Introduction
Lung cancer is the most common malignant neoplasm
and the leading cause of cancer mortality in industrialized
countries (1). Although there have
been advances in the diagnostic and therapeutic approaches against
lung cancer, limited levels of improvement in the treatment outcome
have been accomplished. Recent clinical studies on immunotherapy
indicated favorable therapeutic effects, and suggested that such a
strategy might represent an alternative treatment approach for lung
cancer (2,3). A large number of tumor-associated
antigens have been identified in various human cancers (4–6). A
high level of CD8 T cell infiltration into tumor tissues has been
reported to be associated with a better prognosis in colon
carcinoma and in ovarian cancer, suggesting that antitumor immunity
can be provoked in cancer patients (7,8). A
large number of antigen-based immunotherapy studies have been
conducted; however, Rosenberg et al reported that the
overall response rate for patients with cancer (mainly melanoma) to
vaccines was as low as 2.6% (9).
Such low response rates may be associated with an immunological
escape mechanism.
Overcoming these tumor immunological escape
mechanisms is considered to be necessary to develop effective
immunotherapy using tumor-associated antigens. Regulatory T cells
(Tregs) play a pivotal role in various escape mechanisms, and it is
necessary to suppress the effects of Tregs to provide efficient
cancer immunotherapy. It has been shown that Tregs constitutively
express high levels of the interleukin 2 receptor chain (CD25) and
specifically express the forkhead/winged helix transcription factor
(Foxp3), which inhibits the activation of both self-antigen and
foreign-antigen reactive T cells (10,11).
Tregs are known to have a critical physiological role in the
suppression of autoimmune diseases (12,13).
However, Tregs also play a critical role in suppressing antitumor
immune responses, since most tumor-associated antigens are
self-antigens.
The immunological implications of the population of
Tregs in the local region of the tumor and regional lymph nodes
have not been fully investigated. Therefore, the aim of the present
study was to evaluate the frequency of
CD4+CD25+Foxp3+ T cells in the
tumor-infiltrating lymphocytes (TILs), regional lymph node
lymphocytes (RLNLs) and peripheral blood lymphocytes (PBLs) of
non-small cell lung cancer (NSCLC) patients, and to determine their
influence on the induction of cytotoxic T lymphocytes (CTLs)
against autologous tumor cells.
Materials and methods
Patients
The study protocol was approved by the Human and
Animal Ethics Review Committee of the University of Occupational
and Environmental Health, Japan, and a signed consent form was
obtained from each patient before collecting the tissue samples
used in this study. Between January 2003 and December 2004, 153
patients with NSCLC underwent surgery at the University of
Occupational and Environmental Health. Of these, 84 patients were
enrolled in this study, and their RLNLs and PBLs were collected at
the time of surgery and stored at −80°C until they were analyzed.
The patients' records, including their clinical data, preoperative
examination results, details of surgery, histopathological findings
and TNM staging were also reviewed. The characteristics of the
patients are shown in Table I. The
preoperative assessments included chest roentgenography, computed
tomography (CT) of the chest and upper abdomen, magnetic resonance
imaging (MRI) of the brain, bronchoscopy and bone scintigraphy. All
resected specimens, including the primary tumor and the
systematically dissected hilar and mediastinal lymph nodes, were
examined pathologically to identify the extent of lymph node
metastases. The histopathological findings were classified
according to the World Health Organization criteria, and the TNM
staging system of the International Union Against Cancer (UICC) was
employed (14,15).
 | Table I.Characteristics of the patients with
non-small cell lung cancer. |
Table I.
Characteristics of the patients with
non-small cell lung cancer.
| Characteristic | No. of
patients |
|---|
| Age (years) | 67.8 (44–88) |
| Gender | |
| Male | 54 |
| Female | 30 |
| Histology | |
|
Adenocarcinoma | 59 |
| Squamous cell
carcinoma | 14 |
| Other | 11 |
| pStage | |
| IA | 38 |
| IB | 18 |
| IIA | 1 |
| IIB | 9 |
| IIIA | 10 |
| IIIB | 5 |
| IV | 3 |
Antibodies and flow cytometric analysis
to detect CD4, CD25 and Foxp3
Phycoerythrin-Cy5 (PE-Cy5)-conjugated anti-human CD4
(RPA-T4) and a mouse IgG2b isotype control (eBMG2b) were purchased
from eBioscience (San Diego, CA, USA). A fluorescein isothiocyanate
(FITC)-labeled monoclonal antibody against CD25 (2A3), IgG1 (X40)
and phycoerythrin (PE)-labeled monoclonal antibody against IgG1
(X40) were purchased from BD Bioscience (San Jose, CA, USA). The
PE-labeled monoclonal antibody to Foxp3 (259D) was purchased from
BioLegend (San Diego, CA, USA). A FITC-labeled mouse IgG1 or
PE-labeled mouse IgG1 was used as an isotype-matched control. To
analyze the intracytoplasmic Foxp3 expression, a human regulatory T
cell staining kit was purchased from eBioscience. The fresh and
cultured PBL and RLNL cells were washed with phosphate-buffered
saline (PBS; pH 7.4). Hanks' balanced salt solution containing 0.1%
NaN3 and 1% fetal calf serum (FCS) was used as the staining buffer.
