Introduction
Primary hepatocellular carcinoma (HCC) is a common
malignant tumor, particularly in China and Southeast Asia (1). Although the resection rate of HCC has
improved in the last 20 years, the general therapeutic efficacy is
not yet satisfactory. The mortality rate of HCC ranks second in all
malignant tumors in China, due to postoperative metastatic
recurrence (2). Currently, the
molecular basis for the characteristics of HCC is unknown and there
is a need to identify the cancer biology and develop new
therapeutic strategies.
Ras proteins have essential roles in controlling the
activity of multiple downstream effector pathways that regulate
normal cellular proliferation (3).
Ras-controlled pathways modulate several transcription factors that
eventually link Ras activity to various phases of the cell cycle.
As an impotant member of the Ras family, H-ras mutation has been
widely investigated in multiple types of solid tumor. Over the past
decade, H-ras has emerged as a significant target for anticancer
therapies and has become a hotspot of anticancer research, while
the exact mechanism remains unknown (4).
In the present study, HotStarTaq PCR was adopted to
examine the expression of H-ras in HCC tissues and normal tissues
adjacent to the tumor, and H-ras mutation was analyzed using PCR
direct sequencing. Based on our findings, the essential role of
H-ras in the diagnosis and treatment of HCC patients is discussed,
especially in the Chinese population.
Materials and methods
Samples
A total of 69 paired HCC and normal liver samples
(confirmed by two individual pathological diagnoses) were obtained
from patients treated at the The Affiliated Hospital of Medical
College, Qingdao University (Qingdao, China) for HCC. No patient
had distant metastatic disease at surgery and no patient had been
treated previously with intravesical chemotherapy. For the
selection of surrounding non-tumor liver tissues, specimens were
obtained from tissues at a clear distance from the edge of tumors
(>1 cm), if there was no evidence of nearby tumor invasion. The
cancer tissue and the adjacent microscopically normal samples were
rinsed in sterile PBS and snap frozen in liquid nitrogen
immediately after removal. The research protocol was approved by
the Institutional Review Board and informed consent was obtained
from patients. The average age of the patients in this study,
including 42 males and 27 females, was between 22 and 73 years
(57.9±13.4). Of the 69 patients, the greatest diameter of the tumor
was >5 cm in 48 cases and 5 cm in 21. Tumor capsules were
integrated in 32 and disintegrated in 37 cases. Serum AFP was
positive in 40 and negative in 29; HBsAg was positive in 38 and
negative in 31. Liver cirrhosis was detected in 34 cases. A total
of 24 patients had Edmondson grade I or II disease and the
remaining 45 patients had Edmondson grade III or IV. A total of 28
patients had TNM stage I or II disease and 41 had TNM stage III or
IV. According to the operative records and postoperative
pathological data, 36 HCC patients with cancer emboli, intrahepatic
dissemination (satellite foci or multiple nodules) and/or lymph
node metastasis were classified as high-risk of metastatic
recurrence and the remaining 33 patients without emboli,
dissemination and/or metastasis were described as low-risk of
metastatic recurrence. A total of 63 patients were followed up for
16–35 months after surgery, during which time neoplastic metastasis
or recurrence was found in 38 patients.
RNA extraction and cDNA synthesis
Approximately 100 mg tumor or liver tissue was used
for total RNA isolation using TRIzol reagent (Gibco-BRL, Carlsbad,
CA, USA) according to the manufacturer’s instructions. First-strand
cDNA was synthesized using 5 μl total RNA with oligo (dT) 16
primer in a 50-μl reverse transcription mixture containing
10 μl of 5X first strand buffer, 2.5 μl dNTP mixture
containing 25 mmol/l of each deoxynucleotide triphosphate base
(Pharmacia Biotech Co., Tokyo, Japan), 2.5 μl ribonuclease
inhibitor (Takara Biochemicals, Ohotsu, Japan), 25 μl
ddH2O (managed with DEPC in advance) and 2.5 μl
avian myeloblastosis virus reverse transciptase (Takara
Biochemicals).
