Introduction
Colon cancer is one of the most common malignant
tumors in humans, and the occurrence and development of colon
cancer are closely correlated with lifestyle, genetic factors,
mutations and adenoma. The consumption of heat-treated meat is a
risk factor for colon cancer, as meat cooked at a high temperature
may produce carcinogenic heterocyclic amines. It has been reported
that heterocyclic amines can cause DNA damage in intestinal
epithelial cells in patients with colon cancer (1). Uridine diphosphate glucuronyl
transferase (UGT), one of the most important phase II metabolic
enzymes for biotransformation, catalyzes the binding of N-hydroxy
compounds and glucuronic acid, which in turn prevents the DNA
mutagenesis caused by heterocyclic amines (2). The UGT family, mainly composed of the
UTG1A and UTG2B subfamilies, is expressed in the liver,
gastrointestinal tract, gall bladder and breast (3–5).
Certain UTG1A isoforms, including UTG1A7, UTG1A8 and UTG1A10, are
mainly distributed in colonic mucosa, and exert a protective effect
through the glucuronide reaction (6). A transcription factor, nuclear factor
erythroid-E2-related factor 2 (Nrf2), plays a key role in the
maintenance of cell redox homeostasis. Kelch-like ECH-associated
protein-1 (Keap1) is a negative regulation factor of Nrf2. When the
body is stimulated by chemical substances, carcinogens or oxidative
stress, the Nrf2/Keap1 signaling pathway is activated and Nrf2
enters the nucleus to regulate the gene expression of
detoxification enzymes. Hence, the Nrf2/Keap1 signaling pathway
functions as a key system in response to stress stimulation. It has
been suggested that the Nrf2/Keap1 pathway affects cell
proliferation and the response of the body to chemotherapy in lung,
head and neck, breast and pancreatic cancers (7,8). In
the present study, we investigated the expression of UGT1A, Nrf2
and Keap1 in colon cancer and elucidated their correlation with the
clinicopathological features of patients with colon cancer.
Materials and methods
Materials
All specimens were acquired from surgical patients
or patients undergoing digestive endoscopic inspection admitted to
Qilu Hospital of Shandong University between March 2010 and June
2011. There were 77 patients with colon cancer, including 41 males
and 36 females, who had an average age of 58.6 years. None of the
patients were treated with chemotherapy, radiation therapy or other
biological treatment, and none had a long-term regular medication
history. All patients were pathologically diagnosed with colon
cancer. There were 30 patients with colon adenoma, including 19
males and 11 females, with an average age of 56.1 years. None of
the patients had a long-term regular medication history. All
patients were pathologically diagnosed with colon adenoma
(including tubular, villous and tubular villous adenoma based on
the Morson classification). In addition, normal colonic mucosa
specimens were acquired from 24 healthy subjects (confirmed by
electronic colonoscopy) and volunteers (13 males and 11 females)
who had an average age of 50.0 years. The major clinical medical
records included gender, age, tumor grade, tumor infiltration and
lymph node metastasis. All specimenrs were fixed with 4%
paraformaldehyde and embedded with paraffin and slices at a
5-μm thickness were prepared. The stage and grade of colon
cancer were determined by the American Joint Committee on
Cancer/Union for International Cancer Control, (AJCC/UICC; Table I).
 | Table IExpression of Nrf2, Keap1 and UGT1A
in colonic tissues. |
Table I
Expression of Nrf2, Keap1 and UGT1A
in colonic tissues.
| Group | Cases (n) | UGT1A
| Nrf2
| Keap1
|
|---|
| No. positive | Positive rate
(%) | P-value | No. positive | Positive rate
(%) | P-value | No. positive | Positive rate
(%) | P-value |
|---|
| Normal colonic
mucosa | 24 | 20 | 83.3 | 0.071a | 10 | 41.7 | 0.000a | 13 | 54.2 | 0.200a |
| Adenoma tissue | 30 | 24 | 80.0 | 0.019b | 21 | 70.0 | 0.000b | 21 | 70.0 | 0.002b |
| Adenocarcinoma
tissue | 77 | 41 | 53.2 | 0.023c | 67 | 87.0 | 0.000c | 47 | 61.0 | 0.040c |
Reagents
A rabbit anti-human polyclonal antibody specific for
Keap1 (1:150) was purchased from ABCAM (Cambridge, MA, USA). Rabbit
anti-human polyclonal antibodies specific for Nrf2 (1:100) and
UTG1A (1:150) were purchased from Santa Cruz Biotechnology, Inc.