Following incubation for 30 min with a monoclonal antibody against
Foxp3 (259D) or isotype-matched controls, 1×105 labeled cells in
each sample were analyzed on a FACSCalibur instrument (BD
Bioscience).
In vitro system to induce CTLs against
autologous tumor cells
The lung cancer cell lines, F1121L (adenocarcinoma
cell line) and L1023L (squamous cell carcinoma cell line) were
previously established in our department (16). The culture medium consisted of
RPMI-1640 (Gibco BRL, Grand Island, NY, USA) supplemented with 10%
heat-inactivated FCS (Equitech-Bio, Ingram, TX, USA), 10 mM HEPES,
100 IU/ml penicillin G and 100 mg/ml streptomycin sulfate. The RLNL
cells were obtained at the time of surgery. Each lymph node was
divided into two parts; one for the histological diagnosis and one
for this study. Lymphocytes from each lymph node were mixed and
stored at −80°C until use, as described previously (17). They were then rapidly thawed and
stimulated with an irradiated (100 Gy) CD80-transfected autologous
tumor cell line (F1121L and L1023L) weekly at a tumor-to-lymphocyte
ratio of 1:10 in culture medium as described previously (17). The tumor-specific induction of CTLs
was considered to be successful if the CTLs lysed more than 10% of
the autologous tumor cells at an effector/target ratio of 30/1 and
did not lyse autologous Epstein-Barr virus-transformed B cells
during the 4-h standard 51Cr release assay performed on day 28 of
the mixed lymphocyte-tumor cell culture (MLTC).
The tumor cells (F1121L and L1023L) were plated at
5×105 cells/25 cm2 flask (Falcon; Becton
Dickinson, Oxnard, CA, USA) in culture medium, and were incubated
at 37°C with 5% CO2. At 24 h after plating, almost all
of the tumor cells were adherent to the bottom of the flask, and
the culture medium was completely recovered, centrifuged to remove
cellular debris, and then frozen at −80°C until the measurement of
TGF-β was performed. The level of TGF-β in the supernatant was
measured using an ELISA kit (Amersham Lifescience, Braunschweig,
Germany).
Statistical analysis
The Mann-Whitney U test was used to determine the
differences in the continuous variables between the two groups.
P<0.05 was considered to indicate a statistically significant
difference.
Results
CD4+CD25+Foxp3+ Tregs are more
common in the primary tumor and regional lymph nodes than in the
peripheral blood
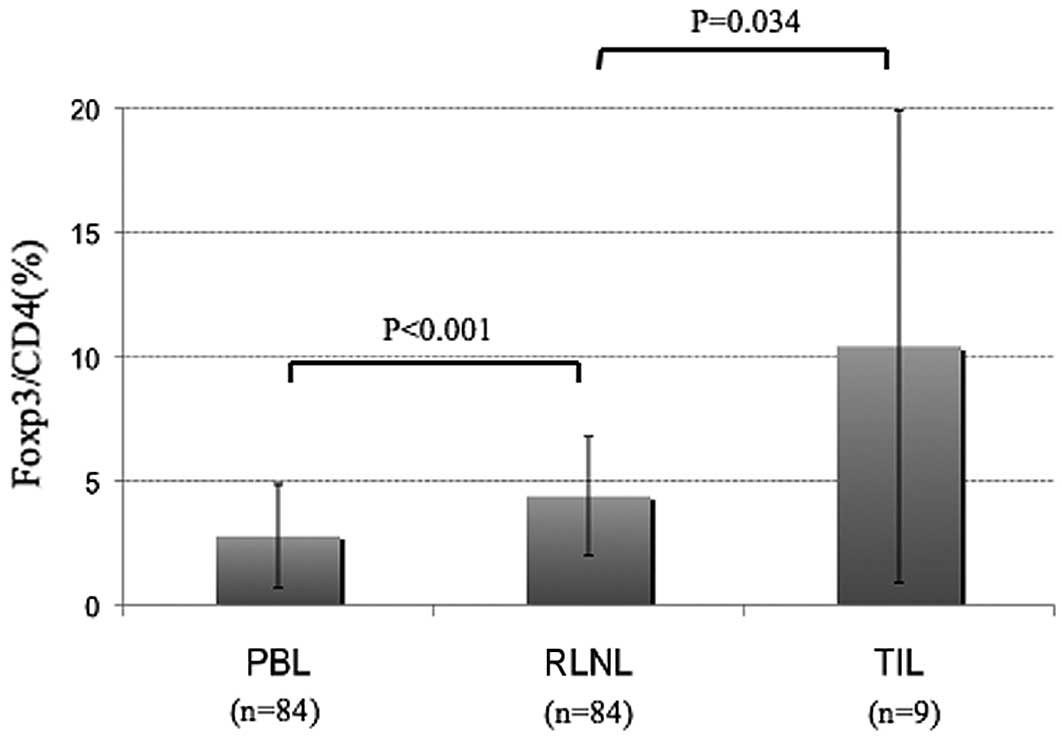
The RLNLs and PBLs of the 84 patients with lung
cancer were analyzed. The Foxp3+/CD4+ cell
ratios in the RLNLs and PBLs were 4.4±2.4 and 2.8±2.1%,
respectively. TILs could be analyzed in 9 cases, and the
Foxp3+ cell/CD4+ cell ratio was 10.4±9.5%.
