Introduction
Primary breast cancer is the most common malignancy
among females worldwide; however, cancer tumors metastasizing to
the breast are fairly rare. The most common cause is spread from a
primary cancer in the contralateral breast (1). Other extramammary tumors often
metastasizing to the breast include lymphoma, malignant melanoma
and lung cancer (2).
Although malignant melanoma involves the skin in the
vast majority of cases, lesions affecting the mucosa of the head
and neck are rare. Involvement of the nasal cavity is even less
common, accounting for less than 1% of all malignant melanomas
(3). The clinical features of
malignant melanoma of the nasal cavity are non-specific and show
poor prognosis due to local recurrence, nodal involvement and
distant metastasis (4). Among
malignant melanomas with metastasis to the breast, cutaneous
melanomas are the most common (5).
We recently treated a patient with a solitary
metastatic breast tumor from a malignant melanoma of the nasal
cavity. We present this rare case and discuss the relevant
literature herein.
Case report
In November 2010, a 78-year-old female presented to
the Department of Otorhinolaryngology at Osaka Medical College
Hospital complaining of occasional epistaxis over the course of 6
weeks. Rhinoscopy revealed a brown-black tumor in the left nasal
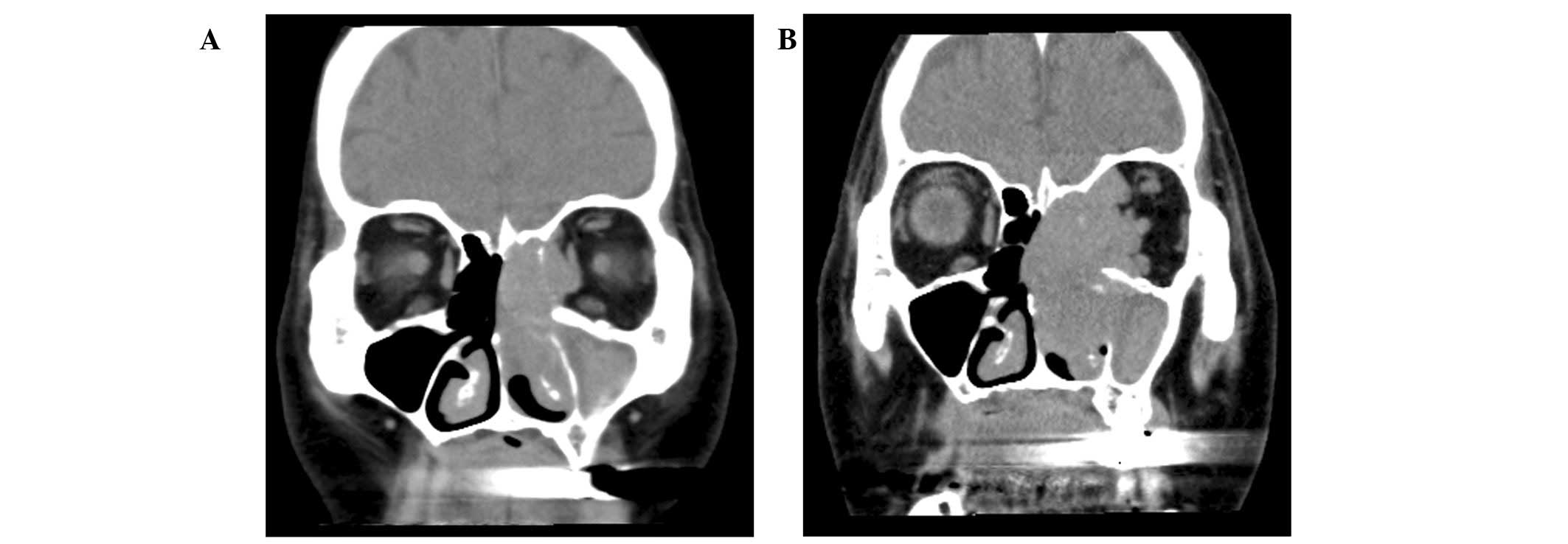
cavity. Head and neck computed tomography (CT) revealed a tumor in
the left nasal cavity with invasion to the orbital cavity (Fig. 1A). Biopsy of the tumor led to a
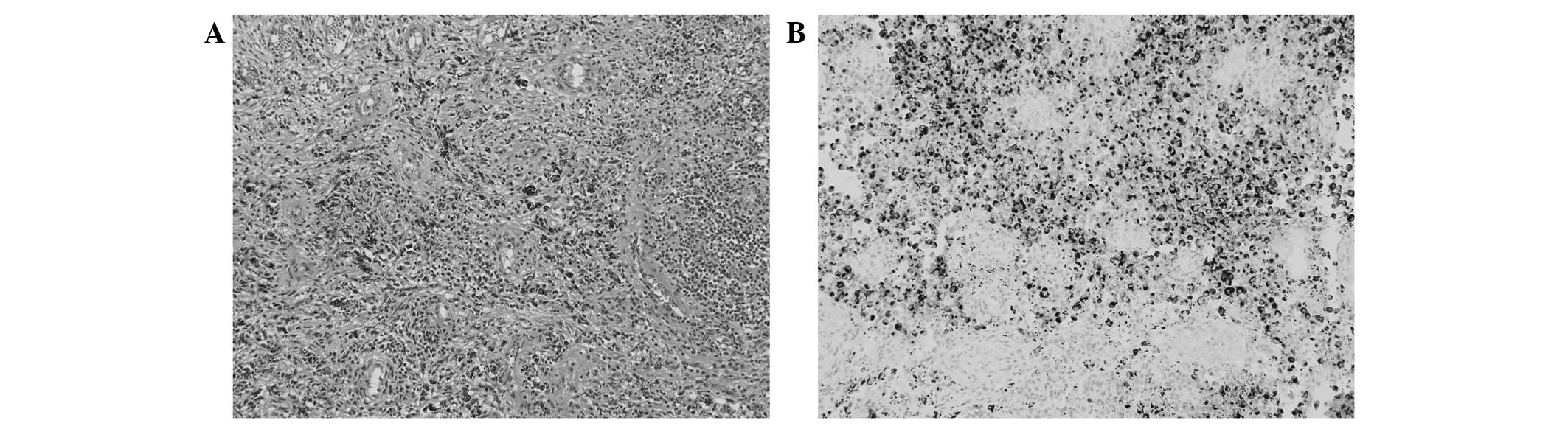
histological diagnosis of malignant melanoma. On pathological
inspection, a hypervascular, pigmented lesion comprising round to
oval-shaped tumor cells was revealed, and a final diagnosis of
malignant melanoma was confirmed by immunohistochemical examination
for HMB-45, a melanoma marker (Fig. 2A
and B). During the investigation of the nasal cavity tumor, the
patient had become aware of a growing lump in the upper region of
the left breast and was referred to our department in December
2010. Physical examination revealed a well-defined, elastic, soft,
immobile tumor in the upper region of the left breast, 4 cm in
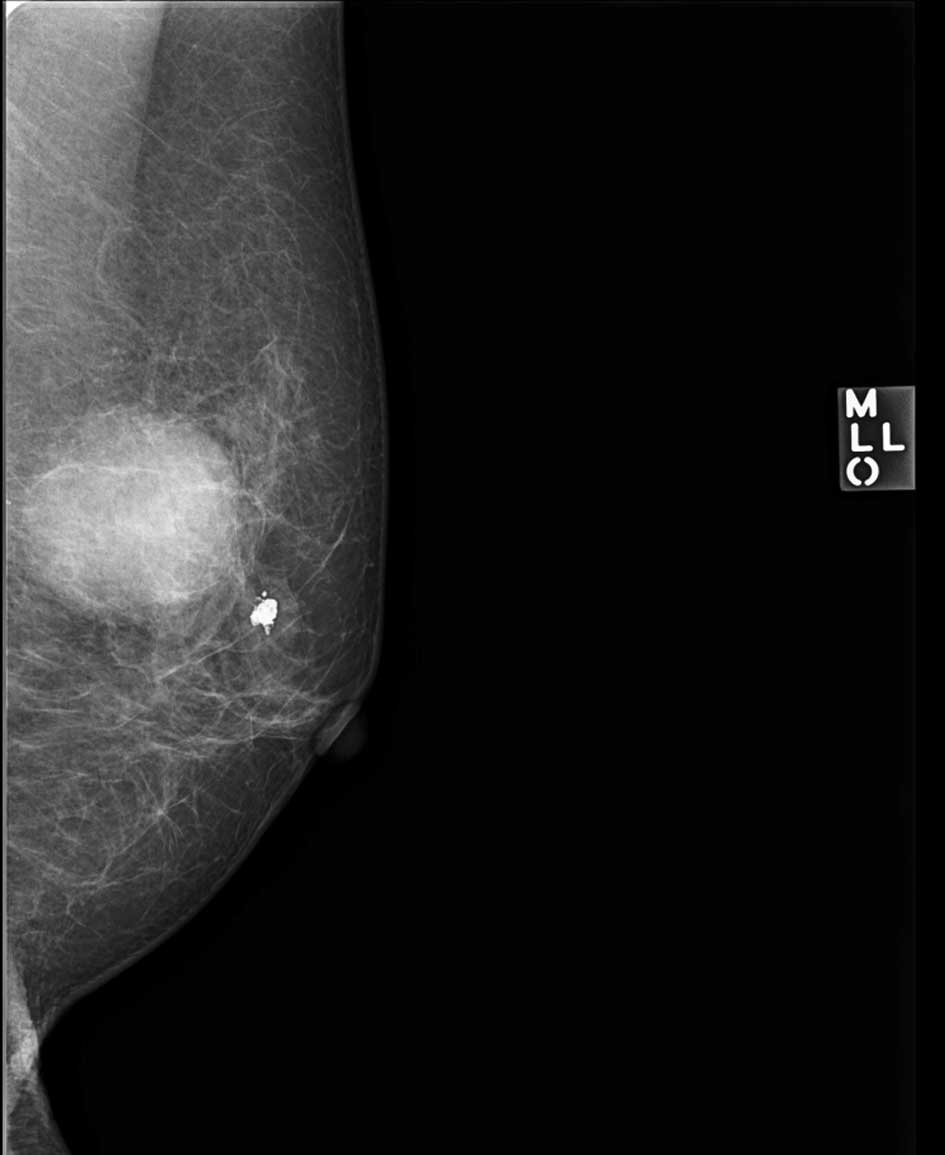
diameter. Mammography revealed an almost oval-shaped,
microlobulated tumor in the left upper breast (Fig. 3). Ultrasonography revealed a
heterogeneous mass with unclear margins. Fine needle aspiration
cytology from the breast tumor was suggestive of metastatic
melanoma, revealing cells similar in character to those of the
