Introduction
Esophageal cancer is a highly aggressive malignant
disease, which is generally diagnosed at an advanced stage.
Esophageal cancer has an extremely poor prognosis with a 5-year
survival rate of less than 10% (1),
and current chemotherapy treatments often have limited success and
fatal outcomes. Efforts are being made to improve the
chemotherapeutic interventions for metastatic esophageal cancer. A
number of esophageal tumors develop resistance to chemotherapy
during the course of therapy. This drug resistance often results in
additional toxicities, of which some are fatal, and are the major
cause of treatment failure (2,3).
Therefore, numerous studies have focused on the molecular
mechanisms of chemotherapy resistance to determine more effective
methods for overcoming this resistance.
5-Fluorouracil (5-FU) remains the most effective
chemotherapeutic option available for the treatment of advanced
esophageal cancer. In numerous patients, esophageal tumors are
either inherently resistant to chemotherapy, or the tumors develop
resistance during treatment when 5-FU is administered alone or in
conjunction with other agents (4).
The ability to inhibit apoptosis appears to be the principal
mechanism by which resistant cancer cells are protected from
chemotherapy and radiation (5–7). The
cellular mechanisms that protect cancer cells from apoptosis are
complex (8,9). The inducible activation of the nuclear
transcription factor NF-κB has been identified to inhibit the
apoptotic response to chemotherapy and radiation (10). Previous studies have demonstrated
that treatment with chemotherapy drugs activates NF-κB, and
inhibition of NF-κB enhances the cytotoxic effect of drugs in
cancer cells (11,12).
Quercetin, the major constituent of the flavonol
subclass of flavonoids (13), has
been identified to induce apoptosis in a number of tumor cell lines
(14) and interact with a broad
range of enzymes, including receptor kinases (15,16).
We propose that NF-κB plays a central role in the chemoresistance
of esophageal cancer cells treated with 5-FU. To study the
molecular mechanisms involved in the anticancer effects of
quercetin on esophageal cancer, we focused our attention on the
effects of quercetin on the chemosensitivity of human esophageal
carcinoma cells.
Materials and methods
Human cells and culture
Human esophageal cancer cells (EC9706 and Eca109)
were cultured in RPMI-1640 medium supplemented with heat
inactivated 10% fetal bovine serum (FBS), 100 U/ml penicillin and
100 mg/ml streptomycin. Cell lines were maintained in a humidified
incubator containing 5% CO2 at 37°C. The cells were
passaged twice a week at an initial density of 1×106
cells/ml.
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
assay
An MTT assay was conducted according to the method
described by Kawada et al (17). A 150 μl aliquot of 1×105
cells suspended in RPMI-1640 medium supplemented with 10% FBS was
added to each well of a 96-well microtiter plate. The cells were
cultured in the absence (control) or presence of various agents,
and the plates were incubated at 37°C in a humidified incubator
containing 5% CO2 for 48 h. Following this, 20 μl of the
MTT reagent (5 mg/ml) was added to each well, and the cells were
allowed to incubate for 4 h. A total of 150 μl of dimethyl
sulfoxide (DMSO) was added to each well in order to dissolve the
formazan crystal that had formed, and the optical density (OD) was
recorded at 490 nm. The growth inhibition rate was expressed as:
Growth inhibition (%) = (1-OD of treated/OD of control) x 100.
Annexin V-FITC/propidium iodide
(PI)-stained fluorescence-activated cell sorting (FACS)
The cells were harvested by trypsinization and
washed twice with cold PBS. Once the cells had been centrifuged at
1,000 x g for 5 min, the supernatant was discarded and the pellet
was resuspended in binding buffer at a density of
1.0×105–1.0xl06 cells/ml. Following this, 100
μl of the sample solution was transferred to a 5-ml culture tube
and incubated with 5 μl of FITC-conjugated Annexin V and 5 μl of PI
for 15 min at room temperature in the dark. Subsequently, 400 μl of
binding buffer was added to each sample and the samples were
analyzed by FACS using CellQuest Research Software (Largo, FL,
USA).
