Introduction
Cancer is widely accepted as a risk factor for
venous thromboembolism (VTE) and, to a lesser extent, arterial
thrombosis. This risk is attributable to factors such as the
expression of prothrombotic factors and compression of the blood
vessels by tumors, inflammatory response to malignancies by the
host, immobility, surgery, indwelling central venous catheters, and
certain antitumor therapies (1). In
surgical oncology patients, VTE is the predominant cause of
mortality during the first 30 postoperative days (2). VTE is also a common complication
associated with gynecologic cancer surgery, and patients with
gynecological malignancies are classified under the highest-risk
group (3).
Coagulation and fibrinolysis in the blood are
complex processes, and numerous markers of thrombin and plasmin
action in patients with VTE have been identified (4). Soluble fibrin (SF) and D-dimer are
sensitive markers for VTE. D-dimer is a stable end-product of
fibrin degradation, and D-dimer levels increase as a result of
fibrin formation and fibrinolysis. Conversely, SF reflects thrombi
activation and the cleavage of fibrinogen during the early stage of
disease and is used as an indicator of coagulation (5). We recently reported the changes in
plasma D-dimer levels over time in gynecologic cancer patients
following surgery (6); however,
changes in the SF levels in these patients following surgery have
not been elucidated.
The purpose of the present study was to evaluate the
changes in the plasma SF levels over time in gynecologic cancer
patients following surgery. Furthermore, we examined the duration
of the coagulation stage and determined a suitable duration for
which chemoprophylaxis with anticoagulant agents should be
administered.
Materials and methods
Study population
We studied 311 patients with invasive gynecologic
cancer who had undergone surgery at Okayama University Hospital
between August 2007 and August 2011. The study was approved by the
ethics committee of Okayama University Graduate School of Medicine,
Okayama, Japan. Informed consent was obtained from each patient
prior to blood collection. The median age of the patients was 55
years (range, 16–81 years). Patients with various types of
gynecologic cancers were enrolled: 65 patients with ovarian cancer,
142 with endometrial cancer, 103 with cervical cancer, and 1 with
vulvar cancer. Patients with preoperative VTE were excluded. All
the patients received general anesthesia. Calf-length external
sequential compression devices (SCD) were placed on both legs of
all the patients from the beginning of the surgery until after the
operation. Chemical anticoagulants were administered to 215
patients following surgery: 54 patients were treated with
unfractionated heparin (UFH; 10,000 U/day); 50 patients with
fondaparinux (FPX; 2.5 mg/day); and 111 patients with enoxaparin
(ENO; 4,000 U/day). UFH, ENO and FPX were administered for a median
of 5 days (range, 3–23 days), 10 days (range, 1–15 days), and 10
days (range, 3–20 days), respectively. UFH was used at the
discretion of the surgeon before regulatory approval of the other
agents was obtained (i.e., before 2008), and ENO and FPX were
routinely used after 2009. In the present study population, VTE was
detected in 23 patients (7.4%).
Measurement of plasma SF levels
The plasma SF levels were measured serially prior to
surgery and on postoperative days 0, 1, 3, 5, 7, 10, 14 and 21;
these levels were also measured after day 28. We used the latex
agglutination method to measure the SF levels with IATRO SF
(Mitsubishi Kagaku Iatron, Inc., Tokyo, Japan), which contains the
monoclonal antibody IF-43 as the reagent. IF-43 recognizes a
segment of the fibrin Aα chain (Aα-17-78 residue segment) exposed
in the E region of the fibrin monomer (FM) when this FM binds to
the D region of another FM or fibrinogen. The antibody is coated
for the SF assay (7). The normal
range is <7.0 μg/ml.
Statistical analyses
We used Fisher's exact test or the Mann-Whitney
U-test to statistically analyze the differences between the groups.
Probability values of <0.05 were considered to indicate
statistical significance.