The frequency of Tregs in the RLNLs was significantly higher than
that in the PBL, and the frequency in the TILs was higher still
(Fig. 1). The frequencies of Tregs
did not correlate with clinicopathological factors such as gender,
histology or pathological stage (Table
II).
 | Table II.Frequency of Foxp3+
cells/CD4+ cells in the lymph nodes and peripheral blood
in patients with non-small cell lung cancer. |
Table II.
Frequency of Foxp3+
cells/CD4+ cells in the lymph nodes and peripheral blood
in patients with non-small cell lung cancer.
| Characteristic | n | RLNL (%) | PBL (%) |
|---|
| Gender | | | |
| Male | 54 | 4.5±2.5 | 2.9±2.1 |
| Female | 30 | 4.1±2.3 | 2.5±2.5 |
| Histology | | | |
|
Adenocarcinoma | 59 | 4.4±2.2 | 2.7±2.2 |
| Squamous cell
carcinoma | 14 | 4.8±2.8 | 2.3±1.7 |
| Other | 11 | 3.9±2.7 | 3.7±1.7 |
| pStage | | | |
| I | 56 | 4.6±2.6 | 2.6±2.0 |
| II–IV | 28 | 4.0±1.9 | 3.2±2.6 |
| pN | | | |
| 0 | 61 | 4.6±2.5 | 2.6±2.0 |
| 1–3 | 23 | 3.8±1.9 | 3.2±2.3 |
Depletion of Tregs improves the
efficiency of CTL induction
The induction of CTLs against L1023L could not be
achieved from the RLNLs of patient L1023. In order to evaluate the
effects of Tregs on the induction of CTLs, autologous MLTC was
performed in the presence or absence of
CD4+CD25+ T cells. The
CD4+CD25+ T cells were isolated from RLNLs
using the AutoMACS magnetic separation system with a human
CD4+CD25+ isolation kit (Miltenyi Biotec,
Auburn, CA, USA). Following depletion of the
CD4+CD25+ T cells, the RLNLs (9×106 cells)
were seeded into 9 wells (1×106/well in 2 ml culture
medium). The 9 wells were used for 3 experimental groups as
follows: no addition of CD4+CD25+ T cells,
addition of 1×104 (1%) CD4+CD25+ T cells, and addition
of 5×104 (5%) CD4+CD25+ T cells.
The induction of tumor-specific CTLs was analyzed by a 51Cr release
assay on day 28 of the MLTC.
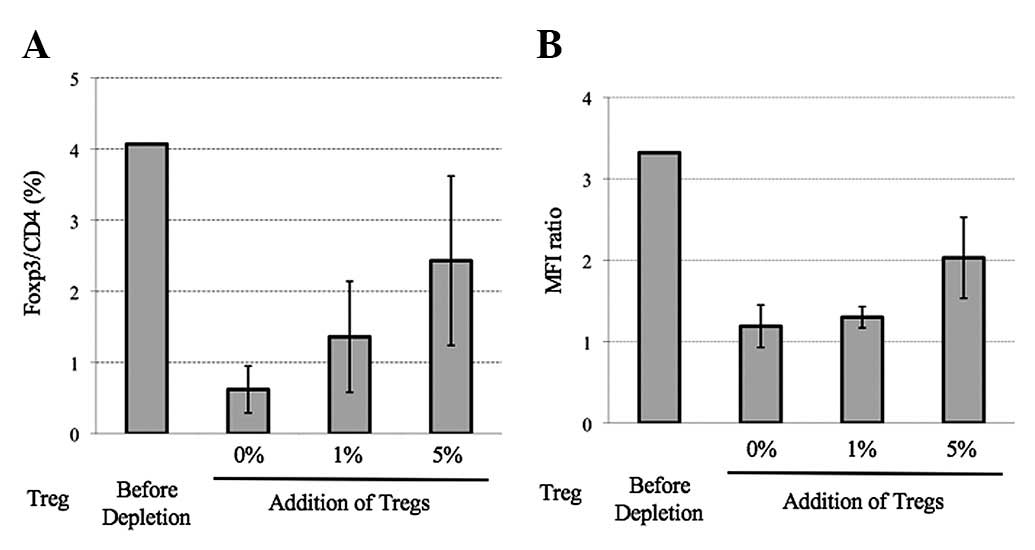
The control group (Treg-depleted group), 1%
Treg-added group and 5% Treg-added group were subjected to MLTC for
4 weeks. After 4 rounds of stimulation with cancer cells, the Tregs
were analyzed in each group by flow cytometry. Although the Tregs
were maintained for 4 weeks after MLTC in each group, the
proportion of Tregs was higher in the 5% Treg-added group than in
the Treg-depleted control group (Fig.
