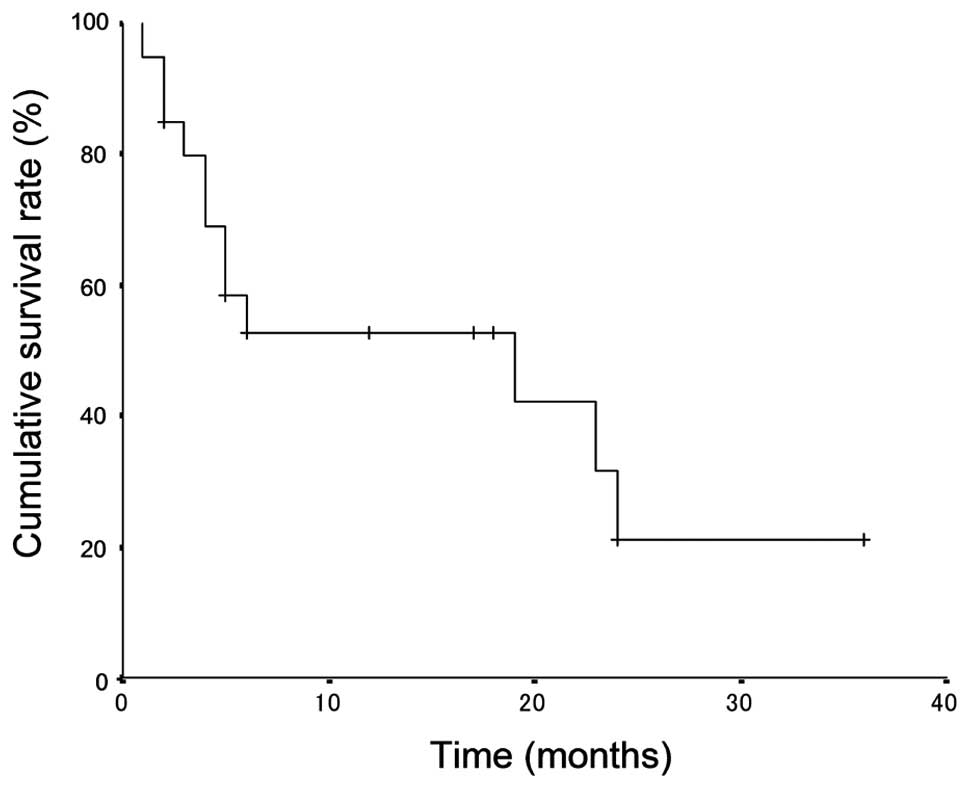
The median survival time was 19 months (range, 1 to
36) and the overall 1- and 3-year survival rates after therapy were
52.6 and 21.0%, respectively (Fig.
1). Clinical characteristics of patients with metastatic
gastric tumors arising from RCC are shown in Table II, together with a comparison of
survival rates among subgroups of prognostic factors. The median
survival period of patients with a protruding type tumor was
significantly worse than those with an ulcerated type tumor (5 vs.
24 months; P=0.030). When patients were divided into two groups
depending on the IGM (i.e., <6.3 or ≥6.3 years), median survival
was 5 months for patients with an IGM <6.3 years compared with
24 months for those with an IGM ≥6.3 years (P=0.017; Fig. 2). A short interval between initial
radical excision of the primary tumor and appearance of gastric
metastasis was found to be significantly associated with a poor
outcome. The 3-year survival rate of patients with a tumor <3 cm
in diameter tended to be higher than those with a tumor in ≥3 cm
diameter (72.9 vs. 11.1%), although the differences did not reach
statistical significance (P=0.067). There were no significant
influences on survival rate by age, gender, tumor location,
bleeding, metastasis type or method of therapy. No independent
prognostic factors were identified by multivariate analysis,
probably due to the small patient number.
 | Table II.Clinical characteristics of patients
following treatment of metastatic gastric tumors arising from renal
cell carcinoma. |
Table II.
