Introduction
Osteosarcoma (OS) is the most common tumor in bone
and the third most common tumor in childhood and adolescence
(1). A significant number of
studies on OS were published during the 1950s and 1960s, revealing
poor 5-year survival rates of less than 15% (2). It was not until the early 1970s that
the introduction of doxorubicin and methotrexate with leucovorin
showed promise in improving the survival rates. Following the
discovery of effective chemotherapy, the 5-year survival rates for
patients treated with intensive multidrug chemotherapy and
aggressive local control have been reported to be 55–80% (3–5);
however, an improvement in survival rates has been limited only to
the patients with a high grade of disease (5). Unfortunately, patients with metastatic
disease have a poor prognosis, particularly those with pulmonary
metastases at diagnosis, with various studies reporting 5-year
survival rates of only 17–23% (6–8).
Therefore, it is necessary to determine the mechanisms contributing
to the metastasis of OS. Although many molecular factors linked to
metastasis have been identified, a significant number still need to
be investigated to provide a new therapeutic target for metastatic
diseases in OS.
Fatty acid synthase (FASN) is an enzyme crucial for
endogenous lipogenesis in mammals and is responsible for catalyzing
the synthesis of long-chain fatty acids. FASN is expressed at high
levels in a variety of human tumors (9–16), but
shows low expression levels in normal tissues. This overexpression
in neoplastic tissues makes FASN a potential diagnostic tumor
marker. The inhibition of FASN activity is selectively cytotoxic to
human cancer cells in vitro and in vivo (17,18).
Thus, FASN is considered a novel and promising target for
antineoplastic therapy. Recent studies revealed that the inhibition
of FASN promotes apoptosis and reduces cell growth and lymph node
metastasis in a mouse melanoma model (19). In Colon 26 and CMT 93 cells, FASN
contributed to metastasis by upregulating the activity of the AKT
pathway (20). However, the
expression of FASN and the correlation between FASN and metastasis
in OS is still uncertain.
In the present study, we investigated FASN and Ki-67
protein expression levels in OS metastases using
immunohistochemistry (IHC). We evaluated the correlation between
FASN and Ki-67 expression levels in OS. Furthermore, the
correlation between the expression levels of FASN and Ki-67 in OS
tissues and OS metastasis was evaluated. Our findings revealed that
there was a significant correlation between FASN, and Ki-67
expression levels and OS metastasis, and between FASN and Ki-67
protein expression levels. This suggests that FASN may be involved
in OS metastasis and may be a promising target for treating OS
metastasis.
Materials and methods
Patients and tumor specimens
From 2005 to 2009, 136 patients with histologically
proven OS of the extremities were treated at the First Affiliated
Hospital of Nanchang University, China. There were 44 cases with
pulmonary metastases and 92 cases without metastases at diagnosis.
All of the specimens were obtained by excision biopsy. The analysis
of pulmonary metastases was performed with CT scans at the time of
the first diagnosis. No patients had a history of prior therapies
with anticancer drugs or radiotherapy. The samples were fixed with
10% formalin, embedded in paraffin and then cut into 2-μm
sections. Twenty-one osteoenchondroma tissue samples were used as
controls. The ethics committee of the First Affiliated Hospital of
Nanchang University approved the study, and patients provided
informed consent.
Immunohistochemistry for Ki-67 and
FASN
Histological sections were cut at 2 μm and
stained with hematoxylin and eosin (H&E). These sections
underwent immunohistochemical analysis which was performed using
the streptavidin-peroxidase (SP) procedure. Antigen retrieval was
performed by heating the deparaffinized rehydrated sections in 10
mm citrate buffer (pH 6.0 for 20 min) followed by blocking with 10%
goat serum. The sections were subsequently incubated overnight at
4°C with the primary antibody (rabbit anti-FASN monoclonal
antibody, Santa Cruz Biotechnology Inc., Santa Cruz, CA, USA;
anti-Ki-67, Dako North America, Carpinteria, CA, USA). For the
negative controls, the sections were incubated with
phosphate-buffered saline (PBS) instead of antibodies. After
washing with PBS three times, the sections were incubated with the
biotinylated secondary antibody for 40 min, followed by incubation
with horseradish peroxidase (HRP)-conjugated streptavidin for 30
min. Finally, the sections were chemiluminescence-stained and
counterstained using H&E. The immunostaining was evaluated by
two independent pathologists without knowledge of the clinical and
pathological parameters.