Western blot analysis
EC9706 and Eca109 cells were collected following
centrifugation at 500 x g for 5 min, and the pellets were
resuspended in lysis buffer containing 1% NP40, 1 mM
phenylmethylsulfonyl fluoride, 40 mM Tris-HCl (pH 8.0), 150 mM NaCl
and 1 mM NaOH at 4°C for 15 min. Cell lysates were resolved on
12.5% SDS-polyacrylamide gels and transferred to nitrocellulose
membranes according to the manufacturer’s instructions. Antibody
binding was detected using an enhanced chemiluminescence kit (ECL)
with a hyper-ECL film. The antibodies against pIκBα and β-actin
were purchased from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA,
USA).
Statistical analysis
The values shown are the mean ± standard error of
mean (SEM). Statistical analyses were conducted using the Student’s
t-test and one-way ANOVA. P<0.05 was considered to indicate a
statistically significant difference and statistical calculations
were conducted using SPSS version 13.0 software (SPSS, Inc.,
Chicago, IL, USA).
Results
Effects of quercetin on the proliferation
and viability of EC9706 and Eca109 cell lines
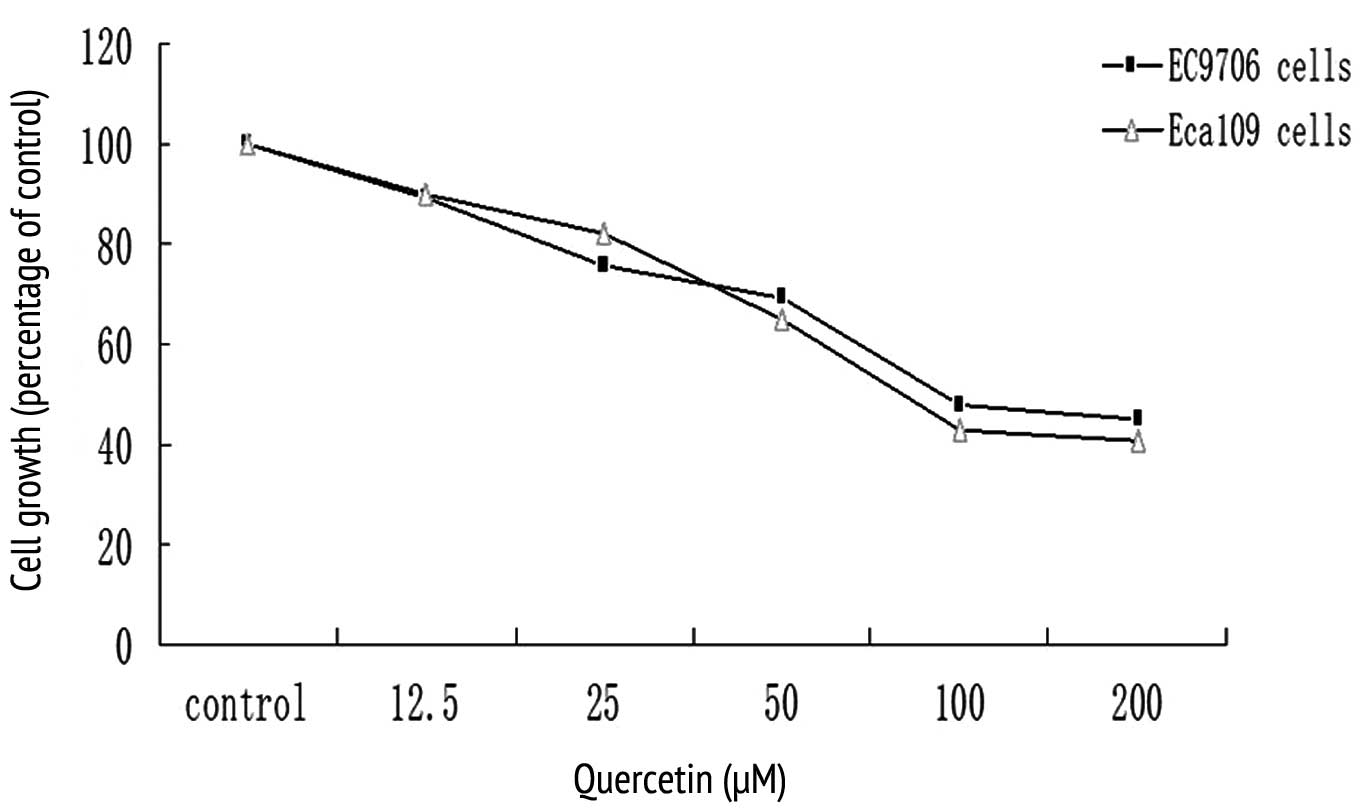
A dose-response study was conducted for EC9706 and
Eca109 cancer cells in response to quercetin. Quercetin inhibited
the growth of both cell lines in a dose-dependent manner, revealing
a maximum inhibition of 60% at 100–200 μM concentrations (Fig. 1). A 50–60% growth inhibition was
observed in both cell lines at a concentration of 100 μM, which was
then used in subsequent studies (Fig.