Clinical characteristics of patients
following treatment of metastatic gastric tumors arising from renal
cell carcinoma.
|
Characteristics | 3-year survival
rate (%) | Median survival
time (months) | P-value |
|---|
| Overall | 21 | 19 | |
| Age (years) | | | 0.631 |
| <68 | 28.6 | 24 | |
| ≥68 | 14.8 | 6 | |
| Gender | | | 0.345 |
| Male | 26.7 | 19 | |
| Female | 0 | 5 | |
| Tumor location | | | 0.102 |
| Upper third | 0 | 24 | |
| Middle third | 38.9 | 23 | |
| Lower third | 20 | 5 | |
| Tumor size
(cm) | | | 0.067 |
| <3 | 72.9 | - | |
| ≥3 | 11.1 | 5 | |
| Gross appearance
type | | | 0.030 |
| Ulcerated | 28.6 | 24 | |
| Protruding | 26.7 | 5 | |
| Bleeding | | | 0.366 |
| Yes | 26.7 | 23 | |
| No | 0 | 4 | |
| Metastasis | | | 0.133 |
| Solitary | - | - | |
| Multiple | 16.7 | 5 | |
| IGM (years) | | | 0.017 |
| <6.3 | 0 | 5 | |
| ≥6.3 | 28.6 | 24 | |
| Therapy | | | 0.318 |
| Resection | 30.7 | 24 | |
| Palliative | 14.8 | 5 | |
Comparison of solitary and multiple
metastases
Table III shows a
comparison of clinical characteristics between solitary and
multiple metastases of 22 patients with metastatic gastric tumors
arising from RCC. The median tumor size was significantly greater
in patients with multiple metastases than in those with solitary
metastasis (4 vs. 2 cm; P=0.036). The incidence of tumor resection
performed as therapy was significantly higher in patients with
solitary metastasis than in those with multiple metastases (100.0
vs. 35.7%; P=0.019). There were no significant differences in age,
gender, tumor location or the IGM between solitary metastasis and
multiple metastases.
 | Table III.Comparison of clinical
characteristics between solitary metastasis and multiple metastases
arising from renal cell carcinoma. |
Table III.
Comparison of clinical
characteristics between solitary metastasis and multiple metastases
arising from renal cell carcinoma.
|
Characteristics | Solitary
metastasis | Multiple
metastases | P-value |
|---|
| Age (years), mean
(range) | 67 (58–78) | 68 (48–83) | 0.933 |
| Gender, n | | | 0.573 |
| Male | 5 | 10 | |
| Female | 1 | 4 | |
| Tumor location,
n | | | 0.964 |
| Upper third | 2 | 3 | |
| Middle third | 3 | 6 | |
| Lower third | 2 | 4 | |
| Median tumor size
(cm), mean (range) | 2 (1–5) | 4 (1.5–8) | 0.036 |
| Median IGM (years),
mean (range) | 10 (1–23) | 6.2 (1.3–20) | 0.447 |
| Therapy, n | | | 0.019 |
| Resection | 7 | 5 | |
| Palliative | 0 | 9 | |
| Outcome, n | | | 0.036 |
| Survived | 5 | 3 | |
| Succumbed to
disease | 1 | 11 | |
Discussion
This study demonstrated that the median IGM of the
22 patients with RCC metastasis to the stomach was 6.3 years
(range, 1–23), meaning that most patients with primary renal cancer
present with metastasis in the stomach several years after the
initial diagnosis and treatment of their original primary cancer.
To date, there are no published studies investigating the treatment
of patients with metastatic gastric tumors following curative
resection for RCC. The particularly noteworthy finding in the
present study was that patients with an IGM <6.3 years had a
significantly poorer prognosis than those with an IGM ≥6.3
years.
