Introduction
Recent advances in chemotherapy have resulted in an
increasing number of patients with colorectal cancer liver
metastases (CLMs) being treated with systemic chemotherapy prior to
hepatic metastasectomy, either as neoadjuvant treatment for
initially resectable lesions or in an attempt to make unresectable
lesions resectable. When used as first-line chemotherapy for CLMs,
new and effective regimens, including 5-fluorouracil
(5-FU)/leucovorin (LV), irinotecan and oxaliplatin in combination
with targeted agents, have yielded a complete response in 1 to 9%
of patients with CLMs (1–3). The optimal treatment strategy in such
cases, however, remains to be determined as, to the best of our
knowledge, little research has been carried out on this topic and
the results thus far have been conflicting.
Patients and methods
Patients
The study protocol conformed to the standards of
good practice and ethics of our institution. Informed consent was
obtained from the individuals included in the study. A
retrospective review of all consecutive patients who had been
diagnosed with CLM and who were treated with first-line
oxaliplatin-based chemotherapy (modified FOLFOX6; mFOLFOX6) with or
without bevacizumab between January 2006 and December 2010 was
carried out. The mFOLFOX6 regimen comprised intravenous infusion of
oxaliplatin (80 mg/m2) over 2 h, followed by rapid
intravenous bolus infusion of 5-FU (400 mg/m2) for 5 min
and continuous intravenous infusion of 5-FU (2,400
mg/m2) over 46 h. This regimen was repeated every 2
weeks. When used in combination with the mFOLFOX6 regimen,
bevacizumab (5 mg/kg) was infused intravenously over 60–90 min
prior to the administration of oxaliplatin. In Japan, the use of
oxaliplatin and bevacizumab for metastatic colonic cancer was
approved by the governmental health insurance system in March 2005
and April 2007, respectively. During this period, mFOLFOX6 with or
without bevacizumab was the standard first-line chemotherapy for
metastatic colorectal cancer at our institute.
Data were collected on patients in whom all CLMs
initially detected by computed tomography (CT) disappeared during
first-line chemotherapy, focusing on time-to-disappearance and
time-to-recurrence on a tumor-by-tumor basis.
The clinicopathological patient data recorded
included age, gender, site of primary lesion, disease stage at
diagnosis of primary lesion, site and number of liver metastases
and carcinoembryonic antigen (CEA) level prior to chemotherapy.
Adverse events during chemotherapy were evaluated according to the
Common Toxicity Criteria of Adverse Events (CTCAE) ver. 4.0
(4). The relative dose intensity of
oxaliplatin was also evaluated.
The extent of metastasis was determined during the
pretreatment workup, which usually involved enhanced triple-phase
helical CT of the chest, abdomen and pelvis in 5-mm thick slices.
CT was periodically performed at 3–4 month intervals. Disappearance
was defined as no further lesion or abnormality, including a low
attenuated mass, calcification and ring enhancement, at the site of
a previously identified CLM. Other imaging modalities, including
intravenously enhanced magnetic resonance imaging (MRI) and
positron emission tomography (PET)/CT, were also used whenever CT
proved inadequate or in order to confirm disappearance on CT.
Statistical analysis
Continuous variables were expressed as the median
and range. Time-to-disappearance and time-to-recurrence were
estimated on a tumor-by-tumor basis. Time-to-disappearance was
defined as the time from the initiation of chemotherapy to
radiographic diagnosis of disappearance. Time-to-recurrence was
defined as the time from disappearance to the time of initial
radiographic evidence of relapse in situ. To calculate the
in situ time-to-recurrence of disappearing CLMs, the CLMs
were censored at the time of the last image in which no evidence of
recurrence was visible. Biopsied lesions without evidence of viable
tumor cells were also censored at the time of surgery. The
cumulative rates of disappearance and recurrence were estimated
using the Kaplan-Meier method.
Results
Patient characteristics and clinical
course
A total of 125 patients diagnosed with CLMs were
treated with mFOLFOX6 with or without bevacizumab. In 5 of the
patients (4%), all CLMs disappeared during chemotherapy. Three of
the patients were female. The primary site was the colon in 3
patients and the rectum in 2. At diagnosis of the primary lesion,
pathological stage was I in 1 patient, II in 1 and IV in 3.