Results
Time course of postoperative plasma SF
levels
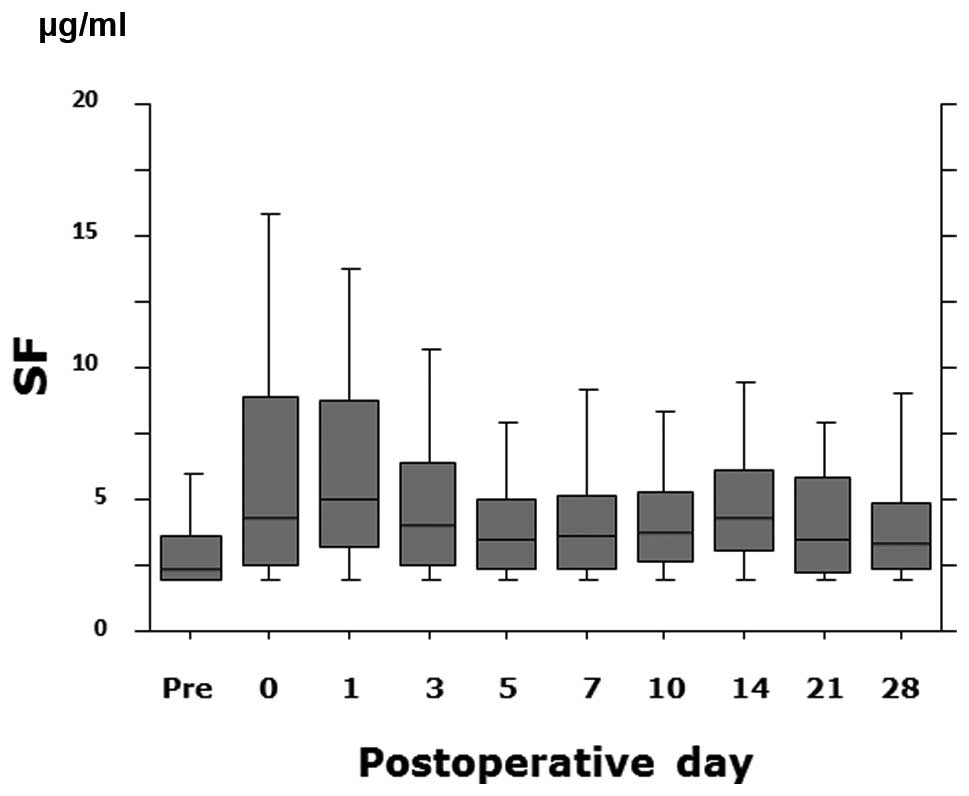
The plasma SF levels increased rapidly, peaked on
postoperative day 1 (median, 4.6 μg/ml), and then decreased
(Fig. 1). The SF levels of
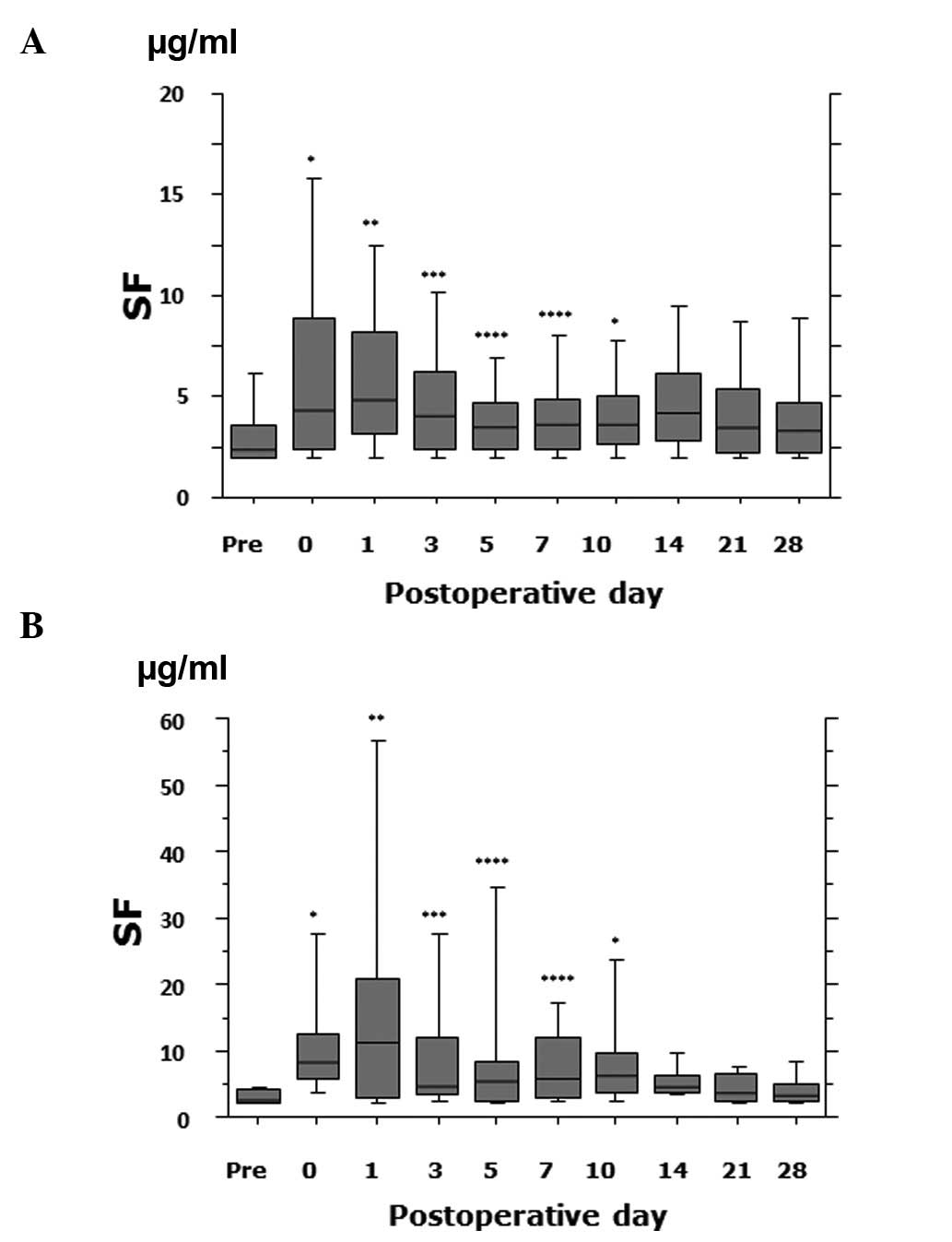
VTE-positive patients were significantly different from those of
VTE-negative patients on postoperative day 0 (medians 8.5
μg/ml and 4.3 μg/ml, respectively; P<0.0001), day
1 (medians 11.3 μg/ml and 4.9 μg/ml, respectively;
P=0.002), day 3 (medians 4.4 μg/ml and 4.0 μg/ml,
respectively; P=0.02), day 5 (medians 5.6 μg/ml and 3.5