tumor in the nasal cavity. No evidence of metastasis besides that
to the left breast was found on positron emission tomography-CT.
Radical resection of both the primary tumor and metastasis was
proposed. The patient declined both radical surgery of the nasal
cavity, in a maximally invasive operation requiring ophthalmectomy,
and palliative surgery including systemic chemotherapy; however,
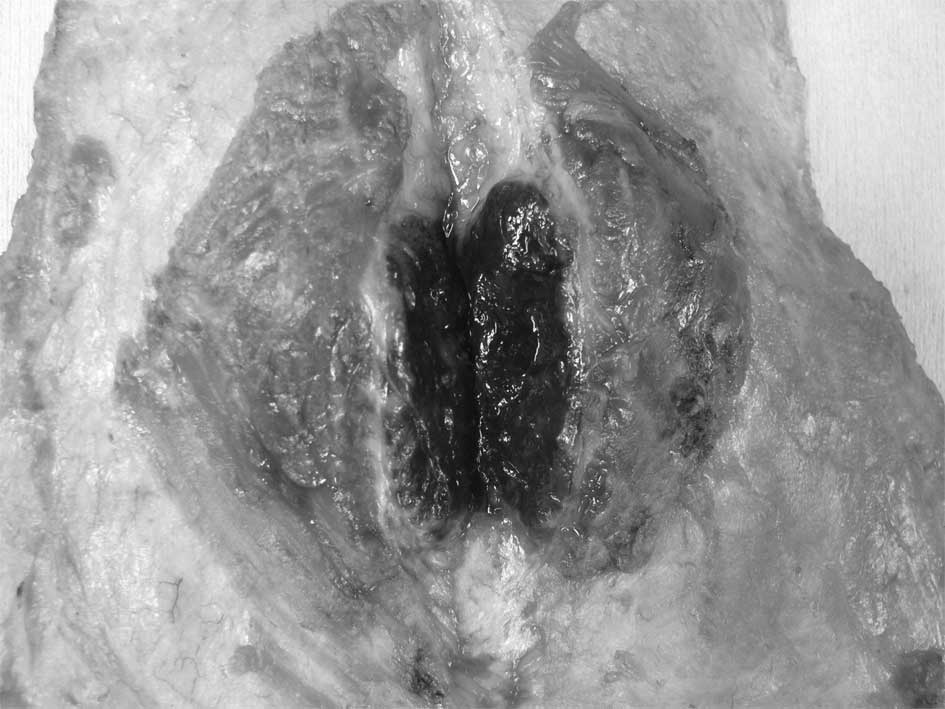
breast surgery was accepted and a left mastectomy with lower
axillary lymph node dissection, involving partial resection of the
pectoralis major muscle, was performed as a result of suspected
muscle invasion (Fig. 4). Although
the dissected axillary lymph nodes were swollen, no metastasis of
malignant melanoma was apparent under microscopy. For treatment of
the primary site, heavy ion radiotherapy was performed in
accordance with the wishes of the patient. The tumor initially
reduced in size by 30% over a 2-month period; however, it showed
regrowth during the month of radiotherapy (Fig. 1B). The disease subsequently
progressed very rapidly. Anticancer therapy was eventually
suspended 3 months after the initial radiotherapy and only
palliative care was administered. Finally, the patient was admitted
into a hospice in August 2011.