1). Similarly, 5-FU also inhibited the growth of the EC9706 and
Eca109 esophageal cancer cells (data not shown). 5-FU was used at a
concentration of 0.2 mM in subsequent experiments.
Annexin V-FITC/PI-stained FACS
analysis
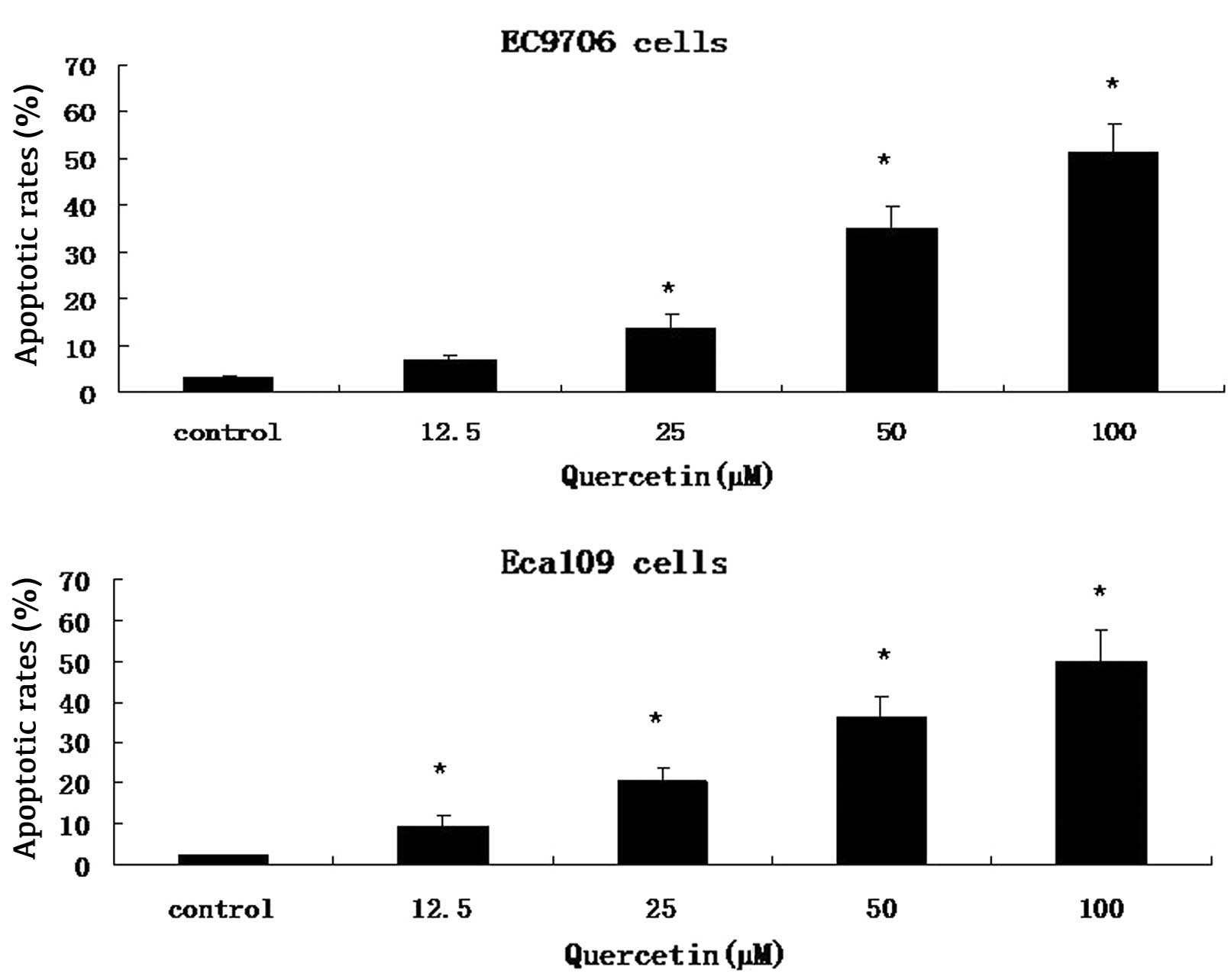
Cells were stained with Annexin V-FITC and PI. A
FACS analysis was conducted to distinguish and quantify the
percentage of viable and apoptotic cells following treatment with
0.2 mM 5-FU, 100 μM quercetin or a combination of both drugs, for
48 h. Quercetin demonstrated a dose-dependent increase in apoptosis
in EC9706 and Eca109 cells (Fig.