Histological examination revealed well- or moderately
differentiated adenocarcinoma in 4 patients and poorly
differentiated adenocarcinoma in 1. The median CEA level (cut-off,
6.7 ng/ml) prior to chemotherapy was 5.1 ng/ml (range, 2.0–14.3).
The median number of liver metastases was 8 (range, 2–16). The
median maximal diameter of liver metastases per patient was 1.8 cm
(range, 1.0–2.4). The median number of cycles of oxaliplatin-based
chemotherapy to disappearance of all CLMs per patient was 12
(range, 9–15), with a median relative dose intensity of oxaliplatin
at 79% (range, 78–88). All patients required a prolonged
chemotherapy interval and/or dose reduction due to neutropenia. No
peripheral neurotoxicities >grade 3 were observed.
The details of treatment for each patient are
summarized in Table I. In Patient
1, CT revealed a large rectal cancer occupying the pelvic space and
16 bilobular metastatic lesions. After 5 and 11 cycles of mFOLFOX6,
12 and 3 CLMs disappeared, respectively. After 15 cycles, the one
remaining lesion also disappeared and the primary lesion showed a
marked reduction in size. Low anterior resection and biopsy of a
scar lesion on the liver surface were performed. Histological
examination revealed viable well-differentiated adenocarcinoma
cells in the primary lesion but no viable tumor cells in the biopsy
specimen. The patient received an additional 6 cycles of mFOLFOX6
postoperatively. At 8 and 9 months after surgery, in situ
relapse was detected in 1 and 3 lesions, respectively, on CT and
MRI. The patient was administered mFOLFOX6 plus bevacizumab for
these 4 lesions, resulting in disappearance of all lesions after 7
cycles. Four months later, one of the 4 lesions reappeared and was
subsequently resected. At the second laparotomy, a scar lesion was
also resected, revealing no viable tumor cells by histological
examination. The patient remains free of disease at 54 months after
the initiation of first-line chemotherapy.
 | Table IClinical features and details of
treatment for each patient. |
Table I
Clinical features and details of
treatment for each patient.
| Case | Age (years) | Gender | Stage at initial
diagnosis | Site of primary
lesion | CEA at initial
diagnosis (ng/ml) | No. CLMs before
mF6 | Maximal diameter of
tumor (cm) | No. mF6 cycles | Bev | Additional mF6+ Bev
after disappearance | Recurrence in
situ |
|---|
| 1 | 69 | M | IV | Rectum | 6.6 | 16 | 2.0 | 15 | − | + | + |
| 2 | 35 | F | IV | Colon | 14.3 | 8 | 1.8 | 12 | + | − | − |
| 3 | 60 | F | II | Colon | 4.5 | 2 | 1.0 | 11 | + | − | + |
| 4 | 68 | M | I | Rectum | 2.0 | 5 | 2.4 | 13 | + | − | + |
| 5 | 68 | F | IV | Colon | 5.1 | 13 | 1.4 | 9 | + | + | − |
In patient 2, CT revealed 8 bilobular synchronous
liver metastases from moderately differentiated adenocarcinoma of
the transverse colon associated with familial adenomatous
polyposis. Chemotherapy comprised mFOLFOX6 plus bevacizumab. After
3 and 12 cycles of mFOLFOX6 plus bevacizumab, 6 and 2 lesions
disappeared, respectively. Two months later, the patient underwent
total colectomy and biopsy of a scar lesion on the liver surface.
Histological examination revealed moderately differentiated
adenocarcinoma in the primary tumor but no viable cells in the
biopsy specimen. No chemotherapy was administered postoperatively.
The patient remains free of disease at 40 months after the
initiation of chemotherapy.
Patient 3 received mFOLFOX6 plus bevacizumab therapy
for 2 recurrent liver metastases detected at 13 months after
Hartmann’s procedure for perforated stage II sigmoid colon
well-differentiated adenocarcinoma. No hepatectomy was performed
due to patient refusal. After 12 cycles of chemotherapy, the 2
metastases disappeared, but reappeared 2 months later. Subsequent
additional chemotherapy included irinotecan plus 5-FU/LV (FOLFIRI)
plus bevacizumab and thereafter irinotecan plus cetuximab. However,
the patient succumbed to progressive disease at 24 months after the
initiation of first-line chemotherapy.
