Introduction
The leucovorin (FOL) and fluorouracil (5-FU) plus
oxaliplatin (l-OHP; FOLFOX) or leucovorin and 5-FU plus irinotecan
(SN-38; FOLFIRI) regimens with or without molecularly-targeted
drugs are widely used as first-line chemotherapy in the treatment
of advanced colorectal cancer (CRC) (1–12).
Whether FOLFOX or FOLFIRI is administered first is not significant,
however, it is crucial that full administration of the targeted
dosages of all 3 drugs, 5-FU, l-OHP and SN-38, is achieved.
However, this is not always possible and second-line chemotherapy
must be abandoned in certain cases due to disease progression,
adverse effects or high medical costs (13,14).
Where possible, the most effective regimen should be
selected as the first line of treatment. A previous study using the
collagen gel droplet embedded culture-drug sensitivity test
(CD-DST) reported that FOLFIRI should be selected as the first line
of chemotherapy in the treatment of poor responders to 5-FU
(15).
The aim of the current study was to determine
whether first-line chemotherapy may be individualized using the
CD-DST.
Patients and methods
Patients
Specimens of primary tumors were obtained between
March 2008 and September 2011 from 43 CRC patients who had received
no preoperative chemotherapy. Informed consent for measuring drug
sensitivity was obtained from all patients. The study was approved
by the ethics committee at Juntendo University School of Medicine,
Tokyo, Japan.
Methods
Tumor tissue was excised from primary surgical
specimens and subjected to the CD-DST. The CD-DST allows for the
evaluation of drug sensitivity using isolated, 3-dimensionally
cultured tumor cells in a small collagen gel droplet and was used
to evaluate the sensitivity of the tumors to 5-FU, performed as
described by Kobayashi et al (16,17).
Each specimen was washed 5 times with 50 ml saline, followed by
further washing 5 times with 50 ml antibiotic fluid containing 1.0
mg/ml piperacillin and 0.5 mg/ml kanamycin. The transport bottle
contained 1.0 mg/ml piperacillin, 0.5 mg/ml kanamycin and 2.5 μg/ml
amphotericin B. Tissue (1 g) was treated for 2 h with a dispersion
enzyme cocktail containing 1.0% collagenase. Dispersed cell
suspensions were inoculated into pre-culture media on
collagen-coated flasks overnight, after which viable tumor cells
were recovered by 0.05% collagenase treatment. Recovered cells were
embedded in 30-μl collagen gel droplets.
The embedded cells were cultivated in culture media
containing 5-FU and l-OHP at 6.0 and 3.0 μg/ml, or 5-FU and SN-38
at 6.0 and 0.2 μg/ml, respectively, for 24 h. Following the removal
of the anticancer agent-containing media, the cells were further
cultured for 7 days in serum-free culture media to prevent the
growth of fibroblasts. Viable cells were stained with neutral red
solution and counted using the imaging colorimetric quantification
method. The surviving cell number ratio between the drug-treated
and control group, which received no drug treatment, was
calculated. A growth rate <0.8 was considered indicative of
successful culture.