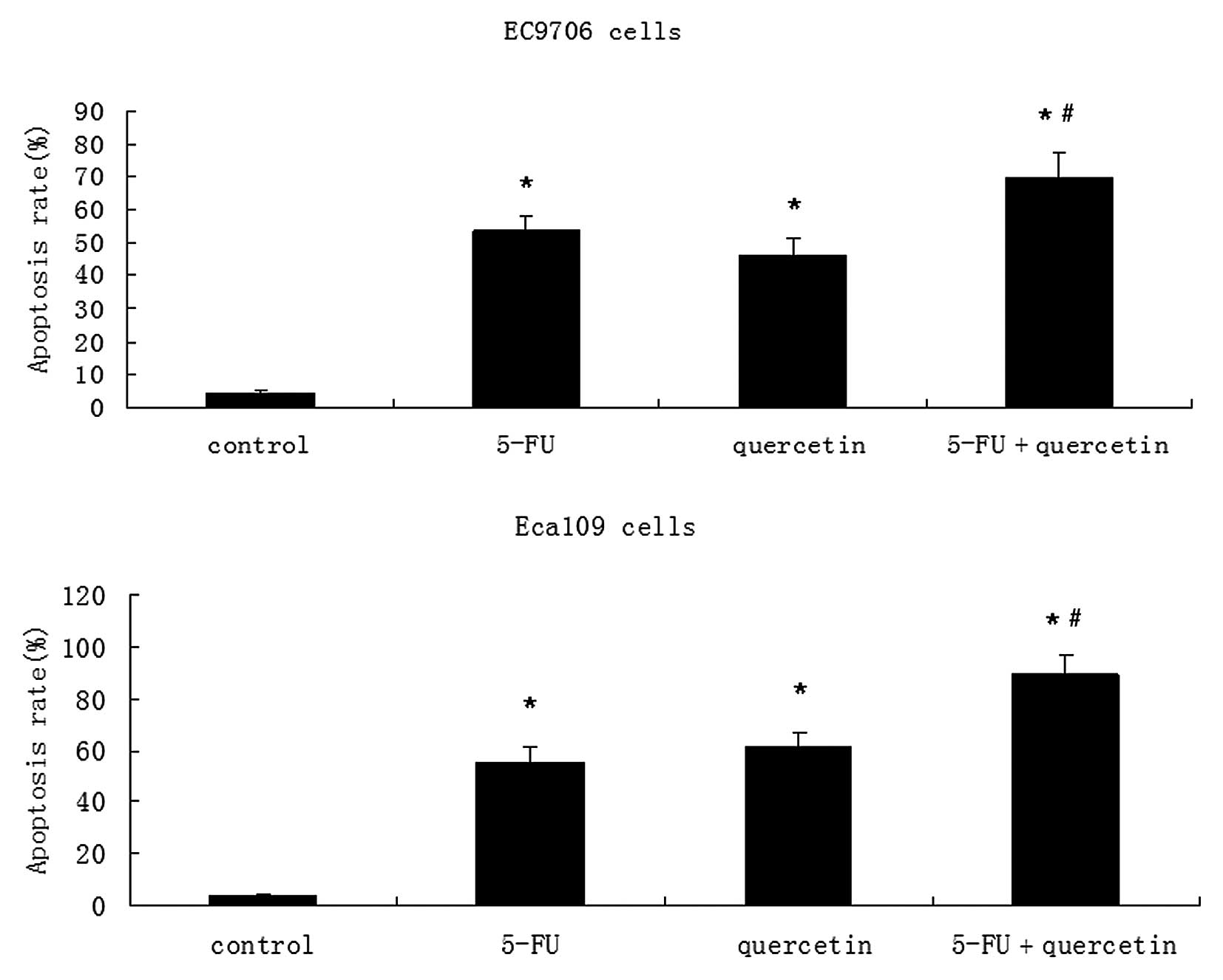
2). In the combinatorial treatment group, there was a greater
degree of apoptosis compared to that is the agent alone or control
groups (Fig. 3).