Patient 4 received mFOLFOX6 plus bevacizumab for 5
recurrent bilobular liver metastases at 6 months after
abdomino-perineal resection for stage I poorly differentiated
adenocarcinoma of the lower rectum. After 12 and 13 cycles of
chemotherapy, 2 and 3 lesions disappeared on CT and/or PET/CT,
respectively. Lymph node metastasis along the right internal iliac
artery was suspected after 13 cycles. Therefore, the patient was
started on FOLFIRI plus bevacizumab. Two metastatic lesions
reappeared during chemotherapy. The patient succumbed to
progressive disease at 17 months after the initiation of first-line
chemotherapy.
Patient 5 received mFOLFOX6 plus bevacizumab for 13
synchonous liver metastases and paraaortic lymph node metastasis at
1 month after resection of moderately differentiated adenocarcinoma
of the ascending colon. After 4 and 6 cycles of chemotherapy, 9 and
3 lesions disappeared, respectively. After 9 cycles, the one
remaining lesion disappeared and a marked reduction was also
observed in the size of the lymph node metastasis. An additional 6
cycles of the same regimen were then administered. Lymph node
metastasis was detected in the hepatoduodenal ligament 3 months
later. Percutaneous transhepatic drainage for obstructive jaundice
due to hepatic lymph node metastasis was successful, but the
patient refused additional chemotherapy. The patient succumbed to
disease at 26 months after initiation of first-line
chemotherapy.
Time-to-disappearance and
time-to-recurrence in situ
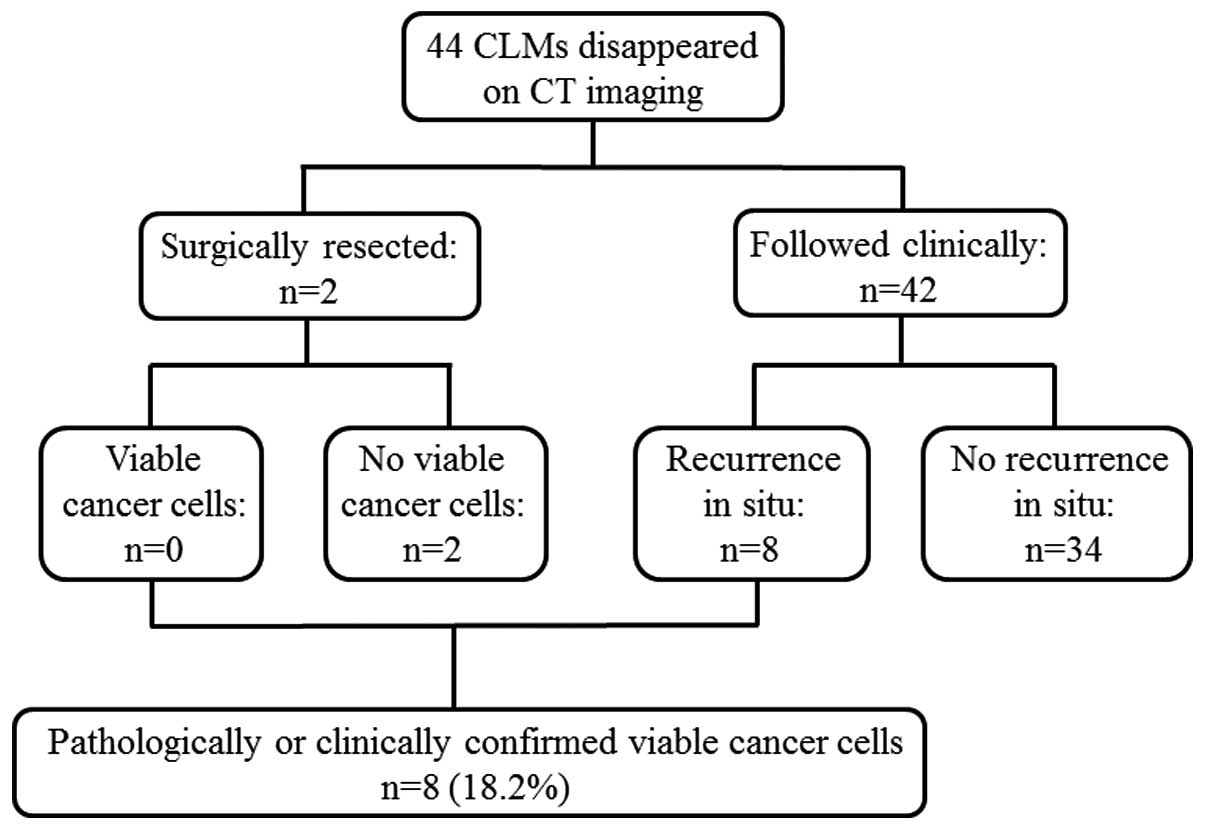
Of the 44 lesions evaluated, 2 were resected,
revealing no viable tumor cells by histological examination. Of the
42 lesions followed clinically with a median follow-up period of
35.4 months (range, 10.5–58.3), 8 recurred in situ and the
remaining 34 did not recur according to radiological evidence. The
crude in situ recurrence rate was 18% (8/44), and the true
complete response rate, meaning either no viable tumor cells on
histological examination or durable local remission of an
unresected site, was 80.5% (36/44; Fig.
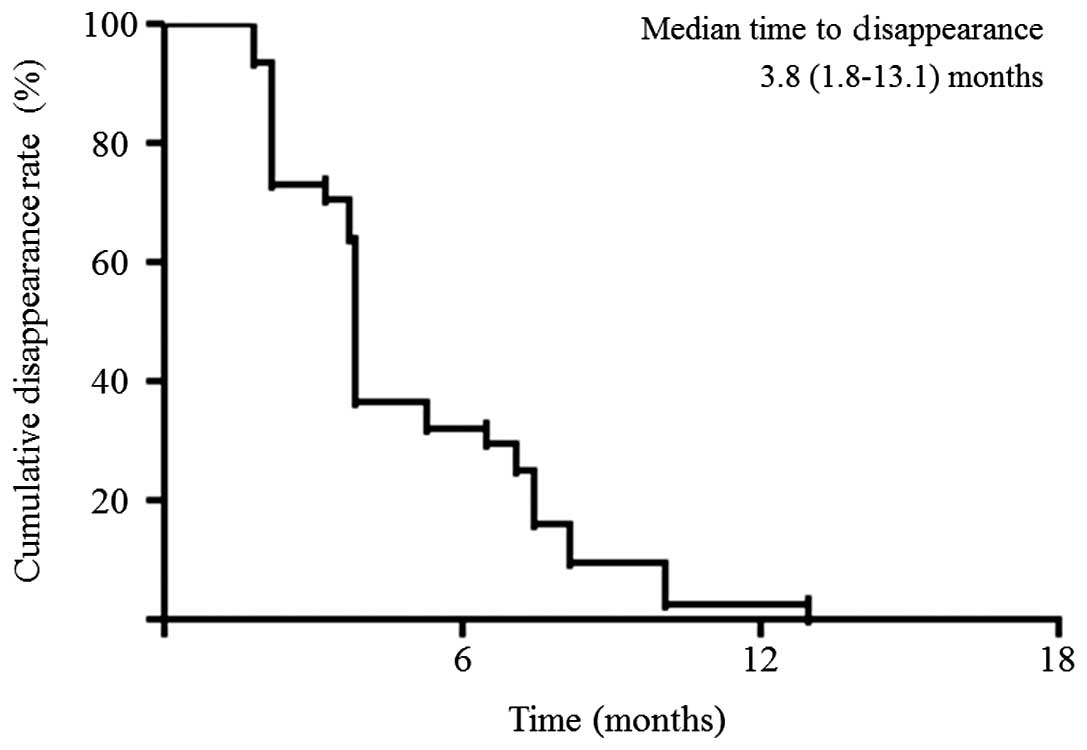
1). The median time-to-disappearance was 3.8 months (1.8–13.1;
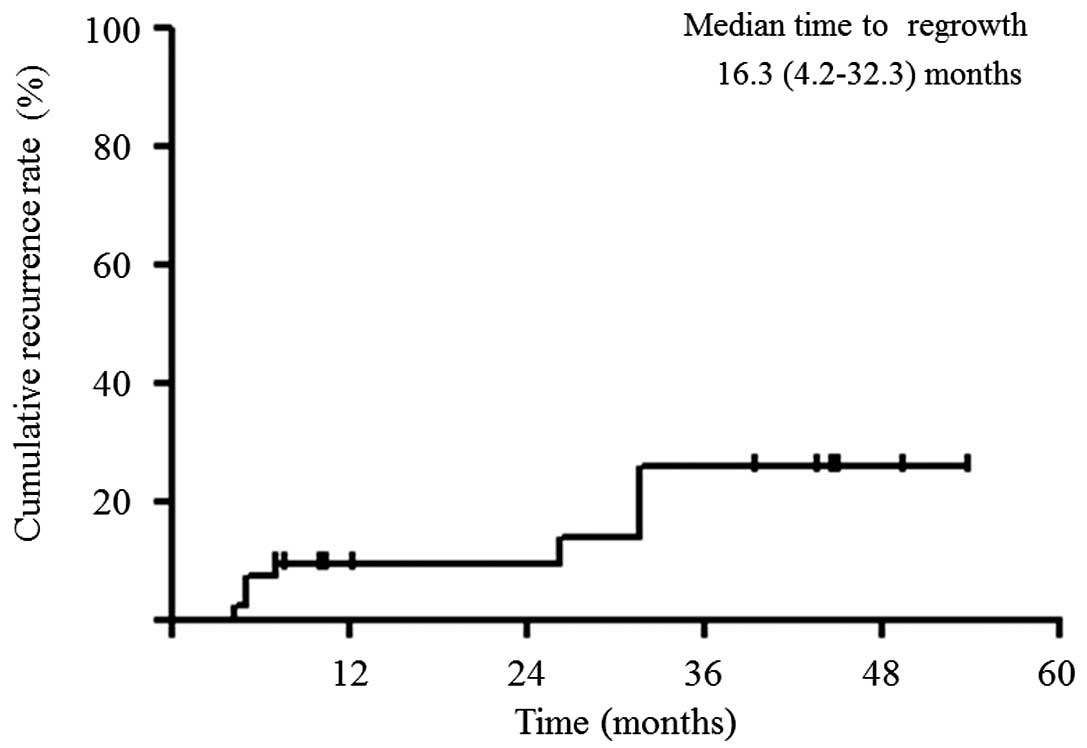
Fig. 2). The cumulative 1-, 2- and
3-year rates of recurrence in situ were 9.1, 9.1 and 31.1%,
respectively (Fig. 3).
Discussion
The optimal treatment strategy for CLMs that have
disappeared due to new and effective chemotherapy regimens remains
to be determined, and a number of problems must be addressed in
deciding what the strategy should be. The number of CLMs which
disappear or show a reduction in size is not important if they are
initially included in the extent of resection. However, when CLMs
involve the entire liver, it becomes necessary to consider how the
lesions should be dealt with when they disappear without apparent
trace. In such cases, a complete cure may be jeopardized if lesions
recur due to incomplete eradication of cancerous cells. In fact, no
data are available on outcome in patients in whom all sites of CLMs
disappearing in situ were left unresected.
In the present study, the true complete response
rate was 18% of disappearing CLMs. The crude recurrence rate in
situ may be influenced by the length of the follow-up period,
making it difficult to compare between studies. Therefore, we
calculated the cumulative rate of in situ recurrence and
demonstrated that the 1-, 2- and 3-year rates were 9.1, 9.1 and
31.1%, respectively.