The time interval between diagnosis of the primary
tumor and diagnosis of gastric metastasis is reported to be 1.3–2.1
years for all metastatic gastric tumors arising from melanoma,
breast and lung cancers (4,24). It has been reported that the
majority of gastric metastases arising from lung cancer and
malignant melanoma were detected within 2 years, most likely due to
the rapid progression of these malignant tumors (2,4). By
comparison, the time interval between the diagnosis of primary
breast cancer and diagnosis of gastric metastasis was reported to
be 4.0–6.5 years and the median survival period following treatment
of these metastases was 10–20 months (25,26).
Consistent with these findings was our finding in the present study
that the IGM was 6.3 years for gastric metastasis arising from RCC
and that the median survival period following treatment was 19
months. Taken together, these results suggest that since metastatic
gastric tumors arising from RCC and those arising from breast
cancer are slow growing, there may be similarities in the clinical
characteristics associated with these cancers.
The present study also confirms the potential of RCC
to lead to resectable solitary stomach metastasis, which comprise
31.8% of all metastatic gastric tumors arising from RCC. Although
RCC spreads hematogenously and is known for its ubiquitous
metastatic patterns, solitary stomach tumors arising from RCC
metastasis is rare. Surgical resection is the preferred treatment
for solitary metastasis in the absence of contraindication related
to the general state (27). In the
present study, patients with solitary gastric metastasis arising
from RCC had good outcomes following treatment compared with those
with multiple metastases. All patients with solitary gastric
metastasis underwent surgical resection of the tumor compared with
only 35.7% of those with multiple metastases. It is possible that
surgical resection of solitary gastric metastases arising from RCC
contributes to the long-term survival of patients.
The clinical presentation of metastatic gastric
tumors is often asymptomatic or non-specific unless the metastases
invade the gastric mucosa or serosa (1). In cases of gastric metastasis arising
from breast cancer, the incidence of hemorrhaging has been reported
to be 12 to 33% (25,26). In the present study,
gastrointestinal bleeding as the presenting symptom, together with
melena or severe anemia, was observed in 81.8% of cases. The
increased incidence of gastrointestinal bleeding in gastric
metastases arising from RCC compared with those arising from breast
cancer is most likely due to the fragile and hypervascular nature
of RCC.
Although gastric metastases may be recognized as
abnormalities on esophagogastroduodenoscopy (EGD), there are no
characteristic appearances that define this disease due to the
variable morphology of the tumors (28). Frequent endoscopic patterns include
volcano-like ulcers, multiple nodules, bull's eye appearance,
extrinsic mass lesions, ulceration and polypoid tumor masses
(4,25). Based on gross appearance, we divided
metastatic gastric tumors into ulcerated and protruding types and
found that 50% were of the protruding type. Gastric metastases
arising from breast cancer are readily recognized as a diffuse
infiltration, such as linitis plastica or type 4 advanced gastric
cancers, at EGD, but are not always confirmed by endoscopic
biopsies (25). Gastric tumors
usually metastasize to other gastrointestinal organs by a
hematogeneous route, lymphatic spread or direct invasion, resulting
in submucosal masses. Tumor cells in the blood may become trapped
in the submucosal layer of the stomach and develop as submucosal
tumors (3). In gastric metastasis
arising from RCC, the protruding type tumors which invade to the
mucosa may be associated with hemorrhaging.
The choice of systemic treatment of metastatic
tumors is based upon presenting symptoms, age, general performance
status and previous systemic treatments. Metastatic breast cancer
with gastrointestinal tract involvement is evidence of a systemic
disease and therefore systemic therapy, such as chemotherapy and/or
hormonal therapy, rather than surgical resection is advised
(25). Surgical resection is not
advised for the treatment of gastric metastasis arising from breast
cancer and surgical palliation is only indicated in emergency
conditions to bypass obstructions in carefully selected patients
(26). Molecular agents targeting
metastatic RCC, including sorafenib and sunitinib, mark the start
of a new era in the management of the disease with the potential to
improve progression-free survival, objective response rate and
quality of life (29). However,
when managing patients with metastatic gastric tumors, it is
important to be aware of the risk of bleeding and tumor perforation
during systemic chemotherapy (30).
