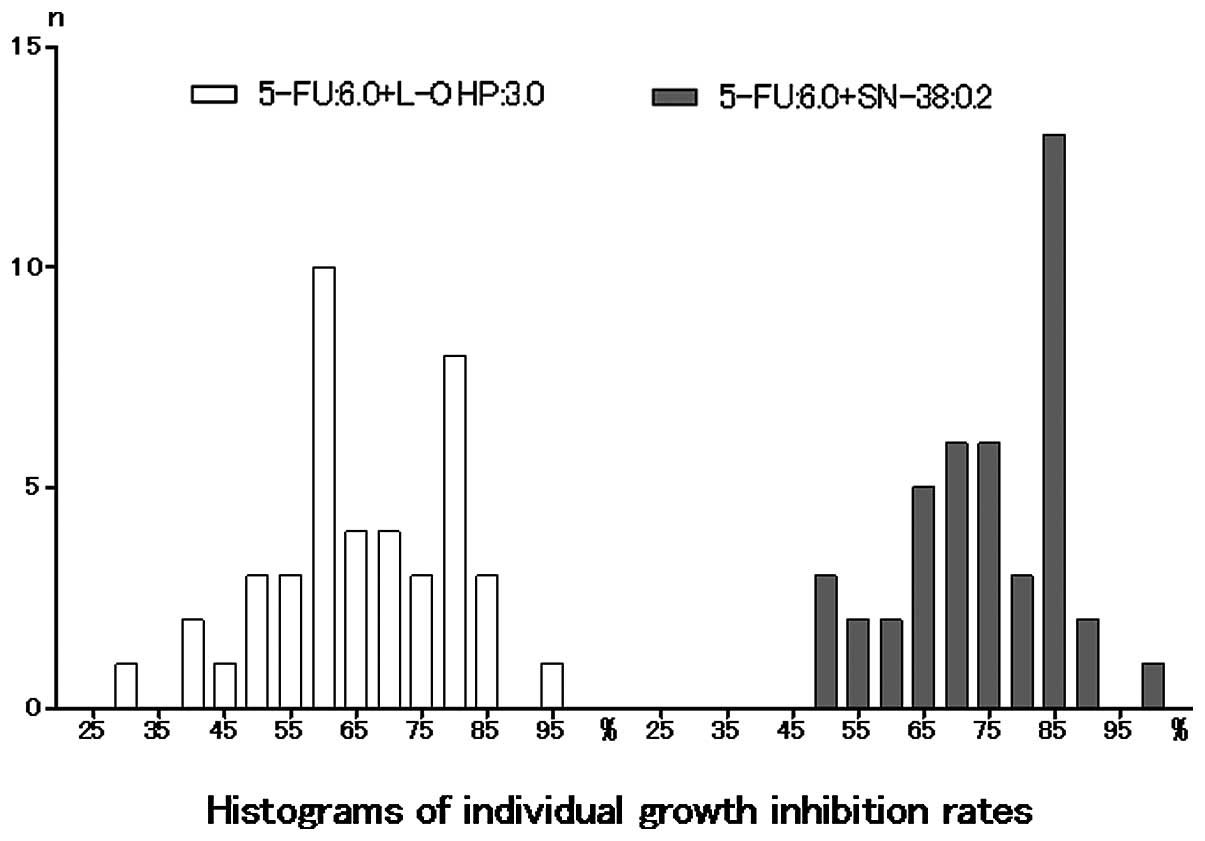
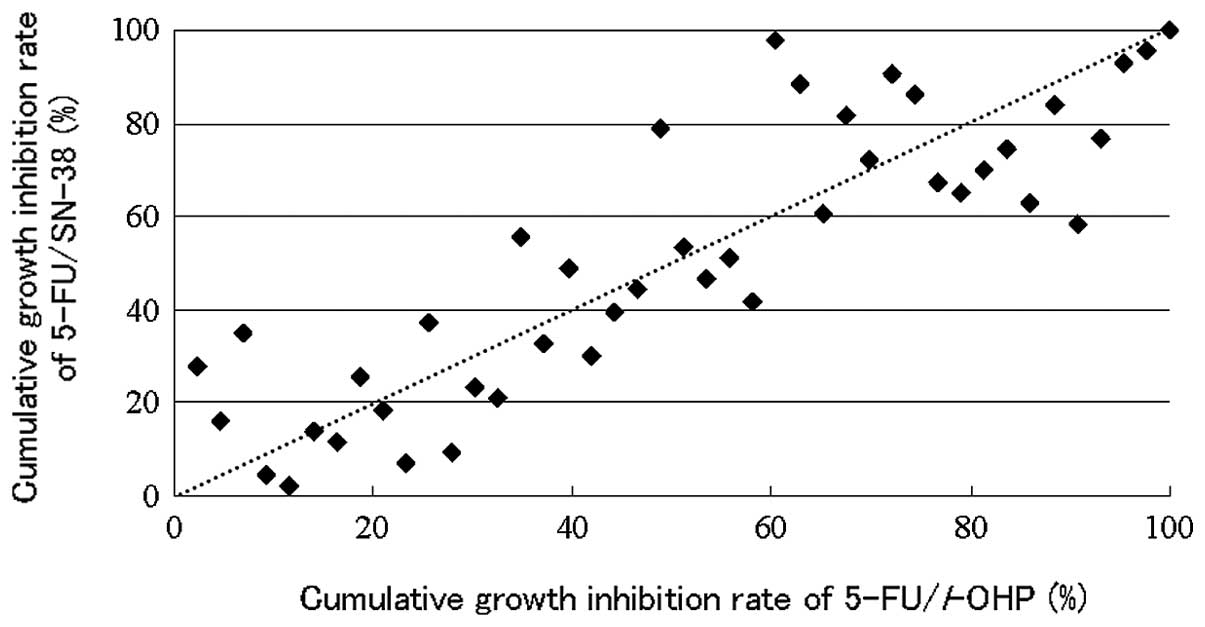
Spandidos Publications style
Ochiai T, Nishimura K, Watanabe T, Kitajima M, Nakatani A, Inou T, Washio M, Sakuyama N, Sato T, Kishine K, Kishine K, et al: Individualized chemotherapy for colorectal cancer based on the collagen gel droplet-embedded drug sensitivity test. Oncol Lett 4: 621-624, 2012.
APA
Ochiai, T., Nishimura, K., Watanabe, T., Kitajima, M., Nakatani, A., Inou, T. ... Nagaoka, I. (2012). Individualized chemotherapy for colorectal cancer based on the collagen gel droplet-embedded drug sensitivity test. Oncology Letters, 4, 621-624. https://doi.org/10.3892/ol.2012.823
MLA
Ochiai, T., Nishimura, K., Watanabe, T., Kitajima, M., Nakatani, A., Inou, T., Washio, M., Sakuyama, N., Sato, T., Kishine, K., Ochi, T., Okubo, S., Futagawa, S., Mashiko, S., Nagaoka, I."Individualized chemotherapy for colorectal cancer based on the collagen gel droplet-embedded drug sensitivity test". Oncology Letters 4.4 (2012): 621-624.
Chicago
Ochiai, T., Nishimura, K., Watanabe, T., Kitajima, M., Nakatani, A., Inou, T., Washio, M., Sakuyama, N., Sato, T., Kishine, K., Ochi, T., Okubo, S., Futagawa, S., Mashiko, S., Nagaoka, I."Individualized chemotherapy for colorectal cancer based on the collagen gel droplet-embedded drug sensitivity test". Oncology Letters 4, no. 4 (2012): 621-624. https://doi.org/10.3892/ol.2012.823