To the best of our knowledge, including the present
study, only 7 studies (5–10) have evaluated the outcome in
disappearing CLMs following chemotherapy. In 3 of these studies,
patients were treated with either systemic or hepatic arterial
chemotherapy, or both. One study (6) evaluated patients treated with hepatic
arterial chemotherapy only. The molecularly-targeted agent
bevacizumab or cetuximab were used in combination with systemic
chemotherapy in 3 studies, including the present study, with a
variety of incidence, ranging from 7.7 to 80% (6,9). In
the present study oxaliplatin-based chemotherapy (mFOLFOX6) was
used in all 5 patients and in combination with bevacizumab in 4
patients, since the use of mFOLFOX6 plus bevacizumab was one of the
standard therapies for metastatic colorectal cancer during the
study period in Japan. Bevacizumab is also known to improve
oxaliplatin-related hepatic injuries, including sinusoidal
dilatation, sinusoidal obstruction and fibrosis (11), and is thus considered to be suitable
for candidates for hepatectomy after oxaliplatin-based
chemotherapy.
The details of the 5 studies that evaluated
disappearing CLMs on a tumor-by-tumor basis, including our study,
are summarized in Table II. Benoist
et al (5) examined data on
38 hepatectomized patients with a total of 66 CLMs that disappeared
after neoadjuvant systemic chemotherapy with various regimens and
reported that persistent macroscopic or microscopic residual
disease or early recurrence in situ were observed in 55
lesions (83%). When the analysis was restricted to lesions left in
place at surgery, 23 (74%) of 31 CLMs were found to have recurred
in situ. Fiorentini et al examined 48 patients with a
total of 106 CLMs that disappeared following 5-FU-based
intra-arterial chemotherapy and reported persistent macroscopic or
microscopic evidence of residual disease or early recurrence in
situ in 86 lesions (81%) (6).
Auer et al (8) examined data
on 39 hepatectomized patients with 118 disappearing CLMs following
neoadjuvant chemotherapy comprising various regimens. In their
study, 75 of 118 disappearing lesions (64%), the sites of which
were left unresected in subsequent surgery, were considered true
complete responses, including 44 pathological complete responses
and 31 durable clinical complete responses. A total of 19
disappearing CLMs (38%) recurred in situ. Tanaka et
al (10) reported microscopic
evidence of persistent metastases or recurrence in situ in
22 (31%) of 72 CLMs no longer radiographically visible after
neoadjuvant chemotherapy, with 11 (41%) of 27 subsequently
unresected lesions recurring in situ. In another study, van
Vledder et al (9) analyzed
data on 17 hepatectomized patients with disappearing CLMs who were
treated with modern anticancer drugs such as oxaliplatin or
irinotecan, among whom 91.1% received concomitant bevacizumab and
41.1% cetuximab. Of the 45 disappearing CLMs that were unresected,
21 (46.7%) recurred in situ during a median follow-up period
of 20 months. The crude rate of recurrence in situ in our
study (18%) appears to be lower than that reported in earlier
studies, which ranges from 38 to 74%. In terms of the cumulative
rate of recurrence in situ, the Kaplan-Meier curve in our
study appeared identical to or slightly more favorable than that
reported in two previous studies (8,9)
 | Table IIStudies that evaluated disappearing
CLMs on a tumor-by-tumor basis. |
Table II
Studies that evaluated disappearing
CLMs on a tumor-by-tumor basis.
| Author (ref.) | Residual cancer in
resected specimen (%) | Regrowth of
clinically followed lesion (%) | Residual cancer in
disappeared CLMs (%) |
|---|
| Benoist (5) | 12/15 (80.0) | 23/31 (74.2) | 55/66 (83.3) |
| Fiorentini
(6) | Not shown | Not shown | 86/106 (81.1) |
| Tanaka (10) | 11/45 (24.4) | 11/27 (40.7) | 22/72 (30.6) |
| Auer (8) | 24/68 (35.3) | 19/50 (38.0) | 43/118 (36.4) |
| van Vledder
(9) | 41/67 (61.2) | 21/45 (46.7) | 62/112 (55.4) |
| Present study | 0/2 (0.0) | 8/42 (19.0) | 8/44 (18.2) |
CT appears to be the most commonly used imaging
modality in the evaluation of the effect of chemotherapy according
to RECIST criteria (12). It has
been reported that the sensitivity of helical CT is 66–84%
(13–16). In patients with persistent
macroscopic disease at surgery, morphological changes in the
structure of the liver due to chemotherapy, including steatosis,
sinusoidal dilatation and fibrosis, may be responsible for
underestimation of liver metastases (17). This raises the question of whether
other imaging modalities, such as MRI with liver-specific contrast
agents or PET/CT, should be used in patients in whom CLMs are no
longer visible on helical CT. Previous studies evaluating the
outcome of disappearing CLMs used enhanced CT routinely in
combination with ultrasonography (8,9),
contrast-enhanced MRI (10,12) or PET/CT (12). In our study, despite a lack of
sufficient data on the usefulness of these alternative diagnostic
modalities, either enhanced MRI or PET/CT was additionally
performed to confirm judgment of the disappearance of lesions on CT
imaging.