Inhibition of NF-κB by quercetin enhances
5-FU-induced apoptosis in esophageal cancer cells
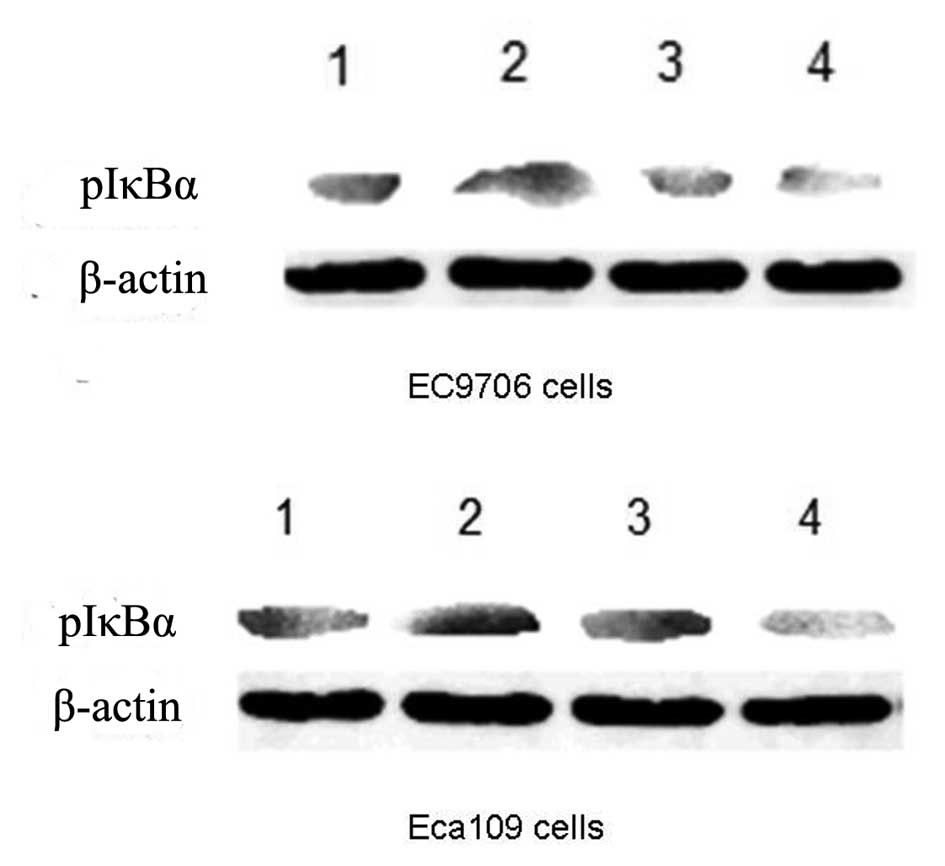
To investigate whether the effect of quercetin on
EC9706 and Eca109 cells was mediated through the NF-κB signaling
pathway, we examined the effects of 50 μM quercetin and 0.2 mM 5-FU
on the expression levels of a phosphorylated inhibitory molecule of
NF-κB (pIκBα) using western blot analysis. As shown in Fig. 4, quercetin significantly suppressed
the expression levels of pIκBα 24 h following drug treatment.
Discussion
It has been well established that the reduced
capacity of tumor cells to undergo apoptotic cell death plays a key
role in the pathogenesis of cancer and in therapeutic failure
(18–20). Evidence suggests that chemotherapy
induces apoptosis as part of its therapeutic effects and that
chemoresistance is likely to involve an antiapoptotic mechanism.
Tumor cells often have multiple alterations in their apoptotic
signaling pathways that lead to chemotherapy failure (21,22).
Overriding these mutations is a significant area of focus in
anticancer drug research.
NF-κB is primarily retained in the cytoplasm as an
inactive complex through direct binding of IκB. Recent data
indicate that activation of NF-κB represents a principal pathway in
inducible chemoresistance. Cell exposure to various stimuli results
in the phosphorylation and degradation of IκBα, the translocation
of active NF-κB to the nucleus (23) and the subsequent induction of target
genes that code for antiapoptotic proteins, including survivin.
This gene activation leads to tumor cell resistance to cytotoxic
therapy (24). NF-κB has been
identified as an antiapoptotic protein, which is activated by
chemotherapy drugs (e.g., 5-FU) in a number of cancer cell lines,
including esophageal cancer cells (2).