Primer design
The resulting cDNA was used for PCR amplification
using Taq polymerse (Takara Biochemicals). The sequence of
the H-ras gene open reading frame was obtained from the gene bank
and the primer was designed using Primer Express 2.0 (Applied
Biosystems, Foster City, CA, USA). The upstream primer is before AT
G, H1=5′ - G G CAG GAGAC C C T GTAG GAG -3′; the downstream primer
was located at exon 2, H2=5′-GGTTCACCTGTACTGGTGGAT-3′; the
amplification area included exons 1 and 2, totaling 613 bp. The
primer was synthesized by Shanghai Sangon Biological Engineering
Technology & Service Co., Ltd. (Shanghai, China) The PCR
conditions included initial denaturation at 95° for 15 min,
followed by 40 cycles of amplification with subsequent denaturation
at 94°C for 60 sec, annealing at 60°C for 60 sec and extension for
60 sec at 72°C. The amplified products were examined by agarose gel
electrophoresis, and recorded by a motored molecular imaging
system. A total volume of 10 l of PCR products underwent
electrophoresis using 12 g/l agarose gel and were visualized by UV
absorption and ethidium bromide.
Sequencing
DNA sequencing was performed on an ALF express DNA
automatic sequencer (Pharmacia Biotech Co.) by the dideoxy terminal
termination method. The sequenced HPA segment was 613 bp in length.
The sequence of the amplified H-ras segment was compared with the
gene bank database and analyzed for homogeneity using the NCBI
BLAST program.
Statistical analysis
The significance of the differences between two
groups was tested using Chi-square analysis. P<0.05 was
considered to indicate a statistically significant result.
Results
Expression of H-ras mRNA in HCC
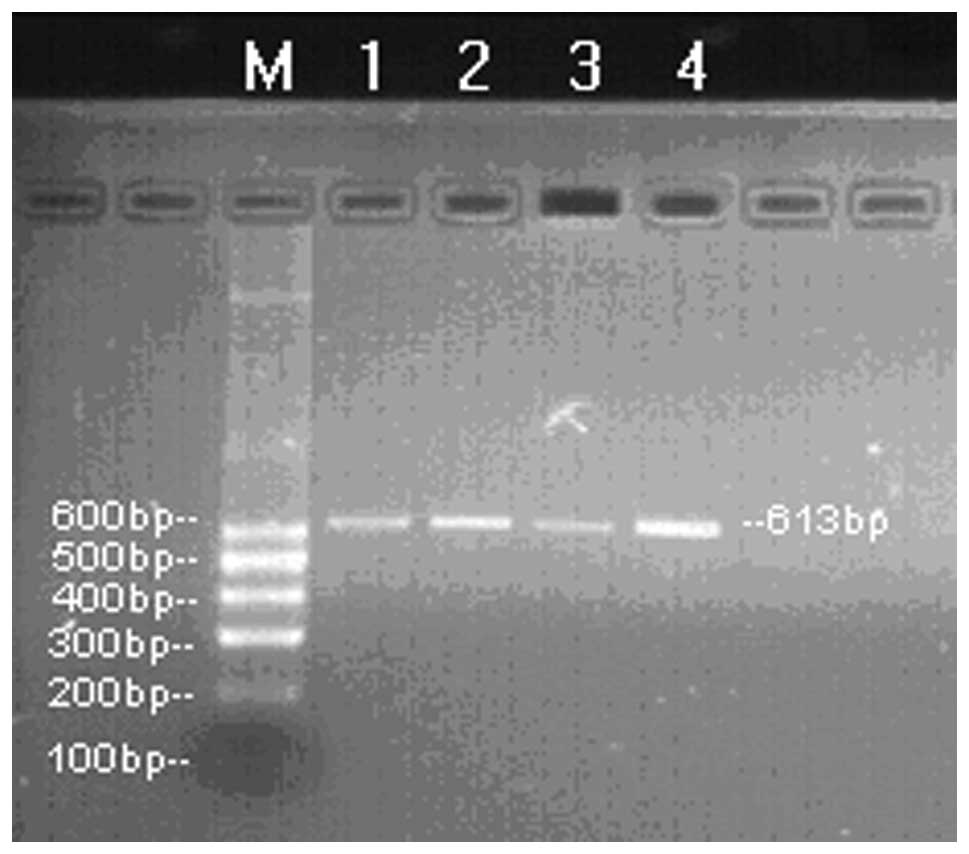
H-ras mRNA was amplified in all the clinical
samples. Electrophoretic analysis showed a bright band ∼600 bp in
length in these patients. The electrophoresis band was clear and
there was no dispersive band, indicating its high specificity
(Fig. 1).