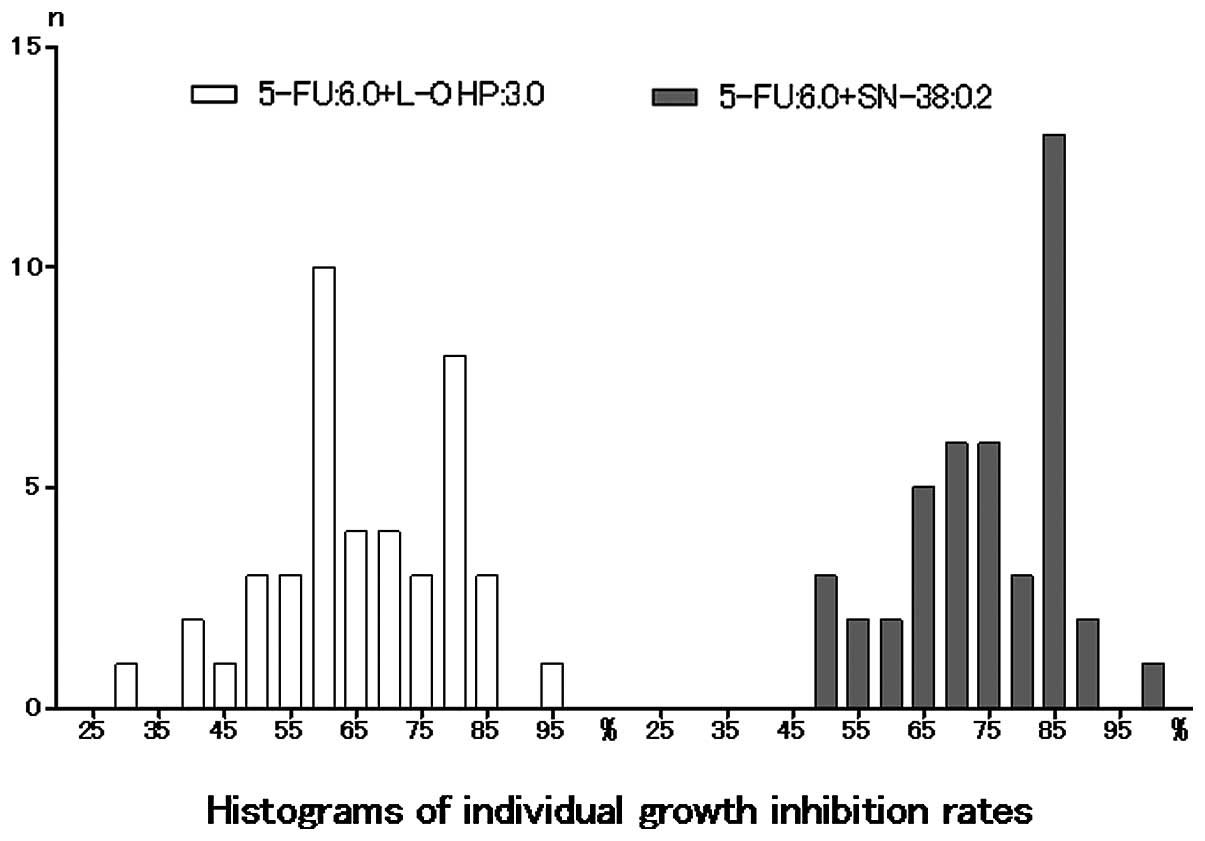
The frequency distributions of the growth inhibition
rates (IRs) under the two conditions were evaluated based on the
evidence that the clinical response rates to FOLFOX and FOLFIRI
were almost the same.
Statistical analysis
Histograms were analyzed with the D’Agostino-Pearson
omnibus normality test using GraphPad Prism (GraphPad Software, La
Jolla, CA, USA). P<0.05 was considered to indicate statistically
significant differences.
Results
Individualization of first-line
chemotherapy is possible in patients with advanced CRC
Patient characteristics are shown in Table I. The individual growth IRs under
each of the two conditions are shown in Table II. With 5-FU and l-OHP at 6.0 and
3.0 μg/ml, respectively, the median, mean, standard deviation and
standard error of the mean were 63.20, 65.69, 14.02 and 2.138,
respectively. With 5-FU and SN-38 at 6.0 and 0.2 μg/ml,
respectively, the median, mean, standard deviation and standard
error of the mean were 74.40, 74.06, 12.12 and 1.848, respectively.
Histograms of the individual growth IRs (%) under each of the two
conditions are shown in Fig. 1. The
histograms passed the normality test (α=0.05; 5-FU and l-OHP,
P=0.7265; 5-FU and SN-38, P=0.3756).
 | Table I.Patient characteristics. |
Table I.
Patient characteristics.
|
Characteristics | n |
|---|
| No. of
patients | 43 |
| Age (years), mean
(range) | 64.3 (42–78) |
| Gender
(male/female) | 24/19 |
| Histological
type | |
|
Well-differentiated carcinoma | 7 |
| Moderately
differentiated carcinoma | 29 |
| Poorly
differentiated carcinoma | 2 |
| Mucinous
carcinoma | 5 |
| Colon/rectum | 35/8 |
| Dukes’ stage
(A/B/C/D) | 2/13/19/9 |
 | Table II.Individual growth inhibition
rates. |
Table II.