(Santa Cruz, CA, USA). The immunohistochemistry kit (PV-9001) and
DAB chromogenic kit were purchased from Beijing Zhongshan Golden
Bridge Biotechnology Company (Beijing, China).
Immunohistochemistry
The slices of 5 μm thickness were dewaxed,
hydrated and washed with PBS for 5 min, twice. The slices were
immersed in a 92–98°C citrate buffer solution for 20–30 min and
then cooled down for 30 min. After being washed with PBS three
times, the slices were treated with 3% H2O2
for 15 min and washed with PBS three times. The slices were
incubated with primary antibodies (diluted in PBS) at 4°C overnight
and a control group was established with the antibody replaced with
PBS. The following day, the slices were incubated at 37°C for 30
min and washed with PBST three times. After being dried, the slices
were treated with reagent 1 (Polymer Helper), incubated at 37°C for
20 min and washed with PBST three times. The slices were then
treated with reagent 2 (poly-HRP anti-rabbit IgG), incubated at
37°C for 20 min and washed with PBST three times. The slices were
then stained with DAB. After being washed with water, the slices
were stained with hematoxylin and washed with water. The slices
were treated with 1% HCl-ethanol to degrade the excessive
hematoxylin for a few seconds and then immersed in ammonia for 3
min. After being washed with water twice, the slices were
dehydrated and mounted with gum. The slices were observed under a
light microscope and images were captured and stored on a computer
for analysis.
Data analysis
The Nrf2-positive cells were determined by brown
stained cytoplasm or nucleus and the Keap1- and UGT1A-positive
cells were determined by brown stained cytoplasm. As described
previously (8,9), the ratio of positive cells and the
intensity of the staining were analyzed as follows: A, score based
on the ratio of stained cells (<1/3 positive cells, 1 point;
1/3–2/3, 2 points; ≥2/3, 3 points); B, score based on the intensity
of staining (no positive cell, 0 point; pale brown, 1 point; brown,
2 points; deep brown, 3 points). The integrated score = A x B. The
final result was determined as negative if the integrated score was
0 and positive if the score was greater than or equal to 1. All
pathological and immunohistochemical analysis was performed by two
pathology professors.
Statistical analysis
All data analysis was performed using SPSS 13.0
software (SPSS, Inc., Chicago, IL, USA). The data were analyzed
using the Chi-square test and Spearman rank correlation analysis.
P<0.05 was considered to indicate a statistically significant
result.
Results
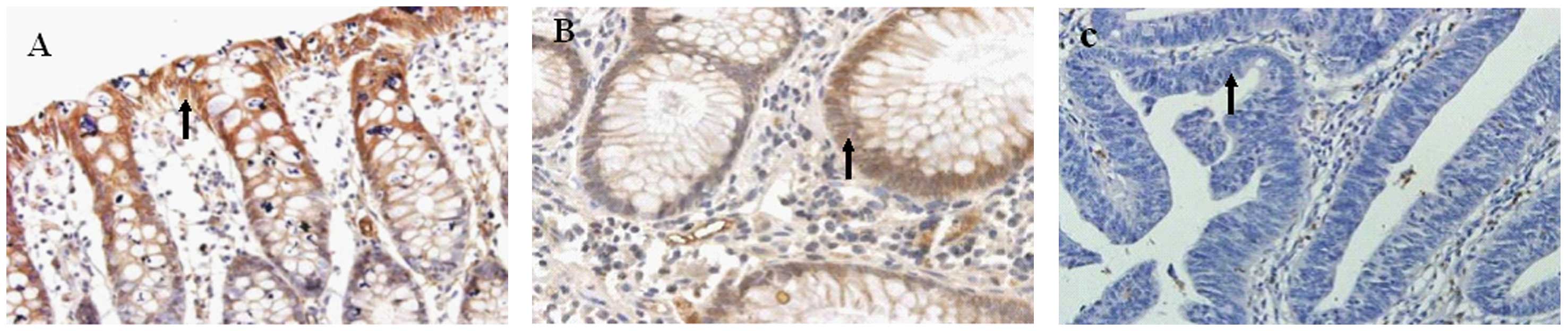
Expression of UGT1A in normal colonic
mucosa, adenoma tissue and adenocarcinoma tissue
In the normal colonic mucosa, UGT1A was distributed
in the epithelial cell cytoplasm, especially the perinuclear area,
with a positive rate of 83.3% (20/24), and the staining intensity
markedly increased from the base of the crypt to the surface
epithelial cells. In adenoma tissue, the positive rate of UGT1A was
80.0% (24/30), but the staining intensity did not markedly increase
from the base of the crypt to the surface epithelial cells
(Table I; Fig. 1). Comparing these two groups, we
found no significant difference (P=0.071). In the adenocarcinoma
tissue, UGT1A showed a slight expression in the cytoplasm with a
significantly reduced positive rate of 53.2% (41/77; Table I; Fig.