Sequencing
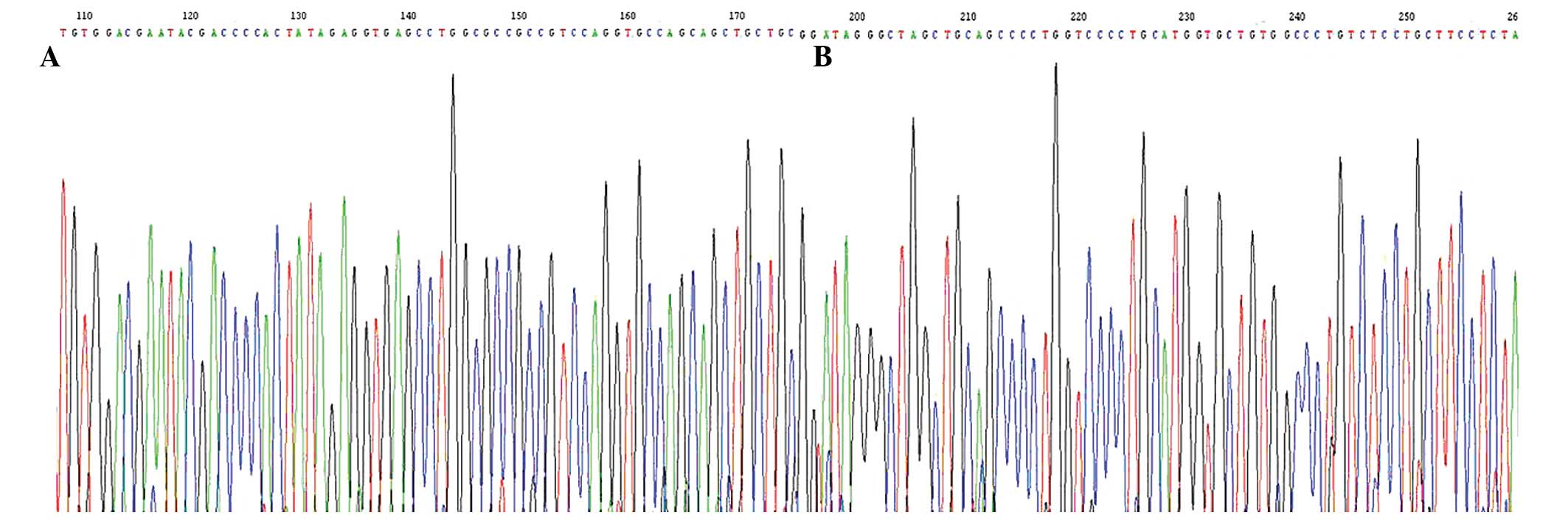
After the sequence of the PCR products of the 69
samples of HCC was examined, the H-ras gene was found to be mutated
in 49 samples of HCC tumor tissue; 19 samples had a mutation in
codon 40 from CTA to CTG; 30 had a mutation in codon 61 of GGC to
AGC. By contrast, only 2 mutations were found in the samples of
normal tissues adjacent to the cancer. The sequencing maps are
shown in Fig. 2. The H-ras mutation
rate in the tumor tissues of HCC was 71.01% (49/69) and was
significantly higher than that in the surrounding non-tumor liver
tissues (P<0.01).
Correlation between H-ras mutation and
clinicopathological indices
By statistical analysis, no significant correlation
of the H-ras mutation rate was identified with tumor size, capsule,
AFP, HBsAg or liver cirrhosis (all P>0.05; Table I). The H-ras mutation rate in the
Edmondson grade I or II group was significantly lower than that in
Edmondson grade III or IV group (P<0.01) and the rate in TNM
stage I or II group was also markedly lower than that in TNM stage
III or IV group (P<0.01; Table
I).
 | Table I.Correlation between H-ras mutation
and the clinicopathological parameters of HCC. |
Table I.
Correlation between H-ras mutation
and the clinicopathological parameters of HCC.
| Parameter | n | H-ras mutation | H-ras normal | P-value |
|---|
| Size of tumor
(cm) | | | | |
| >5 | 48 | 37 | 11 | 0.09 |
| ≤5 | 21 | 12 | 9 | |
| Tumor capusle | | | | |
| Integrated | 32 | 26 | 6 | 0.08 |
|
Disintegrated | 37 | 23 | 14 | |
| AFP | | | | |
| Positive | 40 | 28 | 12 | 0.82 |
| Negative | 29 | 21 | 8 | |
| HBsAG | | | | |
| Positive | 38 | 23 | 15 | 0.07 |
| Negative | 31 | 26 | 5 | |
| Liver
cirrhosis | | | | |
| Yes | 34 | 24 | 10 | 0.94 |
| No | 35 | 25 | 10 | |
| Edmondson
grade | | | | |
| I, II | 24 | 9 | 15 | <0.01 |
| III, IV | 45 | 40 | 5 | |
| TNM staging | | | | |
| I, II | 28 | 11 | 17 | <0.01 |
| III, IV | 41 | 38 | 3 | |
Correlation between H-ras mutation and
metastatic recurrence of HCC
The H-ras mutation rate in the high-risk of
metastatic recurrence group was markedly higher than that in the
low-risk group (P<0.01) and the rate in the metastatic
recurrence group was also significantly higher than that in the
non-metastatic recurrence group (P<0.01; Table II).