Quantitative method
The expression levels of FASN were determined
according to the staining intensity of at least 500 cells in 5
representative areas. Their intensity scores were recorded as: no
staining (0); weak staining (1); moderate staining (2) and intense
staining (3). The percentage scores were recorded as: less than 1%
(score 0), less than 10% (score 1), 11 to 50% (score 2), 51 to 80%
(score 3) and 81 to 100% (score 4). The final score was averaged
with the scores from the two pathologists, which were calculated by
the addition of the intensity score to the percentage score. The
sections with a final score of less than 4 were considered as (−),
score 4 was considered as (+), score 5 as (++) and more than 6 was
considered as (+++). In all cases, brown staining in the cytoplasm
was adopted as the standard for positivity.
For Ki-67 expression, a minimum of 400 cells were
counted on each slide at a magnification of x400, irrespective of
whether they had been stained by anti-Ki-67 or not. In each case, 3
visual fields were evaluated. The percentage of stained cells for
each case was obtained from the ratio between the number of cells
with stained nuclei and unstained nuclei multiplied by 100 (label
index).
Statistical analysis
All analyses were performed using the Statistical
Package for the Social Sciences (SPSS Version 13.0, Chicago, IL,
USA). Two independent samples were used to analyze the difference
between FASN expression levels in OS and osteoenchondroma tissues,
and between OS patients with and without pulmonary metastasis. An
independent samples t-test was used to analyze the difference
between Ki-67 expression levels in OS and in osteoenchondroma
tissues, and between OS patients with and without pulmonary
metastasis. The correlation of FASN with Ki-67 protein in OS
tissues was evaluated using Spearman’s rho. A value of p<0.05
was considered to indicate a statistically significant result.
Results
FASN and Ki-67 protein expression in OS
and osteoenchondroma
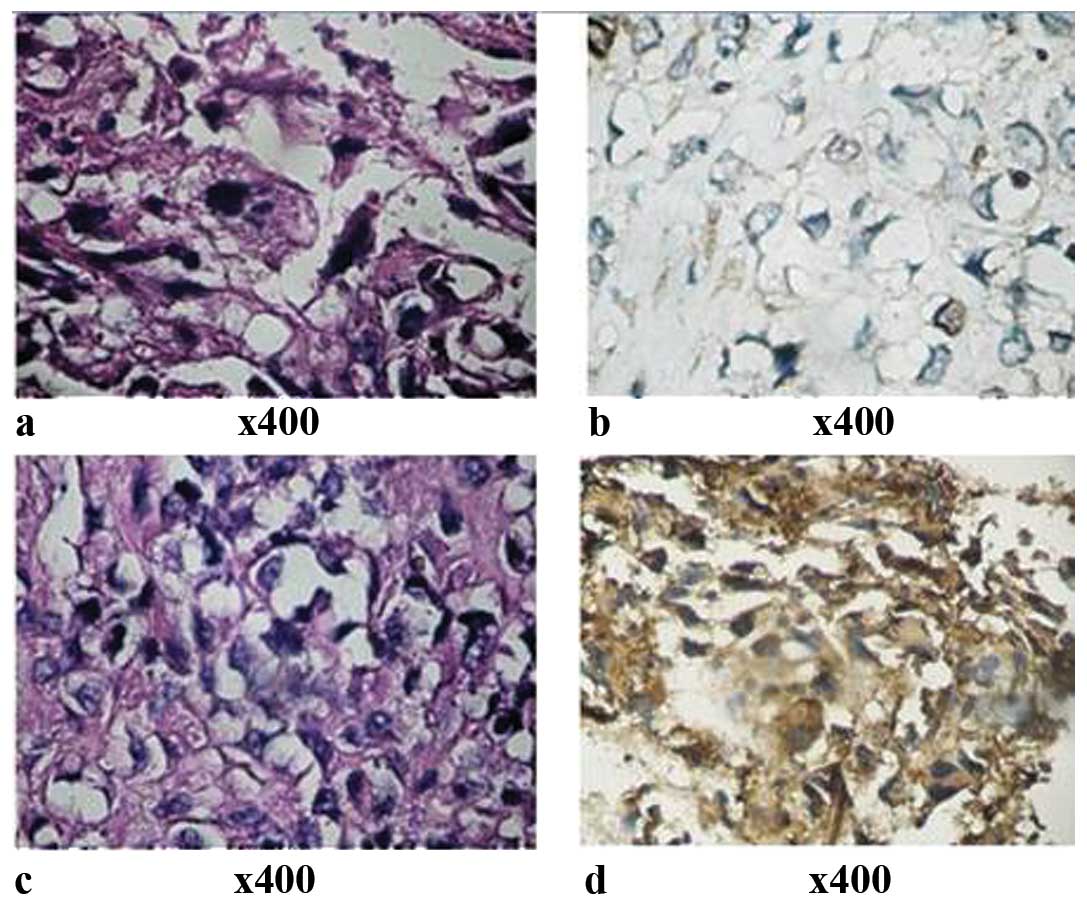
FASN protein was expressed in the cytoplasm and
Ki-67 was expressed in the nuclei of OS and osteoenchondroma
tissues (Fig. 1). The FASN positive
expression rate was significantly higher in OS tissues (63.2%) than
in osteoenchondroma tissues (28.6%) (Table I). The Ki-67 protein expression
level was significantly higher in OS tissues than in
osteoenchondroma tissues (Table I).