The present study had a number of limitations,
including its retrospective nature and small patient sample.
However, the results suggest that outcome in disappearing CLMs
during oxaliplatin-based chemotherapy is more favorable than
previously reported. Although the precise reason for this
improvement remains unclear, one possible explanation is that 4 of
the 5 patients were administered mFOLFOX6 plus bevacizumab and that
3 of the 5 patients received additional chemotherapy. It should be
noted that there are no supporting data from earlier studies for
this supposition. The present data do suggest, however, that
studies are warranted on a larger series of patients with
disappearing CLMs treated with new anticancer drugs and
molecularly-targeted agents.
In terms of the treatment strategy or approach to
disappearing CLMs, owing to the high rate of in situ
recurrence, Benoist et al (5) noted that i) a complete response on
imaging did not mean cure in most patients; ii) medical oncologists
should refer patients with resectable CLMs to surgeons before any
lesions have completely disappeared; and iii) the sites of lesions
disappearing with chemotherapy should be resected. Elias et
al (7) and Auer et al
(8) reported a satisfactory rate of
in situ recurrence with hepatic arterial chemotherapy,
indicating a satisfactory level of efficacy. However, given the
range of new and effective chemotherapy regimens now available
worldwide, this approach should be reconsidered given the
concomitant technical problems associated with placement and
maintenance of the catheter system. van Vleddler et al
(9) proposed that aggressive
surgery should be considered in patients showing a marked response
to chemotherapy, even when all CLM sites could not be
identified.
Despite the favorable results observed in the
present study, we believe that it is prudent to resect all
initially detected sites of CLMs whenever possible. Taking the
results of earlier studies into consideration, the following
strategies may be appropriate: i) if all the lesions are initially
resectable and chemotherapy is administered in an adjuvant setting,
then the duration of chemotherapy should be limited; and ii) where
preoperative chemotherapy is administered to make initially
unresectable lesions resectable, careful follow-up imaging is
important to ensure that they are not reduced in size to the point
where identifying them intraoperatively would be difficult or
impossible for the surgeon. However, the low rate of in situ
recurrence of approximately 30% at 3 years in our study suggests
that the sites of disappearing CLMs may be left untouched, only
resecting should they recur.
In conclusion, given the low risk of recurrence
in situ, the results of the present study suggest that the
sites of disappearing CLMs may be left unresected but should be
carefully monitored during follow-up, with resection an option if
the lesion should recur. These results provide important data on
the treatment of disappearing CLMs in the era of new and effective
chemotherapy. However, to validate such a treatment strategy,
further investigation with larger series of patients is
warranted.
Acknowledgements
The authors would like to thank
Associate Professor Jeremy Williams, Tokyo Dental College, for his
assistance with the English of the manuscript.
References
|
1.
|
B NordlingerH SorbyeB GlimeliusEORTC
Gastro-Intestinal Tract Cancer Group; Cancer Research UK;
Arbeitsgruppe Lebermetastasen und-tumoren in der Chirurgischen
Arbeitsgemeinschaft Onkologie (ALM-CAO); Australasian
Gastro-Intestinal Trials Group (AGITG); Fédération Francophone de
Cancérologie Digestive (FFCD)Perioperative chemotherapy with
FOLFOX4 and surgery versus surgery alone for resectable liver
metastases from colorectal cancer (EORTC Intergroup trial 40983): a
randomised controlled
trialLancet37110071016200810.1016/S0140-6736(08)60455-9
|
|
2.
|
R WongD CunninghamY BarbachanoA
multicentre study of capecitabine, oxaliplatin plus bevacizumab as
perioperative treatment of patients with poor-risk colorectal
liver-only metastases not selected for upfront resectionAnn
Oncol2220422048201110.1093/annonc/mdq71421285134
|
|
3.
|
C BokemeyerI BondarenkoA
MakhsonFluorouracil, leucovorin, and oxaliplatin with and without
cetuximab in the first-line treatment of metastatic colorectal
cancerJ Clin Oncol27663671200010.1200/JCO.2008.20.839719114683
|
|
4.
|
National Cancer InstituteCommon
terminology criteria for adverse events (CTCAE) v4.0http://ctep.cancer.gov/protocolDevelopment/electronic_applications/ctc.htm#ctc_40.