 | Table II.Correlation between H-ras mutation
and metastatic recurrence of HCC. |
Table II.
Correlation between H-ras mutation
and metastatic recurrence of HCC.
| Group | n | H-ras mutation | H-ras normal | P-value |
|---|
| Risk of metastatic
recurrence | | | | |
| High | 36 | 32 | 4 | <0.01 |
| Low | 33 | 17 | 16 | |
| Metastatic
recurrence | | | | |
| Yes | 38 | 35 | 3 | <0.01 |
| No | 31 | 14 | 17 | |
Discussion
The H-ras gene is located at 11p15.5. The possible
carcinogenic mechanism of H-ras is as follows: the mutated H-ras
gene results in overexpression of the H-ras protein. The H-ras
protein inhibits the activation of a nuclease which triggers
apoptosis and leads directly to a significant decrease in cell
death (5). It has been found that
ras mutation occurs in numerous types of human tumors and that the
mutation rate of the ras genes can reach 90% in pancreatic, 40–50%
in colon, 40% in lung and 37% in bladder cancer (5). The present study showed that H-ras
mutation occurred in 49 samples (49/69, 71.01%), of which 19 had a
mutation in codon 40, of CTA to CTG, and 30 had a mutation in codon
61, of GGC to AGC. Compared with the cancer samples, H-ras mutation
was found in only 2 normal tissues adjacent to the cancer. Our
results indicate that H-ras mutation may be a reliable biomarker in
HCC.
No correlation was found between H-ras mutation and
the integrity of the tumor capsule or tumor size in this study. In
addition, H-ras mutation was not found to be associated with AFP,
HBsAg or liver cirrhosis. H-ras mutation was not correlated with
HBsAg, indicating that H-ras mutation may be used for the diagnosis
of HCC patients infected with HBV, as is the case of most HCCs in
China. It has been reported that H-ras mutation in cancerous
tissues is closely correlated with tumor invasion, metastasis and
angiogenesis (6,7). Both metastatic recurrence and tumor
microvessel density (MVD) in tumors may significantly increase with
H-ras mutation (8). In this study,
the mutation rate in HCCs was 71.01% and H-ras mutation was
associated with the pathological classification and TNM stage,
which were similar to the conclusions drawn by a previous study
(9). The marked difference in H-ras
mutation between the low- and high-risk of metastatic recurrence
groups in our study indicated that H-ras mutation was associated
with the invasion and metastasis of HCC. The significant difference
between the metastatic and non-metastatic recurrence groups in our
study was also similar to the results of a previous study (6). This result further showed that H-ras
mutation was associated with metastatic recurrence and that there
was a higher degree of invasiveness and risk of postoperative
recurrence in patients with H-ras mutation compared with normal
H-ras patients, and that H-ras mutation may provide a potential and
valuable index to predict postoperative metastatic recurrence
(10).
Previous studies have reported that H-ras mutation
may be an effective marker in cancer diagnosis and molecular
therapy (11). The exact mechanism
remains unknown. Certain studies have reported the levels of growth
factors, including transforming growth factor-β (TGF-β) and
fibroblast growth factor (bFGF), enhanced DNA synthesis and
anchorage-dependent growth in cells with H-ras mutation (12,13).
Furthermore, H-ras mutation caused progressive tumors in nude mice;
however, those tumors were distinct from v-ras-induced tumors,
indicating that H-ras mutation was directly involved in cell growth
and transformation and the development of tumors (14). Although the present study offers
limited information with regard to the possible correlation between
H-ras mutation and cancer, we may offer extra information
concerning the diagnosis and treatment of HCC, especially in China.
Being a developing country, most Chinese patients lack medical
insurance and the cost of adjuvant therapy is a problem. The
ongoing debates that have not been resolved in China are how to
identify patients who have the highest risk of suffering recurrence
and how to increase the cost efficiency of disease management
(15). According to the above
results, H-ras mutation was not a early event in carcinogenesis and
is associated with invasion and metastatic recurrence, which may be
especially significant in the selection of apporiate candidates for
perioperative comprehensive therapy.