In our study, 5-FU and quercetin demonstrated a
dose-dependent induction of apoptosis in addition to the inhibition
of EC9706 and Eca109 cell growth. Although the anticancer effect of
quercetin was mild, the addition of quercetin to 5-FU significantly
enhanced the cytotoxic and apoptotic cellular responses, even at
low concentrations of 5-FU. This demonstrates that these cells may
be more sensitive to 5-FU when combined with quercetin.
Based on studies suggesting that NF-κB is
constitutively active in a number of cancer cell lines (25-27),
we investigated whether NF-κB was involved in the apoptotic process
induced by 5-FU in EC9706 and Eca109 cells. Our results indicate
that 5-FU mediates the activation of NF-κB through the
phosphorylation of the IκBα protein, leading to increased
expression levels of pIκBα. When the increase in pIκBα reaches a
maximal value at a certain concentration of 5-FU, further dose
increases of the chemotherapy drug may not be effective. Our data
suggest that these cell lines may be resistant to 5-FU treatment,
which has also been observed in patients who are undergoing
treatment.
The last series of experiments was conducted to
determine the potential mechanisms by which quercetin inhibits
NF-κB activation. We revealed that the addition of quercetin
significantly enhanced the cytotoxic and apoptotic responses to
5-FU. To confirm that quercetin plays a converse role in the
5-FU-mediated activation of NF-κB, we sought to determine the
extent to which NF-κB was affected by quercetin and 5-FU, alone or
in combination. Consistent with other studies, 5-FU increased the
expression of pIκBα in EC9706 and Eca109 cells, but expression of
pIκBα was inhibited when quercetin was combined with 5-FU, although
quercetin had little effect on its own.
Our data demonstrated that quercetin acts
synergistically with 5-FU to induce apoptosis in esophageal cancer
cells. This may be attributed to the attenuation of NF-κB
activation, as demonstrated by the decrease in pIκBα expression.
These data suggest that the addition of quercetin to 5-FU may
potentially be a superior therapeutic strategy for the treatment of
esophageal cancer.
References
|
1.
|
RT GreenleeT MurrayS BoldenPA WingoCancer
StatisticsCA Cancer J Clin507332000
|
|
2.
|
J LiDJ MinnichER CampA BrankSL MackaySN
HochwaldEnhanced sensitivity to chemotherapy in esophageal cancer
through inhibition of NF-κBJ Surg Res1321121202006
|
|
3.
|
CT LeeJY SeolSY LeeThe effect of
adenovirus-Ikappa Balpha transduction on the chemosensitivity of
lung cancer cell line with resistance to
cis-diaminedichloroplatinum (II) (cisplatin) and doxorubicin
(adriamycin)Lung
Cancer41199206200310.1016/S0169-5002(03)00227-712871783
|
|
4.
|
HJ ShimSH ChoJE HwangPhase II study of
docetaxel and cisplatin chemotherapy in 5-f luorouracil/cisplatin
pretreated esophageal cancerAm J Clin
Oncol33624628201010.1097/COC.0b013e3181bead9220142726
|
|
5.
|
C FriesenI HerrPH KrammerKM
DebatinInvolvement of the CD95 (APO-1/FAS) receptor/ligand system
in drug-induced apoptosis in leukemia cellsNat
Med2574577199610.1038/nm0596-5748616718
|
|
6.
|
S FuldaSA SusinG KroemerKM
DebatinMolecular ordering of apoptosis induced by anticancer drugs
in neuroblastoma cellsCancer Res584453446019989766678
|
|
7.
|
DE FisherApoptosis in cancer therapy:
crossing the
thresholdCell78539542199410.1016/0092-8674(94)90518-58069905
|
|
8.
|
J LotemL SachsRegulation by bcl-2, c-myc,
and p53 of susceptibility to induction of apoptosis by heat shock
and cancer chemotherapy compounds in differentiation-competent and
-defective myeloid leukemic cellsCell Growth Differ441471993
|
|
9.
|
CY WangMW MayoRG KornelukDV GoeddelAS
Baldwin JrNF-κB antiapoptosis: induction of TRAF1 and TRAF2 and
c-IAP1 and c-IAP2 to suppress caspase-8
activationScience281168016831998
|
|
10.