Surgical resection of metastatic gastric tumors may be recommended
to control hemorrhaging and therefore result in improved quality of
life; however, long-term survival is rare.
The limitations of the present study include the
errors and biases inherent in a small retrospective study design.
Another limitation is the lack of consistency within the study for
treatment following recurrence of the disease, as the choice of
treatment for each case was made independently by each physician.
Since the prognosis of cancer patients is gradually improving, it
is likely that gastrointestinal metastases will be encountered more
frequently in the future. For this reason, special attention should
be paid to the possibility of recurrence of RCC regardless of the
time interval since nephrectomy. While surgical intervention, such
as wedge resection of the stomach to reduce surgical stress, is
considered to be the best approach to prevent bleeding and improve
the postoperative quality of life of patients, it is difficult to
conduct prospective studies to provide evidence in support of this
approach due to the rarity of gastrointestinal metastases.
In conclusion, the results of this current study
suggest that IGM is an important factor in predicting the prognosis
of gastrointestinal metastases, regardless of whether surgical
resection of the tumor is performed.
References
|
1.
|
O KobayashiH MurakamiT YoshidaClinical
diagnosis of metastatic gastric tumors: clinicopathologic findings
and prognosis of nine patients in a single cancer centerWorld J
Surg28548551200410.1007/s00268-004-7216-815366743
|
|
2.
|
LK GreenHematogenous metastases to the
stomach. A review of 67
casesCancer6515961600199010.1002/1097-0142(19900401)65:7%3C1596::AID-CNCR2820650724%3E3.0.CO;2-52311070
|
|
3.
|
LS MenuckJR AmbergMetastatic disease
involving the stomachAm J Dig
Dis20903913197510.1007/BF010708751190198
|
|
4.
|
GD De PalmaS MasoneM RegaMetastatic tumors
to the stomach: clinical and endoscopic featuresWorld J
Gastroenterol1273267328200617143949
|
|
5.
|
MJ PollheimerTA HinterleitnerVS
PollheimerA SchlemmerC LangnerRenal cell carcinoma metastatic to
the stomach: single-centre experience and literature reviewBJU
Int102315319200810.1111/j.1464-410X.2008.07617.x18336607
|
|
6.
|
GJ SadlerMR AndersonMS MossPG
WilsonMetastases from renal cell carcinoma presenting as
gastrointestinal bleeding: two case reports and a review of the
literatureBMC Gastroenterol74200710.1186/1471-230X-7-417266757
|
|
7.
|
WG SullivanEB CabotRE DonohueMetastatic
renal cell carcinoma to
stomachUrology15375378198010.1016/0090-4295(80)90473-27394962
|
|
8.
|
A BoruchowiczP DesreumauxV MaunouryJF
ColombelDysphagia revealing esophageal and gastric metastases of
renal carcinomaAm J Gastroenterol902263226419958540538
|
|
9.
|
MA BlakeA OwensDP O'DonoghueDP
MacErleanEmbolotherapy for massive upper gastrointestinal
haemorrhage secondary to metastatic renal cell carcinoma: report of
three casesGut37835837199510.1136/gut.37.6.835
|
|
10.
|
T OdoriY TsuboiK KatohA solitary
hematogenous metastasis to the gastric wall from renal cell
carcinoma four years after radical nephrectomyJ Clin
Gastroenterol2615315419989563931
|
|
11.
|
M PicchioA PaiolettiE SantiniS IacoponiM
CordahiGastric metastasis from renal cell carcinoma fourteen years
after radical nephrectomyActa Chir Belg100228230200011143327
|
|
12.
|
B MascarenhasB KonetyJT RubinRecurrent
metastatic renal cell carcinoma presenting as a bleeding gastric
ulcer after a complete response to high-dose interleukin-2
treatmentUrology57168200110.1016/S0090-4295(00)00877-311164169
|
|
13.
|
L Kok WeeRY ShyuLF SheuTY HsiehJC YanPJ
ChenMetastatic renal cell cancerGastrointest
Endosc60265200415278061
|
|
14.