1). Compared with the normal colonic mucosa and the adenoma
tissue, the adenocarcinoma tissue was significantly different
(P=0.023 and P=0.019, respectively). Of the 6 cases of negative
adenoma tissue, 4 cases showed intraepithelial neoplasia at
different degrees. Furthermore, the expression of UGT1A in colon
cancer was not correlated with gender, age, tumor infiltration or
lymph node metastasis (P>0.05; Table
II).
 | Table IICorrelation between expression of
Nrf2, Keap1 and UGT1A and the clinicopathological characteristics
of adenocarcinoma. |
Table II
Correlation between expression of
Nrf2, Keap1 and UGT1A and the clinicopathological characteristics
of adenocarcinoma.
| Group | Cases (n) | UGT1A
| Nrf2
| Keap1
|
|---|
| No. positive | Positive rate
(%) | P-value | No. positive | Positive rate
(%) | P-value | No. positive | Positive rate
(%) | P-value |
|---|
| Gender | | | | 0.179 | | | 0.946 | | | 0.982 |
| Male | 41 | 22 | 53.7 | | 36 | 87.8 | | 25 | 61.0 | |
| Female | 36 | 19 | 52.8 | | 31 | 86.1 | | 22 | 61.1 | |
| Age (years) | | | | 0.692 | | | 0.693 | | | 0.433 |
| <60 | 44 | 26 | 59.1 | | 38 | 86.4 | | 25 | 56.8 | |
| ≥60 | 33 | 15 | 45.5 | | 29 | 87.9 | | 22 | 66.7 | |
|
Differentiation | | | | 0.656 | | | 0.549 | | | 0.044 |
| High/medium | 50 | 26 | 52.0 | | 44 | 88.0 | | 35 | 70.0 | |
| Low | 27 | 15 | 55.6 | | 23 | 85.2 | | 12 | 44.4 | |
| Tumor
infiltration | | | | 0.122 | | | 0.318 | | | 0.009 |
| T1-T2 | 33 | 13 | 39.4 | | 29 | 87.9 | | 26 | 78.8 | |
| T3-T4 | 44 | 28 | 63.6 | | 38 | 86.4 | | 21 | 47.7 | |
| Lymph node
metastasis | | | | 0.157 | | | 0.197 | | | 0.275 |
| N0 | 50 | 25 | 50.0 | | 42 | 84.0 | | 33 | 66.0 | |
| Nx (x≥1) | 27 | 16 | 59.3 | | 25 | 92.6 | | 14 | 51.9 | |
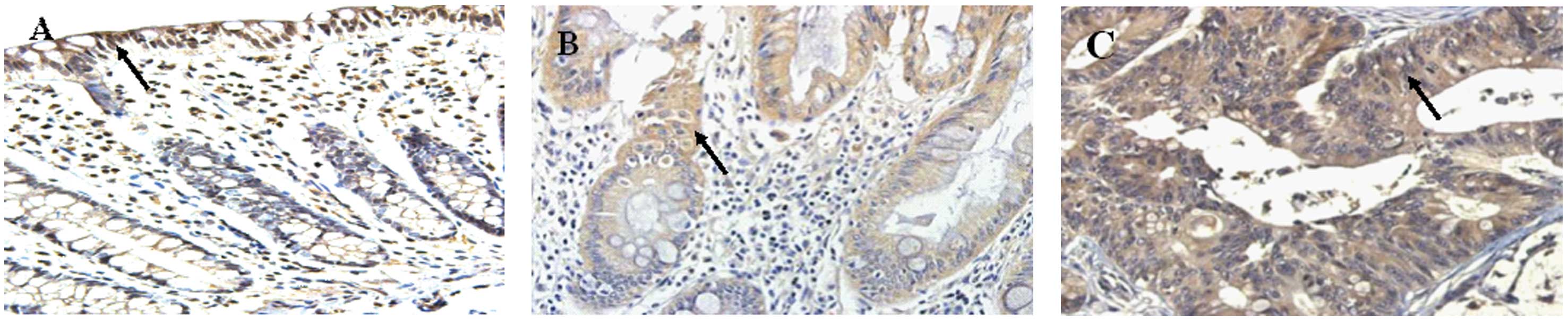
Expression of Nrf2 in normal colonic