2A). The mean fluorescence intensity of Foxp3 in the
CD4+CD25+ T cells was also higher in the 5%
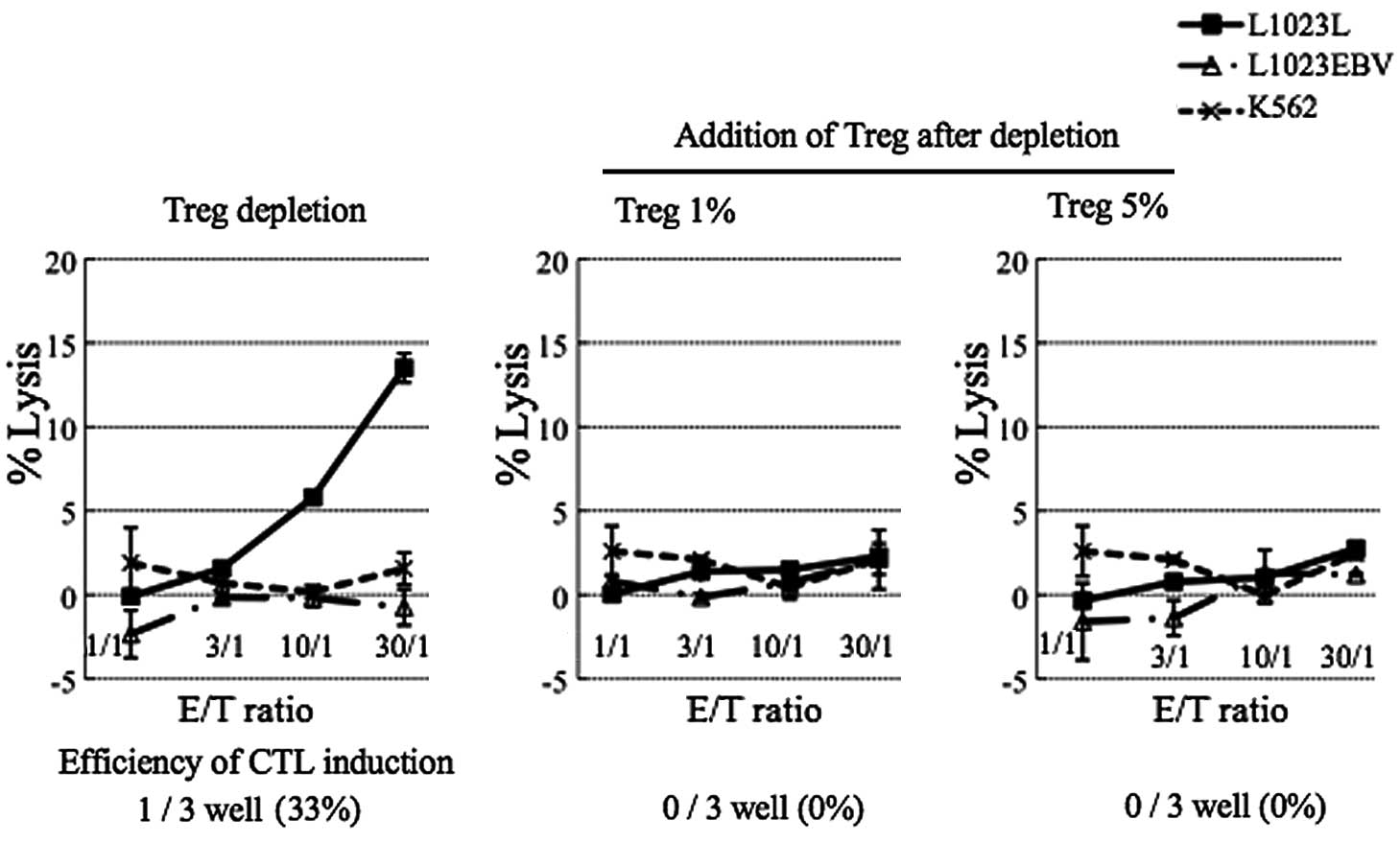
Treg-added group than in the Treg-depleted group (Fig. 2B). The CTL activity in each group
(depletion, 1% and 5% Treg addition), was assessed by the cytolytic
activity against the autologous tumor cell line, L1023L. One of 3
independent wells in the Treg-depleted group showed the successful
development of CTL activity against the autologous tumor (L1023L)
cells. In contrast, the addition of 1 and 5% Tregs completely
inhibited the induction of CTL activity (Fig. 3).