|
GW LambJ MossR EdwardsM AitchisonCase
report: octreotide as an adjunct to embolisation in the management
of recurrent bleeding upper gastrointestinal metastases from
primary renal cell cancerInt Urol
Nephrol37691693200510.1007/s11255-005-0251-z16362580
|
|
15.
|
C RivielloI TaniniG CiprianiUnusual
gastric and pancreatic metastatic renal cell carcinoma presentation
10 years after surgery and immunotherapy: A case report and a
review of literatureWorld J Gastroenterol1252345236200616937540
|
|
16.
|
A PezzoliV MatareseS BocciaL SimoneS
GulliniGastrointestinal bleeding from gastric metastasis of renal
cell carcinoma, treated by endoscopic polypectomyEndoscopy39Suppl
1E52200710.1055/s-2006-94512717323273
|
|
17.
|
RF SaidiSG RemineIsolated gastric
metastasis from renal cell carcinoma 10 years after radical
nephrectomyJ Gastroenterol Hepatol22143144200717201902
|
|
18.
|
D YamamotoY HamadaS OkazakiMetastatic
gastric tumor from renal cell carcinomaGastric
Cancer12170173200910.1007/s10120-009-0519-619890698
|
|
19.
|
R KibriaK SharmaSA AliP RaoUpper
gastrointestinal bleeding revealing the stomach metastases of renal
cell carcinomaJ Gastrointest
Cancer405154200910.1007/s12029-009-9074-y19513859
|
|
20.
|
H SugasawaT IchikuraS OnoIsolated gastric
metastasis from renal cell carcinoma 19 years after radical
nephrectomyInt J Clin Oncol15196200201020229354
|
|
21.
|
P TiwariA TiwariM VijayS KumarAK
KunduUpper gastro-intestinal bleeding - Rare presentation of renal
cell carcinomaUrol
Ann2127129201010.4103/0974-7796.6886420981203
|
|
22.
|
T NamikawaJ IwabuH KitagawaT OkabayashiM
KobayashiK HanazakiSolitary gastric metastasis from a renal cell
carcinoma, presenting 23 years after radical
nephrectomyEndoscopy44Suppl 2E177E178201222622731
|
|
23.
|
EL KaplanP MeierNonparametric estimation
from incomplete observationsJ Am Stat
Assoc53457195810.1080/01621459.1958.10501452
|
|
24.
|
PM CampoliFH EjimaDM CardosoMetastatic
cancer to the stomachGastric
Cancer91925200610.1007/s10120-005-0352-5
|
|
25.
|
BG TaalH PeterseH BootClinical
presentation, endoscopic features, and treatment of gastric
metastases from breast
carcinomaCancer8922142221200010.1002/1097-0142(20001201)89:11%3C2214::AID-CNCR9%3E3.0.CO;2-D11147591
|
|
26.
|
AA AyantundeA AgrawalSL ParsonsNT
WelchEsophagogastric cancers secondary to a breast primary tumor do
not require resectionWorld J
Surg3115971601200710.1007/s00268-007-9099-y17578645
|
|
27.
|
YB ThyavihallyU MahantshettyRS
ChamarajanagarSG RaibhattanavarHB TongaonkarManagement of renal
cell carcinoma with solitary metastasisWorld J Surg
Oncol348200510.1186/1477-7819-3-4816029517
|
|
28.
|
I OdaH KondoT YamaoMetastatic tumors to
the stomach: analysis of 54 patients diagnosed at endoscopy and 347
autopsy casesEndoscopy33507510200110.1055/s-2001-1496011437044
|
|
29.
|
RJ MotzerMD MichaelsonJ RosenbergSunitinib
efficacy against advanced renal cell carcinomaJ
Urol17818831887200710.1016/j.juro.2007.07.03017868732
|
|
30.
|
N SuzakiA HirakiH UeokaGastric perforation
due to metastasis from adenocarcinoma of the lungAnticancer
Res2212091212200212168927
|