mucosa, adenoma tissue and adenocarcinoma tissue
The positive rate of Nrf2 increased from 41.7%
(10/24) in normal colonic mucosa to 70.0% (21/30) in the adenoma
tissue, and further to 87.0% (61/77) in the adenocarcinoma tissue.
Nrf2 was expressed in the cytoplasm and nuclei of the normal
colonic mucosa, but only in the cytoplasm of the adenoma tissue and
the adenocarcinoma tissue (Table I;
Fig. 2). The expression level of
Nrf2 was significantly different among the three types of tissues
(normal colonic vs. adenoma tissue, P=0.000; normal colonic vs.
adenocarcinoma tissue, P=0.000; adenoma vs. adenocarcinoma tissue,
P=0.000). The expression of Nrf2 in tissues with different degrees
of tumor differentiation (high/medium vs. low differentiation) or
infiltration (T1+T2 vs. T3+T4) did not show any significant
difference (P>0.05; Table II).
However, Nrf2 expression was detected in the vascular muscle cells
of tumors, and the expression level increased from the mucosa of
the cancer tissue to the muscle tissue layer. In the adenocarcinoma
tissue, the expression level of Nrf2 was not correlated with age or
gender (Table II).
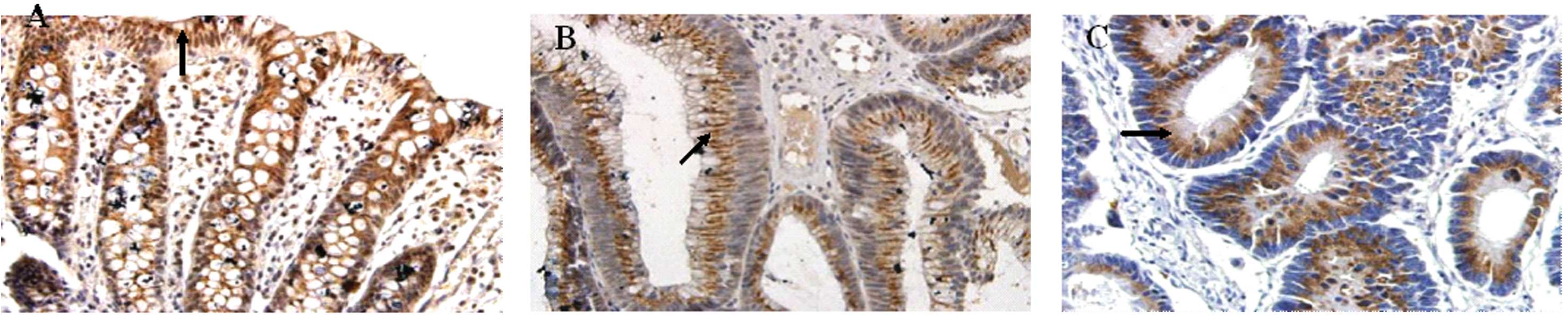
Expression of Keap1 in normal colonic
mucosa, adenoma and adenocarcinoma tissue
In the three types of tissues, Keap1 was only
expressed in the cytoplasm, with a granular distribution (Fig. 3). The positive rate of Keap1 was
54.2% (13/24) in normal colonic mucosa and 70.0% (21/30) in the
adenoma tissue, and no significant difference was found between the
two tissues (P=0.200). In the 77 cases of adenocarcinoma tissue, 47
showed positive Keap1 expression, leading to a positive rate of
61.0%. The expression of Keap1 was significantly different among
the tissues with different degrees of tumor differentiation or
infiltration (P<0.05; Table II).