The statistical analyses revealed a significant positive
correlation between FASN and Ki-67 protein expression (Table II). Our results indicated that FASN
and Ki-67 are overexpressed in the tissues of OS.
 | Table IFASN and Ki-67 protein expression
levels in osteosarcoma and osteoenchondroma. |
Table I
FASN and Ki-67 protein expression
levels in osteosarcoma and osteoenchondroma.
| | FASN expression
levels
|
|---|
| Group (n) | Ki-67 label
index | − | + | ++ | +++ |
|---|
| Osteoenchondroma
(21) | 2.05±0.86 | 15 | 5 | 1 | 0 |
| Osteosarcoma
(136)a | 31.24±11.57 | 50 | 18 | 24 | 44 |
| With metastasis
(44)b | 43.43±10.05 | 6 | 8 | 12 | 18 |
| Without metastasis
(92) | 25.41±6.68 | 44 | 10 | 12 | 26 |
 | Table IICorrelation between FASN ans Ki-67
protein expression levels in osteosarcoma. |
Table II
Correlation between FASN ans Ki-67
protein expression levels in osteosarcoma.
| FASN expression
levels | No. of cases | Ki-67 label
index |
|---|
| − | 50 | 22.38±4.28 |
| + | 18 | 30.22±8.32 |
| ++ | 24 | 35.41±10.37 |
| +++ | 44 | 41.68±10.49 |
The correlation between FASN, Ki-67
protein expression levels and pulmonary metastasis in OS
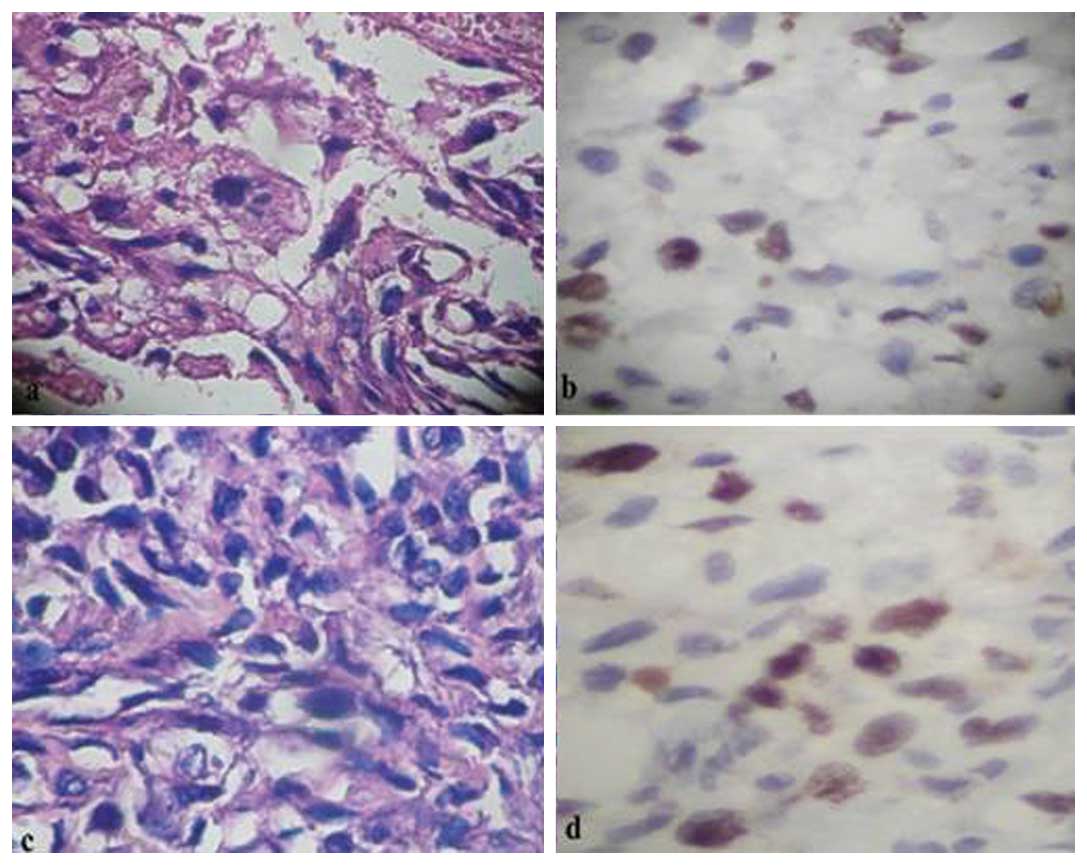
Under light microscopy, there was a greater
concentration of Ki-67-stained nuclei in the cases with pulmonary
metastasis compared to those without (Fig. 2). The Ki-67 proliferation index was
significantly higher in OS tissues with pulmonary metastasis than
in those without (p<0.05, Table
I). Thirty-eight cases (86.4%) with pulmonary metastases at
diagnosis were positive for FASN expression in the OS tissues, but
only 48 cases (52.2%) of those without pulmonary metastases were