In conclusion, the detection of H-ras mutation has
the potential to aid an improved understanding of HCC and to offer
extra information concerning the diagnosis and treatment of
HCC.
Acknowledgements
This study was supported by a grant
from the Natural Science Foundation of Shandong Province, China
(Y2005C45).
References
|
1.
|
JM LukAM LiuProteomics of hepatocellular
carcinoma in Chinese
patientsOMICS15261266201110.1089/omi.2010.009921348761
|
|
2.
|
T YangJ ZhangJH LuLQ YangGS YangMC WuWF
YuA new staging system for resectable hepatocellular carcinoma:
comparison with six existing staging systems in a large Chinese
cohortJ Cancer Res Clin
Oncol137739750201110.1007/s00432-010-0935-320607551
|
|
3.
|
M DaviesSS PrimeJW EvesonN PriceA
GanapathyA D’MelloIC PatersonTransforming growth factor-β enhances
invasion and metastasis in Ras-transfected human malignant
epidermal keratinocytesInt J Exp Pathol931481562012
|
|
4.
|
Y Gus-BrautbarD JohnsonL ZhangH SunP WangS
ZhangL ZhangYH ChenThe anti-inflammatory TIPE2 is an inhibitor of
the oncogenic RasMol
Cell45610618201210.1016/j.molcel.2012.01.00622326055
|
|
5.
|
JH OvermeyerWA MalteseDeath pathways
triggered by activated Ras in cancer cellsFront
Biosci1616931713201110.2741/381421196257
|
|
6.
|
JM CullenC WilliamsL ZadroznyJT OtstotGG
SolomonRC SillsHH HongH-ras consensus sequence and mutations in
primary hepatocellular carcinomas of lemurs and lorisesVet
Pathol48868874201110.1177/030098581038852621123858
|
|
7.
|
XD ZhouRecurrence and metastasis of
hepatocellular carcinoma: progress and prospectsHepatobiliary
Pancreat Dis Int13541200214607620
|
|
8.
|
S ReginaJ RollinC BléchetS IochmannP
ReverdiauY GruelTissue factor expression in non-small cell lung
cancer: relationship with vascular endothelial growth factor
expression, microvascular density, and K-ras mutationJ Thorac
Oncol3689697200810.1097/JTO.0b013e31817c1b21
|
|
9.
|
Q ZuoH HuangM ShiF ZhangJ SunJ BinY LiaoW
LiaoMultivariate analysis of several molecular markers and
clinicopathological features in postoperative prognosis of
hepatocellular carcinomaAnat Rec
(Hoboken)295423431201210.1002/ar.21531
|
|
10.
|
MJ HoenerhoffI ChuD BarkanZY LiuS DattaGP
DimriJE GreenBMI1 cooperates with H-RAS to induce an aggressive
breast cancer phenotype with brain
metastasesOncogene2830223032200910.1038/onc.2009.16519543317
|
|
11.
|
HW LoTargeting Ras-RAF-ERK and its
interactive pathways as a novel therapy for malignant gliomasCurr
Cancer Drug
Targets10840848201010.2174/15680091079335797020718706
|
|
12.
|
G BuhrmanC O’ConnorB ZerbeBM KearneyR
NapoleonEA KovriginaS VajdaD KozakovEL KovriginC MattosAnalysis of
binding site hot spots on the surface of Ras GTPaseJ Mol
Biol413773789201110.1016/j.jmb.2011.09.01121945529
|
|
13.
|
M NakamuraJ KitauraY EnomotoY LuK
NishimuraM IsobeK OzakiY KomenoF NakaharaT OkiTransforming growth
factor-β-stimulated clone-22 is a negative-feedback regulator of
Ras/Raf signaling: Implications for tumorigenesisCancer
Sci10326332012
|
|
14.
|
M LeeDifferential physiological effects of
Raf-1 kinase pathways linked to protein kinase C activation
depending on the stimulus in v-H-ras-transformed cellsCancer Res
Treat403944200810.4143/crt.2008.40.2.39
|
|
15.
|
X YinBH ZhangSJ QiuZG RenJ ZhouXH ChenY
ZhouJ FanCombined hepatocellular carcinoma and cholangiocarcinoma:
clinical features, treatment modalities, and prognosisAnn Surg
OncolMar272012(Epub ahead of print)
|