μg/ml, respectively; P=0.003), day 7 (medians 5.8
μg/ml and 3.6 μg/ml, respectively; P=0.003), and day
10 (medians 6.1 μg/ml and 3.6 μg/ml, respectively;
P<0.0001) (Fig. 2). The SF
levels on each day did not significantly differ between the
patients treated with chemical anticoagulants and those treated
mechanically (data not shown).
Incidence and peak day of elevated plasma
SF
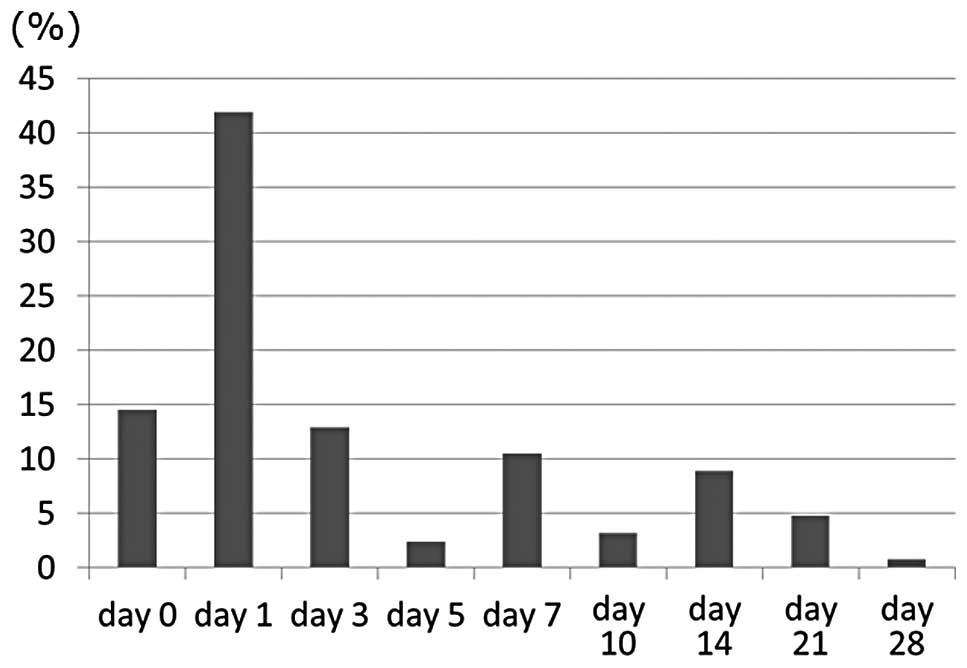
The plasma SF levels were elevated (≥7.0
μg/ml) in 159 of the 311 patients (51.1%) on one of the days
when the levels were measured. The days on which the plasma SF
levels peaked are shown in Fig. 3.