Discussion
The clinically observed incidence of metastatic
breast tumors was 0.5–1.2% in all breast neoplasms and 6% in an
autopsy series (6). Georgiannos
et al reviewed the clinical data for secondary tumors in the
breast, revealing a frequency of 3.2%, with the majority
representing metastasis from a contralateral breast cancer
(7).
Characteristics of metastatic breast tumors usually
include presence in the superficial tissues and well-defined
multinodular masses in the upper outer quadrant of the breast. The
absence of calcification on radiological examination, such as
mammography, is an additional suggestive feature (8). In the present case, a single breast
tumor was present in the upper middle quadrant of the deeper
mammary gland, therefore differentiation from primary breast
neoplasm was required.
Malignant melanomas account for only 2–8% of all
cancers arising in the nasal cavity, and mucosal melanoma of the
nasal cavity is rarely encountered (<1% of all malignant
melanomas) (9). Given the rarity of
this disease, no universally accepted staging system has been
devised. The most fundamental treatment is wide resection of the
primary site. Radiotherapy combined with surgery is recommended in
cases of local recurrence or incomplete lesion removal; however,
the majority of cases are resistant to radiotherapy, as in the
present case. In addition, few standard systemic chemotherapies are
regarded as effective. This disease is aggressive and distant
metastases to the liver, lungs and brain, and regional metastases
to subcutaneous tissues are the major causes of mortality in the
majority of cases (10). In our
institute, the 5-year survival rate for malignant melanoma of the
nasal cavity is 36%. In addition, the 2-year survival rate of
patients with distant metastases is only 13%, compared to 100% in
the absence of distant metastases. Early detection and diagnosis
with appropriate treatment should therefore be emphasized.
Metastases from cutaneous malignant melanoma
represent the majority of cases of melanoma involving the breast
with the most common primary sites associated with breast
involvement on the arms and trunk (5). The mechanism of breast involvement in
these cutaneous malignant melanomas may involve direct lymphatic
and/or vascular drainage routes from the primary site to the
breast. In the present case, breast metastasis was suggested to
have occurred hematogenously, given that the primary tumor showed a
rich blood supply and no axillary lymph node involvement was
detected. As another mechanism, a hormonally-based association with
the progression of melanoma has been suspected. Although the
influence of estrogen in the development and progression of
melanoma has been controversial, epidemiological evidence
implicating estrogens in the etiology of melanoma has been
accumulating (11). Estrogen
receptors have been detected in certain melanoma cells, although at
low levels and infrequently (12).
The peak incidence age of melanoma among females coincides with the
perimenopausal age. In a review of 15 patients with breast
metastasis from cutaneous melanomas, the majority of patients (93%)
were premenopausal women with a mean age of 39 years (5). In another retrospective review of 27
patients with mammary metastasis from malignant melanoma, including
cutaneous melanoma, 70% of patients were premenopausal (13).
In terms of the treatment for metastatic melanoma,
insufficient results have been obtained using systemic
chemotherapies, with response rates below 20% (14). The epidemiological and clinical
evidence of melanoma potentially being an estrogen-dependent tumor
suggests the possible efficacy of adding hormonal therapy to
chemotherapy; however, single-agent hormonal therapy is minimally
active. A preliminary report in which a high response rate was
acquired using chemotherapy concurrent with tamoxifen has attracted
attention (15). In addition, in a
case series on malignant melanomas of the nasal cavity, 3 patients
without distant metastases who responded to tamoxifen concurrent
with chemotherapy were reported in 1997 (16). While a meta-analysis of randomized
controlled trials did not demonstrate any significant improvement
in the overall response rate, complete response rate or survival
rate when tamoxifen was administered along with chemotherapy
regimens for patients with metastatic melanoma; overall response
rates tended to favor the combined regimens (17). In previous clinical trials and
practices, tamoxifen, which was originally used as a target therapy
to treat estrogen receptor-positive breast cancer patients, has
been administered irrespective of the estrogen receptor status of
tumor cells, therefore estrogen receptor-negative cases would have
received no benefit from this hormonal therapy. In the present
case, immunohistochemical staining for estrogen receptors yielded
negative results (data not shown); however, hormonal therapy may be
an option for patients with estrogen receptor-positive tumor cells,
as this therapy is less toxic than chemo- or radiotherapy.