Addition of TGF-β inhibits CTL induction
and increases the number of regulatory T cells
In order to evaluate the inhibitory effect of TGF-β
on the induction of CTLs, autologous RLNLs were subjected to MLTC
in the absence or presence of 100 or 400 pg/ml TGF-β (R&D
Systems, Minneapolis, MN, USA). During the MLTC, various amounts of
TGF-β (0, 100 or 400 pg/ml) were added every day as described
previously (18). In both of the
groups with added TGF-β, the induction of CTLs was inhibited.
However, in the group cultured without TGF-β, CTL induction was
achieved in 2 out of 3 wells, as described previously (18). In the same experiment, we analyzed
the proportion of Tregs 28 days after MLTC, and the proportion of
CD4+CD25+Foxp3+ Tregs in each
sample was analyzed using a FACSCalibur instrument. A higher Treg
population was observed in the groups treated with TGF-β than in
the group without treatment (Table
III).
 | Table III.Increase of Treg population following
addition of TGF-β during mixed lymphocyte-tumor cell culture. |
Table III.
Increase of Treg population following
addition of TGF-β during mixed lymphocyte-tumor cell culture.
| TGF-β (pg/ml) |
CD4+CD25+/CD4+
(%) |
Foxp3+/CD4+ (%) | MFI ratioa |
|---|
| 0 | 19.89±1.24 | 4.59±0.89 | 1.28±0.12 |
| 100 | 28.22±5.97 | 5.83±1.79 | 1.38±0.19 |
| 400 | 24.22±14.0 | 7.19±1.26 | 1.67±0.11 |
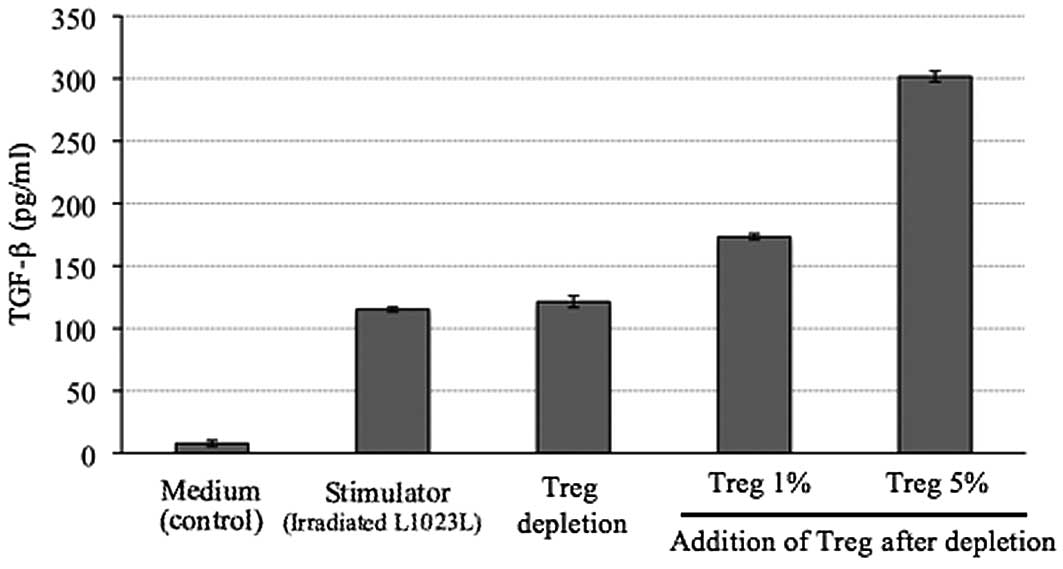
Amount of TGF-β in the supernatant after
4 weeks of MLTC
The level of TGF-β in the supernatant of cells
treated with the autologous tumor cells (L1023L) alone was 115
pg/ml. In the 1% Treg-added group, the TGF-β level in the
supernatant of the culture medium after MLTC was 173 pg/ml, whereas
the TGF-β level in the supernatant of the culture medium after MLTC
was 301 pg/ml in the 5% Treg-added group. The level of TGF-β in the
supernatant was higher in the 1 and 5% Treg-added group than in the
Treg-depleted group (Fig. 4).
Discussion
The accumulation of CD4+CD25+
immunosuppressive Tregs in tumors has been reported in various
types of cancer (19–26). Tregs are known to be a key
contributor to the maintenance of immune tolerance, preventing the
emergence of organ-specific autoimmune diseases (19). These cells constitute 2–3% of the
CD4+ T cells in the peripheral blood (20). Patients with various types of cancer
exhibited higher numbers of Tregs in their peripheral blood than
healthy donors (21), and high
numbers of tumor-infiltrating Tregs have been reported in patients
with hepatocellular, lung, ovarian, gastric, esophageal and breast
cancer (22–25). Emerging evidence suggests that Tregs
have a major effect on suppressing antigen-specific tumor immunity
(24). Furthermore, three recent
studies showed that the amount of tumor-infiltrating Tregs
influenced the prognosis of ovarian and breast cancer, as well as
gastrointestinal stromal tumors (22–26).
Our present study showed that Tregs were present at a higher
frequency in the TILs and RLNLs compared with the PBLs in patients
with lung cancer.