The plasma SF levels of 67 patients (42.1%) peaked on day 1, 23
patients (14.5%) on day 0, and 20 patients (12.6%) on day 3.
However, in 14 patients (8.8%), these levels peaked on day 14 and
in 9 patients (5.7%) on days 21–28. We observed that only 1 of the
14 patients (7.1%) whose levels peaked on day 14 had undergone
chemotherapy following surgery. However, chemotherapy had been
administered to 8 of the 9 (88.9%) patients whose levels peaked on
days 21–28 (P=0.0002).
Discussion
The SF levels reflect the changes that occur during
the early phase of a thrombotic event, while D-dimer levels reflect
secondary fibrinolysis after clot formation (8). Elevated levels of circulating plasma
SF indicate the conversion of fibrinogen to fibrin by thrombin.
Plasma SF levels rapidly increase and then decrease relatively soon
following orthopedic surgery (9,10). In
a previous study, we reported that plasma D-dimer levels gradually
increased, peaked on postoperative days 7–10, and then decreased
(6). To the best of our knowledge,
changes in the plasma SF levels in gynecologic cancer patients have
not yet been described. In the present study, we measured the
plasma SF levels longitudinally and demonstrated that these levels
increased rapidly following surgery, peaked on postoperative day 1,
and then decreased. This observation suggests that increased
intravascular fibrin formation had already occurred during
perisurgery. High plasma levels of D-dimer are maintained for
longer periods than those of SF because the half-life of plasma
D-dimer is relatively long. Furthermore, we demonstrated that the
SF levels in the VTE-positive patients were significantly higher
than those in the VTE-negative patients on postoperative days 0–10.
Therefore, hypercoagulation may exist from surgery to at least 10
days after surgery in certain cases. It is reported that the plasma
concentration of SF following orthopedic surgery is significantly
higher in patients with VTE than in those without VTE on days 1, 4
and 14 (10).
In approximately 70% of the patients, the plasma SF
levels peaked within 3 days of surgery. Kearon reported that in a
large number of cases, VTE occurred between the intraoperative
period and postoperative day 3 (11). The findings of the present study
support this finding. In approximately 95% of the patients, the
plasma SF levels peaked within 14 days of surgery. This finding
suggests that the risk of symptomatic VTE is generally highest
during the 2 weeks following surgery, and therefore, prophylaxis
should be administered to patients with gynecologic cancer for at
least 2 weeks postoperatively.
In the ENOXACAN II study, a double-blind,
multicenter clinical trial involving patients undergoing open
abdominal or pelvic cancer surgery, the incidence of VTE decreased
significantly from 12 to 4.8% when inpatient thromboprophylaxis was
continued for 27–31 days (12).
Most guidelines recommend that cancer patients undergoing elective
major abdominal or pelvic surgery should receive prophylaxis in the
hospital and after discharge for up to 1 month after surgery
(13). However, the exact
indications for such extended prophylaxis have not been defined. In
fact, findings of a systematic review showed that extended
prophylaxis reduced asymptomatic VTE without decreasing the
mortality rate at 3 months, and that the evidence for extended
regimens was limited and of poor quality (13). Notably, approximately 90% of the
patients whose plasma SF levels peaked on days 21–28 had undergone
chemotherapy following surgery. Therefore, the elevated plasma SF
levels were probably not due to surgery, but instead associated
with chemotherapy. Peedicayil et al reported that 75% of VTE
events occur more than a week after surgery and 36% occur after 4
weeks (14). They believed that
early VTE was due to the surgery, while late VTE reflected changes
during the period of recovery and initiation of adjuvant treatments
such as chemotherapy.