Compared with the normal colonic mucosa and the adenoma tissue, the
adenocarcinoma tissue showed a significant difference in the
expression level of Keap1 (normal colonic mucosa vs. adenoma
tissue, P=0.200; normal colonic vs. adenocarcinoma tissue, P=0.040;
adenoma vs. adenocarcinoma, P=0.002).
Correlation between the expression of
Nrf2 and Keap1 in normal colonic mucosa, adenoma tissue and
adenocarcinoma tissue
The expression levels of Keap1 and Nrf2 were
significantly correlated in normal colonic mucosa (r=0.583,
P=0.003), but they were not correlated in either the adenoma
(r=0.067, P=0.723) or the adenocarcinoma tissues (r=0.042,
P=0.715).
Discussion
The binding reaction of N-hydroxy compounds and
glucuronic acid is common in vivo, and significantly affects
the metabolism of numerous drugs, toxins and carcinogens. UGT1A
degrades the majority of toxins and carcinogens (10). Amines in epithelial cells may be
further activated to form DNA adducts, which in turn activate the
carcinogenic process by gene mutation. We found that UGT1A was
highly and continuously expressed in normal colonic mucosa, while
it had low or no expression in the adenoma and adenocarcinoma
tissues with intraepithelial neoplasia. These observations indicate
that UGT1A has a significant inhibitory effect in the early stage
of colonic malignant transformation. The results of our previous
study suggested that the protein and mRNA expression of UGT1A and
its isoforms were decreased in adenocarcinoma tissue (11). In the present study, we found that
although the UGT1A expression level was similar in normal colonic
mucosa and adenoma tissue, 4 of the 6 cases of UGT1A-negative
adenoma tissue showed intraepithelial neoplasia. We further found
that the expression of UGT1A was decreased in the adenocarcinoma
tissue. It has been suggested that UGT1A has only a slight, if any,
expression level in the liver tissues with colon cancer metastases
and the lymph nodes, indicating that the downregulation of UGT1A is
critical for the development and infiltration of tumors (12). In the present study, we investigated
the distribution of UGT1A using the immunohistochemical approach,
which indicates the detoxification function of UGT1A. We found that
UGT1A was mainly distributed in the crypt epithelium and the
endometrial epithelial cells of glandular organs. As the amine
carcinogens in the body are usually in contact with the intestinal
mucosa, the high expression level of UGT1A in the intestinal cells
acts as a chemical barrier against the carcinogenic molecules.
However, UGT1A did not show an marked distribution pattern in
adenoma tissues. Our previous study (13) suggested that UGT1A and its isoforms
had lower catalytic activities compared with normal colonic mucosa
and the UGT1A8 mutant was detected in colon cancer tissues. We thus
proposed that UGT1A had a lower activity and an abnormal expression
level and was unable to degrade the toxic substances quickly, which
in turn prolonged the contact of toxic molecules with the mucosa to
increase the risk of cancer occurrence.
Nrf2 is an important transcription factor which
regulates the activity of phase II metabolic enzymes and plays a
significant role in protecting cells against the oxidative stress
caused by electrophilic substances and reactive oxygen species
(14–16). Our results indicate that Nrf2 was
mostly distributed in the nuclei, not the cytoplasm, in the normal
colonic mucosa, while the opposite was found for the adenocarcinoma
tissue. This was probably caused by gene mutation. A previous study
on squamous cell carcinoma suggested that the gene mutation rate of
Nrf2 was approximately 95.5% (17).
In the adenocarcinoma tissue, the high expression of Nrf2 in the
cytoplasm probably represented the overexpression of Nrf2 mutants,
which resulted in the abnormal nuclear translocation and the less
effective activation of phase II detoxifying enzyme gene
expression, and subsequently promoted the occurrence of
carcinogenesis. Our previous study on colon cancer Caco-2 cells
suggested that the plant chemical sulforaphane promotes the nuclear
translocation of Nrf2, while in the control group, Nrf2 was mostly
located in the cytoplasm (18).