Individual growth inhibition
rates.
| Patient no. | 5-FU/l-OHP 6.0/3.0
μg/ml for 24 h | 5-FU/SN-38 6.0/0.2
μg/ml for 24 h |
|---|
| 1 | 80.1 | 82.9 |
| 2 | 71.3 | 79.2 |
| 3 | 81.2 | 83.4 |
| 4 | 60.0 | 68.7 |
| 5 | 29.9 | 66.5 |
| 6 | 69.7 | 89.6 |
| 7 | 58.7 | 63.2 |
| 8 | 73.0 | 85.2 |
| 9 | 63.2 | 75.9 |
| 10 | 77.9 | 85.5 |
| 11 | 76.3 | 85.6 |
| 12 | 53.6 | 62.6 |
| 13 | 41.9 | 60.7 |
| 14 | 81.3 | 80.9 |
| 15 | 42.3 | 70.2 |
| 16 | 84.8 | 86.8 |
| 17 | 75.9 | 83.9 |
| 18 | 59.2 | 76.4 |
| 19 | 69.9 | 85.5 |
| 20 | 57.0 | 49.7 |
| 21 | 79.2 | 83.0 |
| 22 | 86.1 | 89.1 |
| 23 | 67.3 | 74.4 |
| 24 | 81.3 | 85.2 |
| 25 | 60.4 | 71.9 |
| 26 | 93.4 | 98.6 |
| 27 | 62.6 | 84.9 |
| 28 | 58.5 | 54.8 |
| 29 | 81.2 | 84.0 |
| 30 | 66.5 | 73.3 |
| 31 | 81.3 | 78.1 |
| 32 | 59.9 | 74.1 |
| 33 | 53.3 | 65.0 |
| 34 | 49.3 | 48.3 |
| 35 | 44.7 | 49.3 |
| 36 | 68.8 | 72.1 |
| 37 | 59.7 | 69.4 |
| 38 | 50.8 | 59.3 |
| 39 | 51.6 | 56.5 |
| 40 | 57.9 | 70.2 |
| 41 | 58.5 | 63.7 |
| 42 | 62.4 | 72.5 |
| 43 | 82.9 | 84.3 |
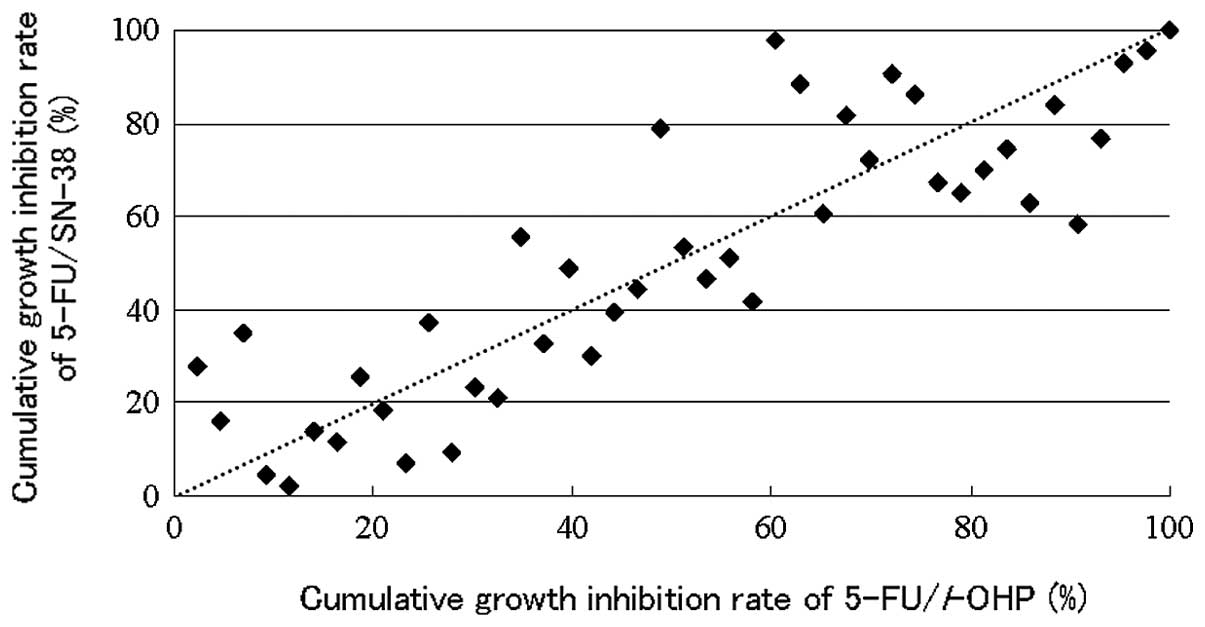
The cumulative distribution of the individual growth
IRs between the two conditions is shown in Fig. 2. There are individual differences of
the efficacies between the two regimens.