To the best of our knowledge, no previous reports in
the English literature have described malignant melanoma of the
nasal cavity with solitary breast metastasis. This case was unusual
not only in that the primary site was the nasal cavity, but also
because the solitary metastasis to the breast occurred in a
postmenopausal woman. At present, radical surgery remains the only
fundamental therapy; however, the establishment of treatment
strategies based on a comprehensive understanding of both etiology
and pathophysiology is needed for rare cases such as this.
References
|
1.
|
K McPhersonCM SteelJM DixonABC of breast
diseases. Breast cancer-epidemiology, risk factors, and
geneticsBMJ321624628200010.1136/bmj.321.7261.62410977847
|
|
2.
|
MN AkçayMetastatic disease in the
breastBreast115265282002
|
|
3.
|
R KhannaRN SrivastavaA AgarwalPrimary
malignant melanoma of the nasal cavityEar Nose Throat
J6965465519902245795
|
|
4.
|
SF HuangCT LiaoCR KanIH ChenPrimary
mucosal melanoma of the nasal cavity and paranasal sinuses: 12
years of experienceJ Otolaryngol36124129200717459285
|
|
5.
|
R AroraWA RobinsonBreast metastases from
malignant melanomaJ Surg Oncol502729199210.1002/jso.2930500110
|
|
6.
|
B OksüzoğluH AbaliN GülerE BaltaliY
OzişikMetastasis to the breast from nonmammarian solid neoplasms: a
report of five casesMed Oncol20295300200314514980
|
|
7.
|
SN GeorgiannosJ ChinAW GoodeM
SheaffSecondary neoplasms of the breast: a survey of the 20th
CenturyCancer9222592266200110.1002/1097-0142(20011101)92:9%3C2259::AID-CNCR1571%3E3.0.CO;2-O11745279
|
|
8.
|
SI HajduJA UrbanCancers metastatic to the
breastCancer2916911696197210.1002/1097-0142(197206)29:6%3C1691::AID-CNCR2820290637%3E3.0.CO;2-44337956
|
|
9.
|
B KungGR DeschenesW KeaneM CunnaneMP
Jacob-AmpueroM RosenParanasal sinus melanoma masquerading as
chronic sinusitis and nasal polyposisEar Nose Throat
J86561564200717970147
|
|
10.
|
SJ BlatchfordCF Koopmann JrSW
CoulthardMucosal melanoma of the head and
neckLaryngoscope96929934198610.1288/00005537-198609000-000013747692
|
|
11.
|
JG MillerS Mac NeilGender and cutaneous
melanomaBr J
Dermatol136657665199710.1111/j.1365-2133.1997.tb03648.x9205495
|
|
12.
|
YN LeeBetter prognosis of many cancers in
female: a phenomenon not explained by study of steroid receptorsJ
Surg Oncol25255262198410.1002/jso.29302504086371384
|
|
13.
|
L RavdelWA RobinsonK LewisR
GonzalezMetastatic melanoma in the breast: a report of 27 casesJ
Surg Oncol94101104200610.1002/jso.2059216847918
|
|
14.
|
MB AtkinsAC BuzaidAN HoughtonSystemic
chemotherapy and biochemotherapyCutaneous MelanomaCM BalchA
HoughtonA SoberS Soong4th editionQuality Medical PublishingSt
Louis5896042003
|
|
15.
|
SA Del PreteLH MaurerJ O’DonnellRJ
ForcierP LeMarbreCombination chemotherapy with cisplatin,
carmustine, dacarbazine, and tamoxifen in metastatic melanomaCancer
Treat Rep681403140519846541973
|
|
16.
|
MB LensT ReimanAF HusainUse of tamoxifen
in the treatment of malignant
melanomaCancer9513551361200310.1002/cncr.1164414508820
|
|
17.
|
W SeoH OgasawaraM SakagamiChemohormonal
therapy for malignant melanomas of the nasal and paranasal
mucosaRhinology35192119979200258
|