Our current findings proved that the plasma SF
levels increased rapidly, peaked on postoperative day 1, and then
decreased. Furthermore, we showed that the levels peaked before
postoperative day 14 in most cases. Therefore, postoperative
chemical thromboprophylaxis may be administered for at least up to
14 days after surgery. Further study is required to determine which
patients should be considered for extended prophylaxis.
References
|
1.
|
A LinJ RyuD HarveyLow-dose warfarin does
not decrease the rate of thrombosis in patients with cervix and
vulvo-vaginal cancer treated with chemotherapy, radiation, and
erythropoietinGynecol
Oncol10298102200610.1016/j.ygyno.2005.11.03116406065
|
|
2.
|
CT BradleyKJ BraselJJ
MillerCost-effectiveness of prolonged thromboprophylaxis after
cancer surgeryAnn Surg
Oncol173139201010.1245/s10434-009-0671-619707830
|
|
3.
|
MH EinsteinEA PrittsEM HartenbachVenous
thromboembolism prevention in gynecologic cancer surgery: a
sysytematic reviewGynecol
Oncol105813819200710.1016/j.ygyno.2007.03.00417449089
|
|
4.
|
A SuzukiH EbinumaM MatsuoThe monoclonal
antibody that recognizes an epitope in the C-terminal region of the
fibrinogen alpha-chain reacts with soluble fibrin and fibrin
monomer generated by thrombin but no with those formed as plasmin
degradation productsThromb
Res121377385200710.1016/j.thromres.2007.05.008
|
|
5.
|
A TsujiH WadaT MatsumotoElevated levels of
soluble fibrin in patients with venous thromboembolismInt J
Haematol88448453200810.1007/s12185-008-0173-518836793
|
|
6.
|
J KodamaN SekiS MasahiroD-dimer level as a
risk factor for postoperative venous thromboembolism in Japanese
women with gynecologic cancerAnn
Oncol2116511656201010.1093/annonc/mdq01220129998
|
|
7.
|
G SoeI KohnoK InuzukaA monoclonal antibody
that recognizes a neo-antigen exposed in the E domain of fibrin
monomer complexed with fibrinogen or its derivatives: its
application to the measurement of soluble fibrin in
plasmaBlood882109211719968822930
|
|
8.
|
H BounameauxP CiraficiP de
MoerlooseMeasurement of D-dimer in plasma diagnostic aid in
suspected pulmonary
embolismLancet337196200199110.1016/0140-6736(91)92158-X1670841
|
|
9.
|
T MisakiI KitajimaT KabataChanges of the
soluble fibrin monomer complex level during the perioperative
period of hip replacement surgeryJ Orthop
Sci13419424200810.1007/s00776-008-1266-y18843455
|
|
10.
|
A SudoH WadaT NoboriCut-off values of
D-dimer and soluble fibrin for prediction of deep vein thrombosis
after orthopaedic surgeryInt J
Hematol89572576200910.1007/s12185-009-0323-419430861
|
|
11.
|
C KearonDuration of venous thromboembolism
prophylaxis after
surgeryChest124386S392S200310.1378/chest.124.6_suppl.386S14668422
|
|
12.
|
D BergqvistG AgnelliAT CohenDuration of
prophylaxis against venous thromboembolism with enoxaparin after
surgery for cancerN Engl J
Meed346975980200310.1056/NEJMoa01238511919306
|
|
13.
|
EA AklI TerrenatoM BarbaExtended
perioperative thromboprophylaxis in patients with cancerThromb
Haemost10011761180200819132245
|
|
14.
|
A PeedicayilA WeaverX LiIncidence and
timing of venous thromboembolism after surgery for gynecological
cancerGynecol
Oncol1216469201110.1016/j.ygyno.2010.11.03821183211
|