Individualization of first-line chemotherapy was
possible in all patients, with 5-FU plus l-OHP and 5-FU plus SN-38
showing higher efficacy in 26 and 15 patients, respectively, and
equal efficacy in 2 cases (Fig.
2).
Discussion
The addition of molecularly-targeted anticancer
agents enhances the effect of FOLFOX/FOLFIRI therapies. Moreover,
individualized chemotherapy with molecularly-targeted anticancer
agents may be implemented based on the genetic characteristics of
the individual patient (6–12,18–20).
Several studies have investigated individualization in 5-FU-based
chemotherapy (21,22). The three enzymes that have been
identified as the most significant in the metabolism of 5-FU are
orotate phosphoribosyl transferase (OPRT), thymidylate synthase
(TS) and dihydropyrimidine dehydrogenase (DPD). The most
significant phosphorylation enzyme of 5-FU is OPRT, while the
degradation enzyme is DPD and the main enzyme of DNA synthesis is
TS. In general, high expression of TS correlates with poor efficacy
of 5-FU, while low expression correlates with good efficacy. The
activity of these enzymes has been reported to be extremely useful
in individualization of 5-FU chemotherapy in CRC (23). Moreover, the individual 50%
inhibitory area under the concentration curve of 5-FU using the
CD-DST has been reported to be useful in determining individualized
chemotherapy in CRC patients (24,25).
Previous studies have investigated the correlation between the
efficacy of irinotecan and topoisomerase-1, or that between the
toxicity of irinotecan and uridine diphosphate
glucuronosyltransferases 1A1 (21,22,26–29).
It has been reported that there is a correlation between the
efficacy of oxaliplatin and the excision cross-complementing gene
(21,22,26).
However, individualization in 5-FU-based chemotherapy remains to be
implemented clinically.
Grothey et al reported that while it was not
significant whether FOLFOX or FOLFIRI was administered first, it
was crucial that full administration of the targeted dosages of all
3 drugs, 5-FU, oxaliplatin and irinotecan, be achieved. However,
certain randomized controlled trials noted that, even with the best
prognosis, full administration of all 3 drugs was not possible in
approximately one-quarter of patients (13,14).
It has also been reported that second-line chemotherapy could not
be carried out in approximately one-third of patients (1,2,4,30,31).
First-line chemotherapy is usually administered over a long period
of time (5). Therefore, a more
effective regimen should be selected. That is, if prognosis is to
be improved, then individualization of first-line chemotherapy is
indispensable.
It has been reported that the clinical response
rates of FOLFOX and FOLFIRI are almost the same, at approximately
50% (5) and thus the efficacies of
FOLFOX and FOLFIRI are considered to be almost equivalent (1,2,4,5,30).
However, it remains to be clarified whether this holds true if
considered on a case-to-case basis. In this study, when the
clinical response rates of FOLFOX and FOLFIRI were 50%, responders
were identified using the median based on the histograms of the
individual growth IRs. The results also demonstrated that the
efficacies of FOLFOX and FOLFIRI were not exactly equivalent in all
the individuals in this study. Therefore, the more effective
regimen for each individual was identified based on the cumulative
distributions of the individual growth IRs between FOLFOX and
FOLFIRI. FOLFOX was recommended as first-line chemotherapy in 26
patients, while FOLFIRI was recommended as first-line chemotherapy
in 15 patients. Thus, individualization of first-line chemotherapy
was possible in all patients.
The results from the present study suggest that this
method has the potential to facilitate the establishment of
individualized first-line chemotherapy for CRC and is likely to
improve the prognosis in such patients. This method requires
further prospective randomized study.
Acknowledgements
The authors would like to thank
Associate Professor Jeremy Williams, Tokyo Dental College, for his
assistance with the English of the manuscript.