positive (Table I). There were also
more cells with a positive expression for FASN protein in the cases
with pulmonary metastasis, as shown by an intensely stained
cytoplasm, compared to the cases without pulmonary metastasis
(Fig. 3). The expression level was
significantly higher in the cases with lung metastasis than in the
cases without metastasis (p<0.01). Our data suggest that FASN
and Ki-67 may be involved in OS metastasis.
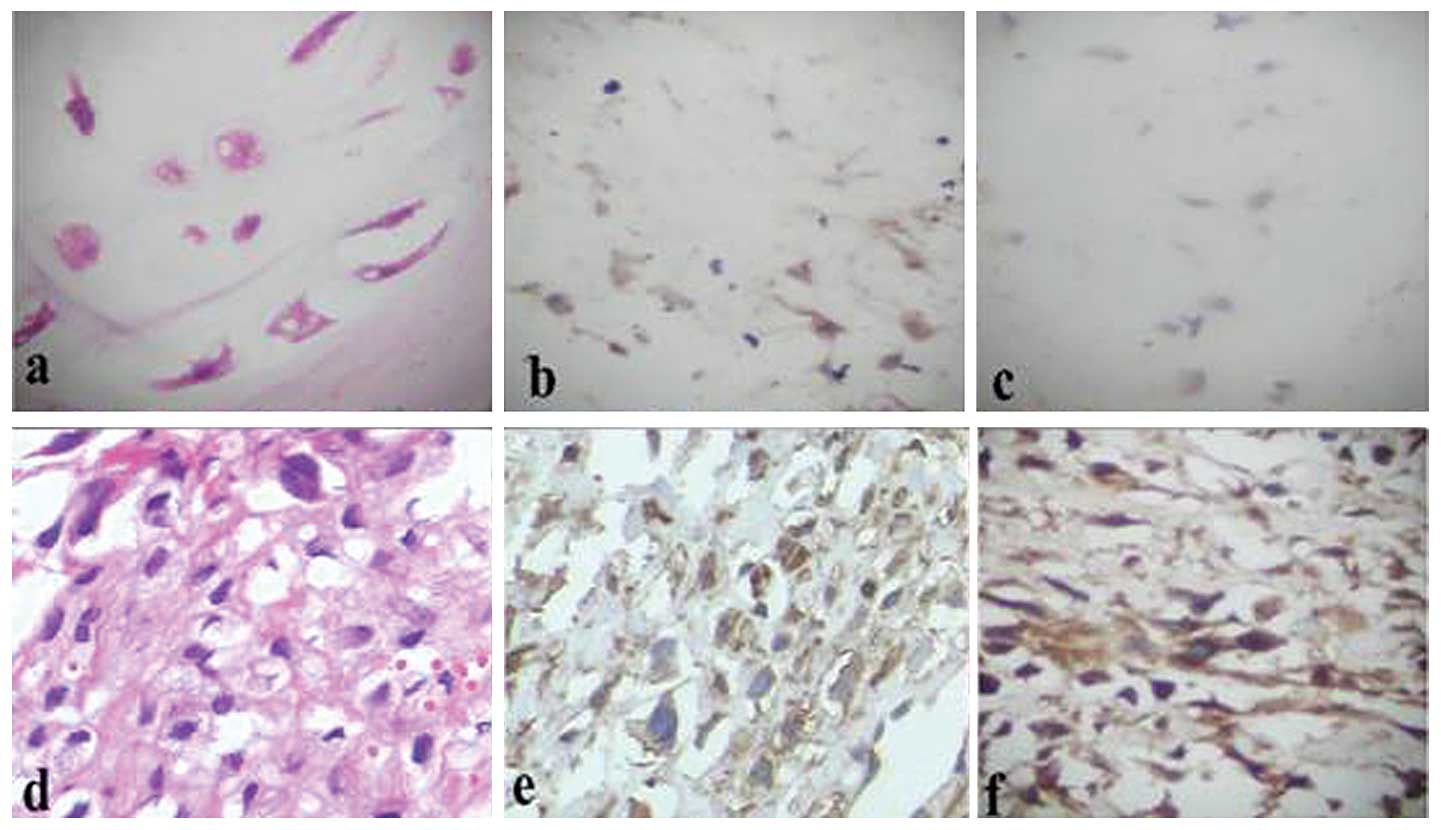
 | Figure 3Fatty acid synthase (FASN) expression
in osteosarcoma (OS) with and without pulmonary metastasis (x400).
Representative images of (a) H&E staining for OS tissues
without pulmonary metastasis showing that OS is cell-rich and has
significant cellular atypia, ansionucleosi, prominent nucleoli,
abundant cytoplasm and a small amount of bone-like matrix; (b) IHC
staining for FASN in OS tissues without pulmonary metastasis,
showing yellow particle deposition in the cytoplasm with only a
small amount of the cells, approximately 10%, colored; (c) H&E
staining for OS tissues with pulmonary metastasis showing that OS
is cell-rich and has significant cellular atypia, ansionucleosi,
prominent nucleoli and abundant cytoplasm; (d) IHC staining for
FASN with lung metastasis showing brown particles deposited in the
cytoplasm with the majority of cells, approximately 92%, colored.
H&E, hematoxylin and eosin; IHC, immunohistochemistry. |
Discussion
Ki-67 is a type of nuclear protein which is
expressed in the G1, S, G2 and M phases of the cellular cycle, but
not in the G0 phase. Ki-67 protein is present in all mitogenic