There have been few investigations regarding the
immunological role of Tregs in the RNLN of NSCLC patients. However,
Tregs were considered to contribute to the suppression of antitumor
immunity by inhibiting the induction of CTLs in the local region of
the tumor and regional lymph nodes. In addition, Petersen et
al reported that stage I NSCLC patients with a higher
proportion of Tregs among their TILs had a significantly higher
risk of recurrence (27).
We have previously reported that TGF-β production
varied among lung cancer cell lines, and that the induction of CTLs
tended to fail against tumor cell lines with a high level of TGF-β
production (18). In that study, as
a higher production of TGF-β in the tumor cells was noted, a lower
induction of CTLs was observed. TGF-β was demonstrated to be
necessary to maintain Tregs in in vitro culture (28). In the present study, the TGF-β
levels in the supernatant of the culture medium following MLTC in
the 1 and 5% Treg-added groups were higher than those in the
Treg-depleted group. Taking into account the background level of
TGF-β derived from the autologous tumor, the high level of TGF-β in
the supernatant in the Treg-added groups was considered to be
ascribable to its production from autologous Tregs. Furthermore,
the efficiency of CTL induction was increased by the depletion of
Tregs in MLTC.
Wieczorek et al reported that the proportion
of Tregs is significantly increased in the peripheral blood of
patients with interleukin 2-treated melanoma and in the tumor
tissue of patients with lung and colon carcinomas (29). Conversely, they showed that
immunosuppressive therapy, including the use of therapeutic
antibodies against CD25 (IL-2Ra), leads to a significant reduction
of Tregs from the peripheral blood of transplantation patients. In
addition, the Treg numbers are elevated in the peripheral blood of
patients with various solid tumors. Foxp3 is currently the only
marker that is exclusively expressed in Tregs. However, Foxp3 is an
intranuclear molecule, and therefore cannot be depleted using a
monoclonal antibody. Further studies on the mechanism(s) of action
for Foxp3 may identify molecular targets that could be useful to
specifically suppress the function of Tregs.
Cytotoxic T lymphocyte-associated antigen 4 (CTLA-4)
is a co-inhibitory molecule expressed by Tregs (30–33).
The CTLA-4 in Tregs plays a significant role in maintaining immune
homeostasis by downregulating T cell signaling to inhibit the
CD28-B7 co-stimulatory pathway, limiting T cell responses and
contributing to the tolerance to self-antigens (34,35). A
blockade of CTLA-4 signaling has been shown to augment
tumor-specific T cell responses, and to induce tumor rejection in a
number of animal models (36–38).
Two monoclonal antibodies against human CTLA-4 were found to elicit
objective and durable tumor responses in clinical trials (39–43).
However, anti-CTLA-4 therapy had a major drawback, as adverse
effects such as autoimmune enterocolitis and depigmentation
occurred. These problems may be resolved by better dose control of
the anti-CTLA-4 antibody and the design of an efficient drug
delivery system for the tumor environment.
Yamaguchi et al reported that natural Tregs
constitutively express high amounts of the folate receptor 4 (FR4),
a subtype of the receptor for the vitamin folic acid, and this high
expression of FR4 distinguishes them from other naïve or activated
T cells (44). In addition,
combinations of FR4 and CD25 may be used to distinguish between
four functionally different CD4+ T cell subpopulations;
i.e., natural Tregs, effector T cells, memory-like T cells and
naïve T cells. The administration of an anti-FR4 monoclonal
antibody specifically reduced Treg cells, provoking effective tumor
immunity in tumor-bearing animals, whereas a similar treatment of
normal young mice elicited autoimmune disease (44). The minimum dose required for tumor
control or the development of a delivery system that specifically
targets the tumor environment should be investigated in further
studies.
In conclusion, the results of this study suggest
that Tregs are present at a high frequency in RLNLs and TILs, and
they are considered to contribute to the suppression of antitumor
immunity by inhibiting the induction of CTLs in the regional lymph
nodes. The TGF-β production of Tregs was associated with the
suppression of CTL induction, and the depletion of Tregs could
result in a more efficient induction of CTLs.
Acknowledgements
This study was supported in part by a
UOEH Research Grant for the Promotion of Occupational Health and a
Grant-in-Aid for scientific research from the Ministry of
Education, Culture, Sports, Science and Technology, Japan. We thank
Yukari Oshibuchi, Misako Fukumoto and Aya Katayama for their expert
technical assistance.