References
|
1.
|
A de GramontA FigerM SeymourLeucovorin and
fluorouracil with or without oxaliplatin as first-line treatment in
advanced colorectal cancerJ Clin Oncol18293829472000
|
|
2.
|
RM GoldbergDJ SargentRF MortonA randomized
controlled trial of fluorouracil plus leucovorin, irinotecan, and
oxaliplatin combinations in patients with previously untreated
metastatic colorectal cancerJ Clin
Oncol222330200410.1200/JCO.2004.09.046
|
|
3.
|
ML RothenbergAM OzaRH BigelowSuperiority
of oxaliplatin and fluorouracil-leucovorin compared with either
therapy alone in patients with progressive colorectal cancer after
irinotecan and fluorouracil-leucovorin: interim results of a phase
III trialJ Clin Oncol2120592069200310.1200/JCO.2003.11.126
|
|
4.
|
JY DouillardD CunninghamAD RothIrinotecan
combined with fluorouracil compared with fluorouracil alone as
first-line treatment for metastatic colorectal cancer: a
multicentre randomized
trialLancet35510411047200010.1016/S0140-6736(00)02034-1
|
|
5.
|
C TournigandT AndréE AchilleFOLFIRI
followed by FOLFOX6 or the reverse sequence in advanced colorectal
cancer: a randomized GERCOR studyJ Clin
Oncol22229237200410.1200/JCO.2004.05.11314657227
|
|
6.
|
HS HochsterLL HartRK RamanathanSafety and
efficacy of oxaliplatin and fluoropyrimidine regimens with or
without bevacizumab as first-line treatment of metastatic
colorectal cancer: results of the TREE StudyJ Clin
Oncol2135233529200810.1200/JCO.2007.15.413818640933
|
|
7.
|
LB SaltzS ClarkeE Díaz-RubioBevacizumab in
combination with oxaliplatin-based chemotherapy as first-line
therapy in metastatic colorectal cancer: a randomized phase III
studyJ Clin Oncol2620132019200810.1200/JCO.2007.14.993018421054
|
|
8.
|
BJ GiantonioPJ CatalanoNJ
MeropolBevacizumab in combination with oxaliplatin, fluorouracil
and leucovorin (FOLFOX4) for previously treated metastatic
colorectal cancer: results from the eastern cooperative oncology
group study E3200J Clin
Oncol2515391544200710.1200/JCO.2006.09.6305
|
|
9.
|
C BokemeyerI BondarenkoA
MakhsonFluorouracil, leucovorin and oxaliplatin with and without
cetuximab in the first-line treatment of metastatic colorectal
cancerJ Clin Oncol27663671200910.1200/JCO.2008.20.839719114683
|
|
10.
|
TS MaughanR AdamsCG SmithIdentification of
potentially responsive subsets when cetuximab is added to
oxaliplatin-fluoropyrimidine chemotherapy (CT) in first-line
advanced colorectal cancer (aCRC)J Clin Oncol28Suppl 5abs.
35022010
|
|
11.
|
CS FuchsJ MarshallE MitchellRandomized,
controlled trial of irinotecan plus infusional, bolus, or oral
fluoropyrimidines in first-line treatment of metastatic colorectal
cancer: results from the BICC-C StudyJ Clin
Oncol2547794786200710.1200/JCO.2007.11.3357
|
|
12.
|
E Van CutsemCH KöhneE HitreCetuximab and
chemotherapy as initial treatment for metastatic colorectal cancerN
Eng J Med36014081417200919339720
|
|
13.
|
A GrotheyD SargentRM GoldbergSurvival of
patients with advanced colorectal cancer improves with the
availability of fluorouracil-leucovorin, irinotecan and oxaliplatin
in the course of treatmentJ Clin
Oncol2212091214200410.1200/JCO.2004.11.03715051767
|
|
14.
|
A GrotheyD SargentOverall survival of
patients with advanced colorectal cancer correlates with
availability of fluorouracil, irinotecan and oxaliplatin regardless
of whether doublet or single-agent therapy is used first lineJ Clin
Oncol2394419442200510.1200/JCO.2005.04.4792
|
|
15.
|
T OchiaiK NishimuraT WatanabeLeucovorin
and fluorouracil plus oxaliplatin or leucovorin and fluorouracil
plus irinotecan as individualized first-line therapy based on a
drug sensitivity testExp Ther
Med1325329201010.3892/etm_0000005022993545
|
|
16.
|
H KobayashiK TanisakaO DoiAn in vitro
chemosensitivity test for solid tumors using collagen gel droplet
embedded culturesInt J Oncol11449455199721528231
|
|
17.