cells of normal and tumor tissues. Ki-67 protein expression levels
not only reflect the activity of tumor cells, but are also
correlated with the development, metastasis and prognosis of
cancer. The present study evaluated the proliferation of Ki-67 in
OS and osteoenchondroma tissues and the results revealed that the
percentage of positively stained cells for Ki-67 protein expression
was 31.24±11.57 and 2.05±0.86% in OS and osteoenchondroma tissues,
respectively (p<0.01). The expression levels of Ki-67 protein in
OS were significantly higher than in osteoenchondroma tissues.
Furthermore, the correlation between Ki-67 protein expression
levels and pulmonary metastasis was investigated in this study and
the results showed a significant correlation between Ki-67 protein
expression levels and pulmonary metastasis of OS (p<0.01), which
was consistent with the results from a previous study (21). The results suggest that Ki-67
protein may be involved in OS metastasis.
Fatty acid metabolic pathways have an important role
in carcinogenesis (22). Human FASN
is a 270 kDa cytosolic dimeric enzyme that is responsible for fatty
acid synthesis. Endogenous fatty acid synthesis from the small
carbon precursors acetyl-CoA and malonyl-CoA is dependent on the
activity of FASN. FASN is downregulated by dietary fatty acids in
the majority of cells, with the exception of lipogenic tissues such
as liver, lactating breast, fetal lung and adipose tissues. Recent
studies have provided compelling evidence that neoplastic
lipogenesis is essential for cancer cell survival (23). Various studies have reported FASN
overexpression in a variety of human tumors (24–29).