Accessed April 23, 2012.
|
|
5.
|
S BenoistA BrouquetC PennaComplete
response of colorectal liver metastases after chemotherapy: does it
mean cure?J Clin
Oncol2439393945200610.1200/JCO.2006.05.872716921046
|
|
6.
|
G FiorentiniA Del ConteM De SimoneComplete
response of colorectal liver metastases after intra-arterial
chemotherapyTumori94489492200818822683
|
|
7.
|
D EliasD GoereV BoigeN Kohneh-SharhiD
MalkaG TomasicC DromainM DucreuxOutcome of posthepatectomy-missing
colorectal liver metastases after complete response to
chemotherapy: impact of adjuvant intra-arterial hepatic
oxaliplatinAnn Surg
Oncol1431883194200710.1245/s10434-007-9482-9
|
|
8.
|
RC AuerRR WhiteNE KemenyPredictors of a
true complete response among disappearing liver metastases from
colorectal cancer after
chemotherapyCancer11615021509201010.1002/cncr.2491220120032
|
|
9.
|
MG van VledderMC de JongTM PawlikRD
SchulickLA DiazMA ChotiDisappearing colorectal liver metastases
after chemotherapy: should we be concerned?J Gastrointest
Surg1416911700201020839072
|
|
10.
|
K TanakaH TakakuraK TakedaK MatuoY NaganoI
EndoImportance of complete pathologic response to prehepatectomy
chemotherapy in treating colorectal cancer metastasesAnn
Surg250935942200910.1097/SLA.0b013e3181b0c6e419953712
|
|
11.
|
M KlingerS EpieldauerS HackerBevacizumab
protects against sinusoidal obstruction syndrome and does not
increase response rate in neoadjuvant XELOX/FOLFOX therapy of
colorectal cancer liver metastasesEur J Surg
Oncol35515520200910.1016/j.ejso.2008.12.013
|
|
12.
|
EA EisenhauerP TherasseJ BogaertsNew
response evaluation criteria in solid tumors: revised RECIST
guideline (version 1.1)Eur J
Cancer45228247200910.1016/j.ejca.2008.10.026
|
|
13.
|
S BhattacharjyaT BhattacharjyaS BaberJM
TibballsAF WatkinsonBR DavidsonProspective study of
contrast-enhanced computed tomography, computed tomography during
arterioportography, and magnetic resonance imaging for staging
colorectal liver metastases for liver resectionBr J
Surg9113611369200410.1002/bjs.4699
|
|
14.
|
S BipatMS van LeeuwenEF ComansColorectal
liver metastases: CT, MR imaging, and PET for diagnosis -
meta-analysisRadiology237123131200510.1148/radiol.237104206016100087
|
|
15.
|
C VallasE AndíaA SánchezHepatic metastases
from colorectal cancer: Preoperative detection and assessment of
respectability with helical
CTRadiology2185560200110.1148/radiology.218.1.r01dc115511152779
|
|
16.
|
D EliasL SiderisM PocardIncidence of
unsuspected and treatable metastatic disease associated with
operable colorectal liver metastases discovered only at laparotomy
(and not treated when performing percutaneous radiofrequency
ablation)Ann Surg Oncol12298302200510.1245/ASO.2005.03.020
|
|
17.
|
FG FernandezJ RitterJW GoodwinDC LinehanWG
HawkinsSM StrasbergEffect of steatohepatitis associated with
irinotecan or oxaliplatin pretreatment on resectability of hepatic
colorectal metastasesJ Am Coll
Surg200845853200510.1016/j.jamcollsurg.2005.01.024
|