|
CY WangMW MayoAS Baldwin JrTNF- and cancer
therapy-induced apoptosis: potentiation by inhibition of
NF-κBScience27478478719968864119
|
|
11.
|
R VoborilSN HochwaldJ LiA BrankJ WeberovaF
WesselsLL MoldawerER CampSL MacKayInhibition of NF-kappa B augments
sensitivity to 5-fluorouracil/folinic acid in colon cancerJ Surg
Res120178188200410.1016/j.jss.2003.11.02315234211
|
|
12.
|
JC Cusack JrR LiuM HoustonEnhanced
chemosensitivity to CPT-11 with proteasome inhibitor PS-341:
implications for systemic nuclear factor-κB inhibitionCancer
Res6135353540200111325813
|
|
13.
|
MG HertogPC HollmanMB KatanD
KromhoutIntake of potentially anticarcinogenic flavonoids and their
determinants in adults in the NetherlandsNutr
Cancer202129199310.1080/016355893095142678415127
|
|
14.
|
B CsokayN PrajdaG WeberE OlahMolecular
mechanisms in the antiprolife- rative action of quercetinLife
Sci6021572163199710.1016/S0024-3205(97)00230-09188758
|
|
15.
|
F CasagrandeJM DarbonEffects of
structurally related flavonoids on cell cycle progression of human
melanoma cells: regulation of cyclin-dependent kinases CDK2 and
CDK1Biochem
Pharmacol6112051215200110.1016/S0006-2952(01)00583-411322924
|
|
16.
|
TT NguyenE TranTH NguyenPT DoTH HuynhH
HuynhThe role of activated MEK-ERK pathway in quercetin-induced
growth inhibition and apoptosis in A549 lung cancer
cellsCarcinogenesis25647659200410.1093/carcin/bgh05214688022
|
|
17.
|
K KawadaT YoneiH UeokaComparison of
chemosensitivity tests: clonogenic assay versus MTT assayActa Med
Okayama56129134200212108583
|
|
18.
|
MH LamQ LiuSJ ElledgeJM RosenChk1 is
haploinsufficient for multiple functions critical to tumor
suppressionCancer
Cell64559200410.1016/j.ccr.2004.06.01515261141
|
|
19.
|
Y YamamuraWL LeeMX GohY ItoRole of
TAp73alpha in induction of apoptosis by transforming growth
factor-beta in gastric cancer cellsFEBS
Lett58226632667200810.1016/j.febslet.2008.06.04618593586
|
|
20.
|
M KurokawaC ZhaoT ReyaS
KornbluthInhibition of apoptosome formation by suppression of
Hsp90beta phosphorylation in tyrosine kinase-induced leukemiasMol
Cell Biol2854945506200810.1128/MCB.00265-0818591256
|
|
21.
|
D HanahanRA WeinbergThe hallmarks of
cancerCell1005770200010.1016/S0092-8674(00)81683-9
|
|
22.
|
KC ZimmermannDR GreenHow cells die:
apoptosis pathwaysJ Allergy Clin Immunol108Suppl
499103200110.1067/mai.2001.11781911586274
|
|
23.
|
F ChenK BeezholdV CastranovaTumor
promoting or tumor suppressing of NF-kappa B, a matter of cell
context dependencyInt Rev
Immunol27183204200810.1080/0883018080213032718574736
|
|
24.
|
C JobinA PanjaC HellerbrandInhibition of
proinflammatory molecule production by adenovirus-mediated
expression of a nuclear factor kappaB super-repressor in human
intestinal epithelial cellsJ Immunol1604104181998
|
|
25.
|
M HinzP LoserS MathasD KrappmannB DorkenC
ScheidereitConstitutive NF-kappaB maintains high expression of a
characteristic gene network, including CD40, CD86, and a set of
antiapoptotic genes in Hodgkin/Reed-Sternberg
cellsBlood9727982807200110.1182/blood.V97.9.279811313274
|
|
26.
|
S HuangCA PettawayH UeharaCD BucanaIJ
FidlerBlockade of NF-kappaB activity in human prostate cancer cells
is associated with suppression of angiogenesis, invasion, and
metastasisOncogene2041884197200110.1038/sj.onc.120453511464285
|
|
27.
|
X BianAW Opipari JrAB
RatanaproeksaConstitutively active NFkappa B is required for the
survival of S-type neuroblastomaJ Biol
Chem2774214442150200210.1074/jbc.M20389120012198114
|