In this study, we demonstrated that FASN was positively expressed
in 86 of 136 OS cases (63.2%); however, the rate of positive
expression was 28.6% in osteoenchondroma tissues. The difference in
FASN expression level between OS and osteoenchondroma was
significant (p<0.01). Recent studies have shown that FASN
expression levels are associated with tumor cells metastasis in
vivo (20,30). In our study, FASN was overexpressed
in 38 of 44 patients with pulmonary metastasis, but only 48 of the
92 patients without metastasis demonstrated positive FASN
expression. The difference between expression levels of FASN in
cases with pulmonary metastasis was significantly higher than in
those without metastasis (p=0.002), showing similar results to
previous studies. This suggests that FASN may be involved in OS
metastasis.
As the correlation between Ki-67 protein and OS
metastasis has been revealed in previous studies, we investigated
the correlation of FASN and Ki-67 protein expression levels in OS
tissue to indirectly provide evidence of the correlation between
FASN and OS metastasis. Our results revealed a positive correlation
between FASN and Ki-67 protein expression levels and therefore we
believe that FASN may be involved in OS metastasis.
To our knowledge, this is the first study to
evaluate the correlation between FASN expression levels and
metastasis of OS. Although further studies are required, the
present findings show that FASN may be involved in proliferation
and metastasis of OS, thereby possibly suggesting FASN as a
promising target for treating OS metastasis.
Acknowledgements
This study was supported by
Grants-in-Aid from the Technology Department, Education Office of
Jiangxi Province (No. GJJ11316). We would like to thank associate
pathologist Dr Li Weihua (Yuyao People’s Hospital, Zhejiang, China)
for the immunostaining evaluation.
References
|
1.
|
A LonghiC ErraniM De PaolisPrimary bone OS
in the pediatric age: State of the artCancer Treatment
Reviews32423436200610.1016/j.ctrv.2006.05.00516860938
|
|
2.
|
R MckennaCP SchwinnKY SoongSarcomata of
the osteogenic series (osteosarcoma, fibrosarcoma, chondrosarcoma,
parosteal osteogenic sarcoma, and sarcomata arising in abnormal
bone) an analysis of 552 casesJ Bone Joint Surg481261966
|
|
3.
|
Y IwamotoK TanakaK IsuMultiinstitutional
phase II study of neoadjuvant chemotherapy for OS (NECO study) in
Japan: NECO-93J and NECO-95JJ Orthop
Sci14397404200910.1007/s00776-009-1347-619662473
|
|
4.
|
PA MeyersCL SchwartzM KrailoOS: A
randomized, prospective trial of the addition of ifosfamide and/or
muramyl tripeptide to cisplatin, doxorubicin, and high-dose
methotrexateJ Clin
Oncol2320042011200510.1200/JCO.2005.06.03115774791
|
|
5.
|
MU JawadMC CheungOsteosarcoma: improvement
in survival limited to high-grade patients onlyJ Cancer Res Clin
Oncol137597607201110.1007/s00432-010-0923-720514491
|
|
6.
|
V MialouT PhilipC KalifaMetastatic OS at
diagnosis: prognostic factors and long-term outcome - the French
pediatric experienceCancer10411001109200516015627
|
|
7.
|
M HegyiAF SemseiZ JakabGood prognosis of
localized OS in young patients treated with limb-salvage surgery
and chemotherapyPediatr Blood
Cancer57415422201110.1002/pbc.2317221563300
|
|
8.
|
MP StokkelMF LinthorstJJ BormA
reassessment of bone scintigraphy and commonly tested pretreatment
biochemical parameters in newly diagnosed osteosarcomaJ Cancer Res
Clin Oncol128393399200210.1007/s00432-002-0350-512136254
|
|
9.
|
PL AloM AminiF PiroImmunohistochemical
expression and prognostic significance of fatty acid synthase in
pancreatic carcinomaAnticancer Res2725232527200717695548
|
|
10.
|
T KusakabeA NashimotoK HonmaFatty acid
synthase is highly expressed in carcinoma, adenoma and in
regenerative epithelium and intestinal metaplasia of the
stomachHistopathology407179200210.1046/j.1365-2559.2002.01289.x11903600
|
|
11.
|
K WalterSM HongS NyhanSerum fatty acid
synthase as a marker of pancreatic neoplasiaCancer Epidemiol
Biomarkers
Prev1823802385200910.1158/1055-9965.EPI-09-014419723916
|
|
12.