This was likely caused by the Nrf2 gene mutations induced by
oxidative stress or carcinogenic damage. In the present study, we
found that Nrf2 was expressed in the vascular muscle cells of
tumors, and that the expression level increased from the mucosa of
the cancer tissue to the muscle tissue layer, indicating that Nrf2
is involved in cancer invasion and metastasis. It has been
previously suggested that Nrf2 is involved in the invasion and
metastasis of gastric cancer (19).
Usually, the expression of Nrf2 is regulated by its
negative regulating factor, Keap1, to maintain a stable low
expression level. We found that Keap1 was highly expressed in the
adenoma and adenocarcinoma tissues, and its expression level was
correlated with the infiltration and differentiation of
adenocarcinoma, indicating that Keap1 is involved in the
development of cancer. The expression levels of Nrf2 and Keap1 were
correlated in normal colonic mucosa, but not in the adenoma or
adenocarcinoma tissues. These results indicate that the imbalance
in their expression may be involved in the development of cancer.
In this case, the imbalance may be caused by the upregulated
expression of proteins that compete for the binding sites of
Nrf2/Keap1, including sequestosome-1 and prorhymosin-α (20–22),
which results in the slower degradation of Keap1 and the
overexpression of Nrf2. It has been suggested that HNE modifies
Keap1, allowing Nrf2 to enter the nucleus to take effect (23,24).
Future cellular studies are required to elucidate the effects of
abnormal regulation and expression of the Nrf2/Keap1 pathway on the
development of cancer. Changes in the expression of UGT1A, Nrf2,
and Keap1 may be used as early indicators for the development of
cancer to facilitate cancer intervention therapy on a molecular
basis.
Acknowledgements
This study was supported by grants
from Science Bonus Funds for Young Scientists of Shandong Province
(No. 2007BS03017) and Natural Science Foundation of Shandong
Province (No. Y2008C115).
References
|
1.
|
TS DanielMG LisaS BarbaraInhibition of
fried meat-induced colorectal DNA damage and altered systemic
genotoxicity in humans by crucifera, chlorophyllin, and yogurtPLoS
One64111201121541030
|
|
2.
|
MA MalfattiJS FeltonN-glucuronidation of
2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine (PhIP) and
N-hydroxy-PhIP by specific human
UDP-glucuronosyltransferasesCarcinogenesis22108710932001
|
|
3.
|
L GiulianiP GazzangaF CaporuscioCan
down-regulation of UDP-glucuronosyltransferases in the urinary
bladder tissue impact the risk of chemical cacinogenesis?Int J
Cancer91141143200110.1002/1097-0215(20010101)91:1%3C141::AID-IJC1005%3E3.0.CO;2-H11149414
|
|
4.
|
WM McDonnellE HitomiFK
AskariIdentification of bilirubin UDP-GTs in the human alimentary
tract in accordance with the gut as a putative metabolic
organBiochem Pharmacol51483488199610.1016/0006-2952(95)02224-4
|
|
5.
|
SA GestlMD GreenDA ShearerExpression of
UGT2B7, a UDP-glucuronosyltransferase implicated in the metabolism
of 4-hydroxyestrone and all-trans retinoic acid, in normal human
breast parenchyma and in invasive and in situ breast cancersAm J
Pathol16014671479200210.1016/S0002-9440(10)62572-211943730
|
|
6.
|
CP StrassburgN NguyenMP MannsRH
TukeyPolymorphic expression of UDP-glucuronosyltransferases UGT1A
gene locus in human gastric epitheliumMol
Pharmacol5464765419989765507
|
|
7.
|
LM SolisC BehrensW DongNrf2 and Keap1
abnormalities in non-small cell lung carcinoma and association with
clinicopathologic featuresClin Cancer
Res1637433753201010.1158/1078-0432.CCR-09-335220534738
|
|
8.
|
A ListerT NedjadiNR KitteringhamNrf2 is
overexpressed in pancreatic cancer: implications for cell
proliferation and therapyMol
Cancer1037201110.1186/1476-4598-10-3721489257
|
|
9.