References
|
1.
|
A JemalR SiegelE WardT MurrayJ XuMJ
ThunCancer statistics, 2007CA Cancer J
Clin574366200710.3322/canjclin.57.1.43
|
|
2.
|
R SanghaC ButtsL-BLP25: a peptide vaccine
strategy in non small cell lung cancerClin Cancer
Res1346524654200710.1158/1078-0432.CCR-07-0213
|
|
3.
|
J VansteenkisteM ZielinskiA LinderFinal
results of a multi-center, double-blind, randomized,
placebo-controlled Phase II study to assess the efficacy of MAGE-A3
immuno-therapeutic as adjuvant therapy in stage IB/II non-small
cell lung cancer (NSCLC)J Clin OncolSuppl 25398s2007
|
|
4.
|
P van der BruggenC TraversariP ChomezA
gene encoding an antigen recognized by cytolytic T lymphocytes on a
human melanomaScience254164316471991
|
|
5.
|
Y IchikiM TakenoyamaM MizukamiSimultaneous
cellular and humoral immune response against mutated p53 in a
patient with lung cancerJ
Immunol17248444850200410.4049/jimmunol.172.8.484415067062
|
|
6.
|
M TakenoyamaJF BaurainM YasudaA point
mutation in the NFYC gene generates an antigenic peptide recognized
by autologous cytolytyic T lymphocytes on a human squamous cell
lung carcinomaInt J
Cancer11819921997200610.1002/ijc.2159416287085
|
|
7.
|
F PagesA BergerM CamusEffector memory T
cells, early metastasis, and survival in colorectal cancerN Engl J
Med35326542666200510.1056/NEJMoa05142416371631
|
|
8.
|
L ZhangJR Conejo-GarciaD
KatsarosIntratumoral T cells, recurrence, and survival in
epithelial ovarian cancerN Engl J
Med348203213200310.1056/NEJMoa02017712529460
|
|
9.
|
SA RosenbergJC YangNP RestifoCancer
immunotherapy: moving beyond current vaccinesNat
Med10909915200410.1038/nm110015340416
|
|
10.
|
MK LevingsR SangregorioMG RoncaroloHuman
cd25(+) cd4(+) t regulatory cells suppress naïve and memory T cell
proliferation and can be expanded in vitro without loss of
functionJ Exp Med193129513022001
|
|
11.
|
EM ShevachCertified professionals:
CD4(+)CD25(+) suppressor T cellsJ Exp Med1934146200111390442
|
|
12.
|
H JonuleitE SchmittG SchulerJ KnopAH
EnkInduction of interleukin 10-producing, non proliferating CD4(+)
T cells with regulatory properties by repetitive stimulation with
allogeneic immature human dendritic cellsJ Exp
Med192121312222000
|
|
13.
|
S ReadF PowrieCD4(+) regulatory T
cellsCurr Opin Immunol136446492001
|
|
14.
|
AR GibbsFB ThunnissenHistological typing
of lung and pleural tumours: third editionJ Clin
Pathol54498499200110.1136/jcp.54.7.49811429418
|
|
15.
|
CF MountainRevisions in the international
system for staging lung
cancerChest11117101717199710.1378/chest.111.6.17109187198
|
|
16.
|
M SugayaM TakenoyamaT OsakiEstablishment
of 15 cancer cell lines from patients with lung cancer and the
potential tools for
immunotherapyChest122282288200210.1378/chest.122.1.28212114371
|
|
17.
|
M TakenoyamaI YoshinoR EifukuSuccessful
induction of tumor-specific cytotoxic T lymphocytes from patients
with non-small cell lung cancer using CD80-transfected autologous
tumor cellsJpn J Cancer
Res92309315200110.1111/j.1349-7006.2001.tb01096.x11267941
|
|
18.
|
T FukuyamaY IchikiS YamadaCytokine
production of lung cancer cell lines: correlation between their
production and the inflammatory/immunological responses both in
vivo and in vitroCancer
Sci9810481054200710.1111/j.1349-7006.2007.00507.x
|
|
19.
|
S SakaguchiM OnoR
SetoguchiFoxp3+ CD25+ CD4+ natural
regulatory T cells in dominant self-tolerance and auto-immune
diseaseImmunol Rev2128272006
|
|
20.
|
JD FontenotMA GavinAY RudenskyFoxp3
programs the development and function of
CD4+CD25+ regulatory T cellsNat
Immunol4330336200310.1038/ni90412612578
|
|
21.
|
AM WolfD WolfM SteurerG GastlE GunsiliusB
Grubeck-LoebensteinIncrease of regulatory T cells in the peripheral
blood of cancer patientsClin Cancer Res9606612200312576425
|
|
22.
|
LA OrmandyT HillemannH WedemeyerMP MannsTF
GretenF KorangyIncreased populations of regulatory T cells in
peripheral blood of patients with hepatocellular carcinomaCancer
Res6524572464200510.1158/0008-5472.CAN-04-323215781662
|
|
23.
|
EY WooCS ChuTJ GoletzRegulatory
CD4(+)CD25(+) T cells in tumors from patients with early-stage
non-small cell lung cancer and late-stage ovarian cancerCancer
Res61476647722001
|
|
24.
|
TJ CurielG CoukosL ZouSpecific recruitment
of regulatory T cells in ovarian carcinoma fosters immune privilege
and predicts reduced survivalNat
Med10942949200410.1038/nm109315322536
|
|
25.