|
H KobayashiM HigashiyamaK
MinamigawaExamination of in vitro chemosensitivity test using
collagen droplet culture method with colorimetric endpoint
quantificationJpn J Cancer
Res92203210200110.1111/j.1349-7006.2001.tb01083.x11223550
|
|
18.
|
E Van CutsemCH KöhneI LángCetuximab plus
irinotecan, fluorouracil and leucovorin as first-line treatment for
metastatic colorectal cancer: updated analysis of overall survival
according to tumor KRAS and BRAF mutation statusJ Clin
Oncol2920112019201121502544
|
|
19.
|
C BokemeyerI BondarenkoJT HartmannEfficacy
according to biomarker status of cetuximab plus FOLFOX-4 as
first-line treatment for metastatic colorectal cancer: the OPUS
studyAnn Oncol2215351546201110.1093/annonc/mdq63221228335
|
|
20.
|
JY DouillardS SienaJ CassidyRandomized,
phase III trial of panitumumab with infusional fluorouracil,
leucovorin, and oxaliplatin (FOLFOX4) versus FOLFOX4 alone as
first-line treatment in patients with previously untreated
metastatic colorectal cancer: the PRIME studyJ Clin
Oncol2846974705201010.1200/JCO.2009.27.4860
|
|
21.
|
M KoopmanS VenderboschH van
TinterenPredictive and prognostic markers for the outcome of
chemotherapy in advanced colorectal cancer, a retrospective
analysis of the phase III randomized CAIRO studyEur J
Cancer4519992006200910.1016/j.ejca.2009.04.01719457654
|
|
22.
|
M KoopmanS VenderboschID NagtegaalJH van
KriekenCJ PuntA review on the use of molecular markers of cytotoxic
therapy for colorectal cancer, what have we learned?Eur J
Cancer4519351949200910.1016/j.ejca.2009.04.02319473832
|
|
23.
|
T OchiaiK NishimuraH NoguchiPrognostic
impact of orotate phosphoribosyl transferase among 5-fluorouracil
metabolic enzymes in resectable colorectal cancers treated by oral
5-fluorouracil-based adjuvant chemotherapyInt J
Cancer11830843088200610.1002/ijc.21779
|
|
24.
|
T OchiaiK NishimuraT WatanabeEvaluation of
the individual 50% inhibitory area under the concentration curve of
5-fluorouracil based on the collagen gel droplet embedded culture
drug sensitivity test in colorectal cancerMol Med
Rep24054092009
|
|
25.
|
T OchiaiK NishimuraT WatanabePersonalized
adjuvant chemotherapy for colorectal cancer (CRC) based on an
individual 50% inhibitory area under the concentration curve (AUC)
using collagen gel droplet embedded culture-drug sensitivity test
(CD-DST)J Clin Oncol28Suppl 15abs. 35822010
|
|
26.
|
MS BraunSD RichmanP QuirkePredictive
biomarkers of chemotherapy efficacy in colorectal cancer: results
from the UK MRC FOCUS trialJ Clin
Oncol2626902698200810.1200/JCO.2007.15.558018509181
|
|
27.
|
H MinamiK SaiM SaekiIrinotecan
pharmacokinetics/pharmacodynamics and UGT1A genetic polymorphisms
in Japanese: roles of UGT1A1*6 and *28Pharmacogenet
Genomics17497504200717558305
|
|
28.
|
Y AndoH SakaM AndoPolymorphisms of
UDP-glucuronosyltransferase gene and irinotecan toxicity: a
pharmacogenetic analysisCancer Res6069216926200011156391
|
|
29.
|
K SaiJ SawadaH MinamiIrinotecan
pharmacogenetics in Japanese cancer patients: roles of UGT1A1 *6
and *28Yakugaku Zasshi1285755842008(In Japanese)
|
|
30.
|
MT SeymourFluorouracil, oxaliplatin and
CPT-11 (irinotecan), use and sequencing (MRC FOCUS): a 2135-patient
randomized trial in advanced colorectal cancer (ACRC)J Clin
Oncol23Suppl 16abs. 35182005
|
|
31.
|
CH KöhneE Van CutsemJ WilsPhase III study
of weekly high-dose infusional fluorouracil plus folinic acid with
or without irinotecan in patients with metastatic colorectal
cancer: European organization for research and treatment of cancer
gastrointestinal group study 40986J Clin Oncol23485648652005
|