|
Y OkawaT HideshimaH IkedaFatty acid
synthase is a novel therapeutic target in multiple myelomaBritish
Journal of
Haematology141659671200810.1111/j.1365-2141.2008.07114.x
|
|
13.
|
T MigitaS RuizA FornariFatty acid
synthase: a metabolic enzyme and candidate oncogene in prostate
cancerJ Natl Cancer
Inst101519532200910.1093/jnci/djp03019318631
|
|
14.
|
S YanW KefengSY ShengExpression of fatty
acid synthase in bladder transitional cell carcinoma and its
significanceChina Oncology173953972007
|
|
15.
|
SD SilvaIW CunhaRN YounesErbB receptors
and fatty acid synthase expression in aggressive head and neck
squamous cell carcinomasOral
Dis16774780201010.1111/j.1601-0825.2010.01687.x20604875
|
|
16.
|
P CrispinoPL AlòM RiveraEvaluation of
fatty acid synthase expressionin oesophageal mucosa of patients
with oesophagitis, Barrett’s oesophagus and adenocarcinomaJ Cancer
Res Clin Oncol13515331541200919471962
|
|
17.
|
H OritaJ CoulterE TullyInhibiting fatty
acid synthase for chemoprevention of chemically induced lung
tumorsClin Cancer
Res1424582464200810.1158/1078-0432.CCR-07-417718413838
|
|
18.
|
DT ColemanR BigelowJA CardelliInhibition
of fatty acid synthase by luteolin post-transcriptionally
down-regulates c-Met expression independent of proteosomal
lysosomal degradationMol Cancer
Ther8214224200910.1158/1535-7163.MCT-08-072219139131
|
|
19.
|
MA CarvalhoKG ZecchinF SeguinFatty acid
synthase inhibition with Orlistat promotes apoptosis and reduces
cell growth and lymph node metastasis in a mouse melanoma modelInt
J Cancer12325572565200810.1002/ijc.2383518770866
|
|
20.
|
S MurataK YanagisawaK FukunagaFatty acid
synthase inhibitor cerulenin suppresses liver metastasis of colon
cancer in miceCancer
Sci10118611865201010.1111/j.1349-7006.2010.01596.x20491775
|
|
21.
|
C LinLW MingLF BaoExpressions of P21(WAF1)
protein, CNA and Ki-67 and their significance in osteosarcomaChina
Oncology111091122001
|
|
22.
|
CS YehJY WangTL ChengFatty acid metabolism
pathway play an important role in carcinogenesis of human
colorectal cancers by Microarray Bioinformatics analysisCancer
Lett233297308200610.1016/j.canlet.2005.03.05015885896
|
|
23.
|
R FlavinS PelusoPL NguyenM LodaFatty acid
synthase as a potential therapeutic target in cancerFuture
Oncol6551562201210.2217/fon.10.1120373869
|
|
24.
|
T TakahiroK ShinichiS ToshimitsuExpression
of fatty acid synthase as a prognostic indicator in soft tissue
sarcomasClin Cancer Res922042212200312796387
|
|
25.
|
T KusakabeM MaedaN HoshiFatty acid
synthase is expressed mainly in adult hormone-sensitive cells or
cells with high lipid metabolism and in proliferating fetal cellsJ
Histochem
Cytochem48613622200010.1177/00221554000480050510769045
|
|
26.
|
JA MenendezR LupuFatty acid synthase and
the lipogenic phenotype in cancer pathogenesisNat Rev
Cancer7763777200710.1038/nrc222217882277
|
|
27.
|
S RossiW OuD TangGastrointestinal stromal
tumours overexpress fatty acid synthaseJ
Pathol209369375200610.1002/path.198316583360
|
|
28.
|
SD SilvaDE PerezIN NishimotoFatty acid
synthase expression in squamous cell carcinoma of the tongue:
clinicopathological findingsOral
Dis14376382200810.1111/j.1601-0825.2007.01395.x18410580
|
|
29.
|
PL AlòP ViscaML
FramarinoImmunohistochemical study of fatty acid synthase in
ovarian neoplasmsOncol Rep713831388200011032949
|
|
30.
|
K SelvendiranS AhmedA DaytonHO-3867, a
synthetic compound, inhibits the migration and invasion of ovarian
carcinoma cells through downregulation of fatty acid synthase and
focal adhesion kinaseMol Cancer
Res811881197201010.1158/1541-7786.MCR-10-0201
|