|
GJ BatesSB FoxC HanQuantification of
regulatory T cells enables the identification of high-risk breast
cancer patients and those at risk of late relapseJ Clin
Oncol2453735380200610.1200/JCO.2006.05.958417135638
|
|
26.
|
F GhiringhelliC MenardM
TermeCD4+CD25+ regulatory T cells inhibit
natural killer cell functions in a transforming growth
factor-h-dependent mannerJ Exp Med20210751085200516230475
|
|
27.
|
RP PetersenMJ CampaJ SperlazzaTumor
infiltrating Foxp3+ regulatory T-cells are associated
with recurrence in pathologic stage I NSCLC
patientsCancer10728662872200617099880
|
|
28.
|
JC MarieJJ LetterioM GavinAY
RudenskyTGF-beta 1 maintains suppressor function and Foxp3
expression in CD4+CD25+ regulatory T cellsJ
Exp Med20110611067200510.1084/jem.2004227615809351
|
|
29.
|
G WieczorekA AsemissenF ModelQuantitative
DNA methylation analysis of FOXP3 as a new method for counting
regulatory T cells in peripheral blood and solid tissueCancer
Res69599608200910.1158/0008-5472.CAN-08-236119147574
|
|
30.
|
TL WalunasCY BakkerJA BluestoneCTLA-4
ligation blocks CD28-dependent T cell activationJ Exp
Med1832541255019968676075
|
|
31.
|
MF KrummelJP AllisonCD28 and CTLA-4 have
opposing effects on the response of T cells to stimulationJ Exp
Med182459465199510.1084/jem.182.2.459
|
|
32.
|
S ReadV MalmstromF PowrieCytotoxic T
lymphocyte-associated antigen 4 plays an essential role in the
function of CD25(+)CD4(+) regulatory cells that control intestinal
inflammationJ Exp Med1922953022000
|
|
33.
|
B SalomonDJ LenschowL RheeB7/CD28
costimulation is essential for the homeostasis of the
CD4+CD25+ immunoregulatory T cells that
control autoimmune
diabetesImmunity12431440200010.1016/S1074-7613(00)80195-810795741
|
|
34.
|
NJ KarandikarCL VanderlugtTL WalunasSD
MillerJA BluestoneCTLA-4: A negative regulator of autoimmune
diseaseJ Exp Med184783788199610.1084/jem.184.2.7838760834
|
|
35.
|
MC BrunnerCA ChambersFK ChanJ HankeA
WinotoJP AllisonCTLA-4-Mediated inhibition of early events of T
cell proliferationJ Immunol16258135820199910229815
|
|
36.
|
DR LeachMF KrummelJP AllisonEnhancement of
antitumor immunity by CTLA-4
blockadeScience27117341736199610.1126/science.271.5256.17348596936
|
|
37.
|
A van ElsasAA HurwitzJP AllisonCombination
immunotherapy of B16 melanoma using anti-cytotoxic T
lymphocyte-associated antigen 4 (CTLA-4) and granulocyte/macrophage
colony-stimulating factor (GM-CSF)-producing vaccines induces
rejection of subcutaneous and metastatic tumors accompanied by
autoimmune depigmentationJ Exp Med1903553661999
|
|
38.
|
A van ElsasRP SutmullerAA
HurwitzElucidating the autoimmune and antitumor effector mechanisms
of a treatment based on cytotoxic T lymphocyte antigen-4 blockade
in combination with a B16 melanoma vaccine: comparison of
prophylaxis and therapyJ Exp Med1944814892001
|
|
39.
|
FS HodiMC MihmRJ SoifferBiologic activity
of cytotoxic T lymphocyte-associated antigen 4 antibody blockade in
previously vaccinated metastatic melanoma and ovarian carcinoma
patientsProc Natl Acad Sci
USA10047124717200310.1073/pnas.0830997100
|
|
40.
|
GQ PhanJC YangRM SherryCancer regression
and autoimmunity induced by cytotoxic T lymphocyte-associated
antigen 4 blockade in patients with metastatic melanomaProc Natl
Acad Sci USA10083728377200310.1073/pnas.153320910012826605
|
|
41.
|
FS HodiM ButlerDA ObleImmunologic and
clinical effects of antibody blockade of cytotoxic T
lymphocyte-associated antigen 4 in previously vaccinated cancer
patientsProc Natl Acad Sci
USA10530053010200810.1073/pnas.071223710518287062
|
|
42.
|
AJ KormanKS PeggsJP AllisonCheckpoint
blockade in cancer immunotherapyAdv
Immunol90297339200610.1016/S0065-2776(06)90008-X16730267
|
|
43.
|
A RibasDC HansonDA NoeTremelimumab
(CP-675,206), a cytotoxic T lymphocyte associated antigen 4
blocking monoclonal antibody in clinical development for patients
with
cancerOncologist12873883200710.1634/theoncologist.12-7-87317673618
|
|
44.
|
T YamaguchiK HirotaK NagahamaControl of
immune responses by antigen-specific regulatory T cells expressing
the folate
receptorImmunity27145159200710.1016/j.immuni.2007.04.01717613255
|