|
S DangoW SienelM SchreiberElevated
expression of carcinoembryonic antigen-related cell adhesion
molecule 1 (CEACAM-1) is associated with increased angiogenic
potential in non-small-cell lung cancerLung
Cancer60426433200810.1016/j.lungcan.2007.11.01518215438
|
|
10.
|
J MinersRA McKinnonPI MackenzieGenetic
polymorphisms of UDP-glucuronosyltransferases and their functional
significanceToxicology181453456200210.1016/S0300-483X(02)00449-312505351
|
|
11.
|
M WangYQ LiDF SunPolymorphic expression of
UDP-glucuronosyltransferase UGT1A gene locus in human colorectal
epitheliumZhongguo Bing Li Sheng Li Xue Hui21131513202005
|
|
12.
|
L GiulianiM CiottiA
StoppacciaroUDP-glucuronosyltransferases 1A expression in human
urinary bladder and colon cancer by immunohistochemistryOncol
Rep13185191200515643497
|
|
13.
|
M WangDF SunYQ LiPolymorphism of
UDP-glucuronosyltransferase UGT1A8 gene in human colorectal
cancerShi Jie Hua Ren Xiao Hua Za Zhi13181918232005
|
|
14.
|
K ChanXD HanYW KanAn important function of
Nrf2 in combating oxidative stress: detoxification of
acetaminophenProc Natl Acad Sci
USA9846114616200110.1073/pnas.08108209811287661
|
|
15.
|
T RangasamyCY ChoRK ThimmulappaGenetic
ablation of Nrf2 enhances susceptibility to cigarette smoke-induced
emphysema in miceJ Clin
Invest11412481259200410.1172/JCI20042114615520857
|
|
16.
|
M Ramos-GomezMK KwakPM DolanSensitivity to
carcinogenesis is increased and chemoprotective efficacy of enzyme
inducers is lost in nrf2 transcription factor-deficient miceProc
Natl Acad Sci USA9834103415200110.1073/pnas.05161879811248092
|
|
17.
|
YR KimJE OhMS KimOncogenic NRF2 mutations
in squamous cell carcinomas of oesophagus and skinJ
Pathol220446451201010.1002/path.265319967722
|
|
18.
|
M WangYQ LiN ZhongInduction of uridine
5′diphosphate-glucuronosyltransferase gene expression by
sulforaphane and its mechanism: experimental study in human colon
cancer cellsZhonghua Yi Xue Za Zhi858198242005
|
|
19.
|
HB WangCJ ZhouSZ SongEvaluation of Nrf2
and IGF-1 expression in benign, premalignant and malignant gastric
lesionsPathol Res
Pract207169173201110.1016/j.prp.2010.12.00921367536
|
|
20.
|
IM CoppleA ListerAD ObengPhysical and
functional interaction of sequestosome 1 with Keap1 regulates the
Keap1-Nrf2 cell defense pathwayJ Biol
Chem2851678216788201010.1074/jbc.M109.09654520378532
|
|
21.
|
M KomatsuH KurokawaS WaguriThe selective
autophagy substrate p62 activates the stress responsive
transcription factor Nrf2 through inactivation of Keap1Nat Cell
Biol12213223201020173742
|
|
22.
|
RN KarapetianAG EvstafievaIS AbaevaNuclear
oncoprotein prothymosin alpha is a partner of Keap1: implications
for expression of oxidative stress-protecting genesMol Cell
Biol2510891099200510.1128/MCB.25.3.1089-1099.200515657435
|
|
23.
|
CM MahaffeyH ZhangA
RinnaMultidrug-resistant protein-3 gene regulation by the
transcription factor Nrf2 in human bronchial epithelial and
non-small-cell lung carcinomaFree Radic Biol
Med4616501657200910.1016/j.freeradbiomed.2009.03.02319345732
|
|
24.
|
S PiccirilloG FilomeniB BrüneRedox
mechanisms involved in the selective activation of Nrf2-mediated
resistance versus p53-dependent apoptosis in adenocarcinoma cellsJ
Biol Chem2842772127733200910.1074/jbc.M109.014837
|