Introduction
Head and neck cancer is one of the most common
cancerous diseases worldwide. Each year more than 500,000 new cases
are diagnosed (1). These are mostly
malignancies of the upper aerodigestive tract, including the mouth,
larynx and pharynx region from the base of the skull up to the
upper oesophagus. Currently, standard treatment regimes include
surgery, radiation and chemotherapy. In approximately 50% of all
cases a tumour relapse has been observed to correlate with a poor
prognosis (2,3). Standard chemotherapeutical regimes
including cisplatin and 5-FU are often associated with adverse
toxicity. In the last decade there has been an introduction of
well-tolerated agents in head and neck cancer therapy, such as
cetuximab, a chimeric monoclonal inhibitor of the epidermal growth
factor (EGF) receptor. This agent binds with high affinity to the
extracellular domain of the EGF receptor being overexpressed in
squamous cell carcinoma of the head and neck (SCCHN). The addition
of cetuximab to conventional chemotherapy or standard radiation
therapy was found to significantly enhance patient survival
(4). However, despite the success
of cetuximab for use in SCCHN patients, improvement in overall
survival with the use of this agent has only been incremental in
the past. Thus, there is a strong need for alternative or additive
drug regimens for the treatment of head and neck cancer (5,6). In
previous reports we showed a significant anti-proliferative and
apoptotic effect of Bortezomib and BI2536 in SCCHN (7,8).
Nuclear factor-κB (NF-κB) is a protein which is overexpressed in
SCCHN (9). In several gene
expression studies it was shown that tumours with high risk of
recurrence harboured a higher expression of genes associated with
the activation of NF-κB signalling (10). Bortezomib is a small-molecule
proteasome inhibitor. While it affects various signalling pathways,
one of the mechanisms of antitumour activity is NF-κB inhibition
(11). BI2536 is another selective
and potent small-molecule drug that inhibits polo-like-kinase-1
(PLK-1), which plays a key role in processes such as cell division
and checkpoint regulation of mitosis. Approximately 80% of human
tumours express high levels of PLK transcripts, while PLK mRNA is
mostly absent in healthy surrounding tissue (12,13).
This PLK overexpression is associated with poor prognosis and a
lower overall survival rate. The aim of the present study was to
illuminate the combinational effects of Bortezomib and BI2536 in
SCCHN.
Material and methods
Nine different squamous carcinoma cell lines,
predominantly of head and neck origin, were tested in this study.
A-431 cells were obtained from American Type Culture Collection
(ATCC). PE/CA-PJ 15, PE/CA-PJ 34, PE/CA-PJ 41 and PE/CA-PJ 49 cells
were obtained from European Collection of Cell Cultures (ECACC),
and Cal-27 and Kyse-140 cells from DSMZ GmbH (Braunschweig,
Germany). CLS-354 and UM-SCC-14C cells were obtained from Cell
Lines Service (CLS; Eppelheim, Germany). The fibroblast cell line
was a gift from the Department of Dermatology, University Hospital,
Frankfurt/Main, Germany. Bortezomib (Velcade®) was
supplied by Millenium Pharmaceuticals Inc. (Cambridge, MA, USA) and
Johnson & Johnson Pharmaceuticals (Raritan, NJ, USA). BI2536
was provided by Boehringer Ingelheim GmbH (Ingelheim am Rhein,
Austria). Squamous carcinoma cell lines were cultivated, according
to the instructions of the suppliers, with antibiotics at 37°C in
the cell type-specific medium Quantum 263 with L-glutamine (PAA
Laboratories GmbH, Pasching, Austria). Cells were seeded in 96-well
plates (1×105 cells/well), and after incubation for 24
h, the cells were treated with Bortezomib and/or BI2536 for 24, 48
and 72 h, respectively. In the experiments described in this
publication, Bortezomib and BI2536 were used in each cell line at a
fixed, cell line-specific concentration that had produced maximum
growth inhibition in previous systematic investigations in our
laboratory. The concentration for all cell lines investigated was
2.5 nmol/l for BI2536, which showed maximal growth inhibition in
our previous dose escalation studies. The concentration for
Bortezomib ranged from 1.25 to 5 μM (Table I). Cells were counted in a Rosenthal
chamber at 24, 48 and 72 h after treatment. Apoptosis was detected
by microscopic cytohistology as well as by Human Apoptose Array kit
(R&D Systems, Abingdon, UK) as previously described by other
groups (14,15).
 | Table I.Concentration of Bortezomib and
BI2536 in different cell lines. |
Table I.
Concentration of Bortezomib and
BI2536 in different cell lines.
| Tumour cell
line | Concentration of
Bortezomib (μM) | Concentration of
BI2536 (nM) |
|---|
| PE/CA-PJ 15 | 2.50 | 2.50 |
| PE/CA-PJ 34 | 5.00 | 2.50 |
| PE/CA-PJ 41 | 2.50 | 2.50 |
| PE/CA-PJ 49 | 1.25 | 2.50 |
| CLS-354 | 1.25 | 2.50 |
| UM-SCC-14C | 2.50 | 2.50 |
| Cal-27 | 2.50 | 2.50 |
| Kyse-140 | 2.50 | 2.50 |
| A-431 | 2.50 | 2.50 |
Each experiment was performed in triplicate. For
statistical analysis, a Wilcoxon test for matched pairs (dependent
samples) was performed using SPSS 19.0 software for Windows.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Nine different squamous carcinoma cell lines,
predominantly of head and neck origin, were tested in this study.
After incubation for 24 h, the cells were treated with Bortezomib
or BI2536 alone, or with a combination of both agents for 24, 48
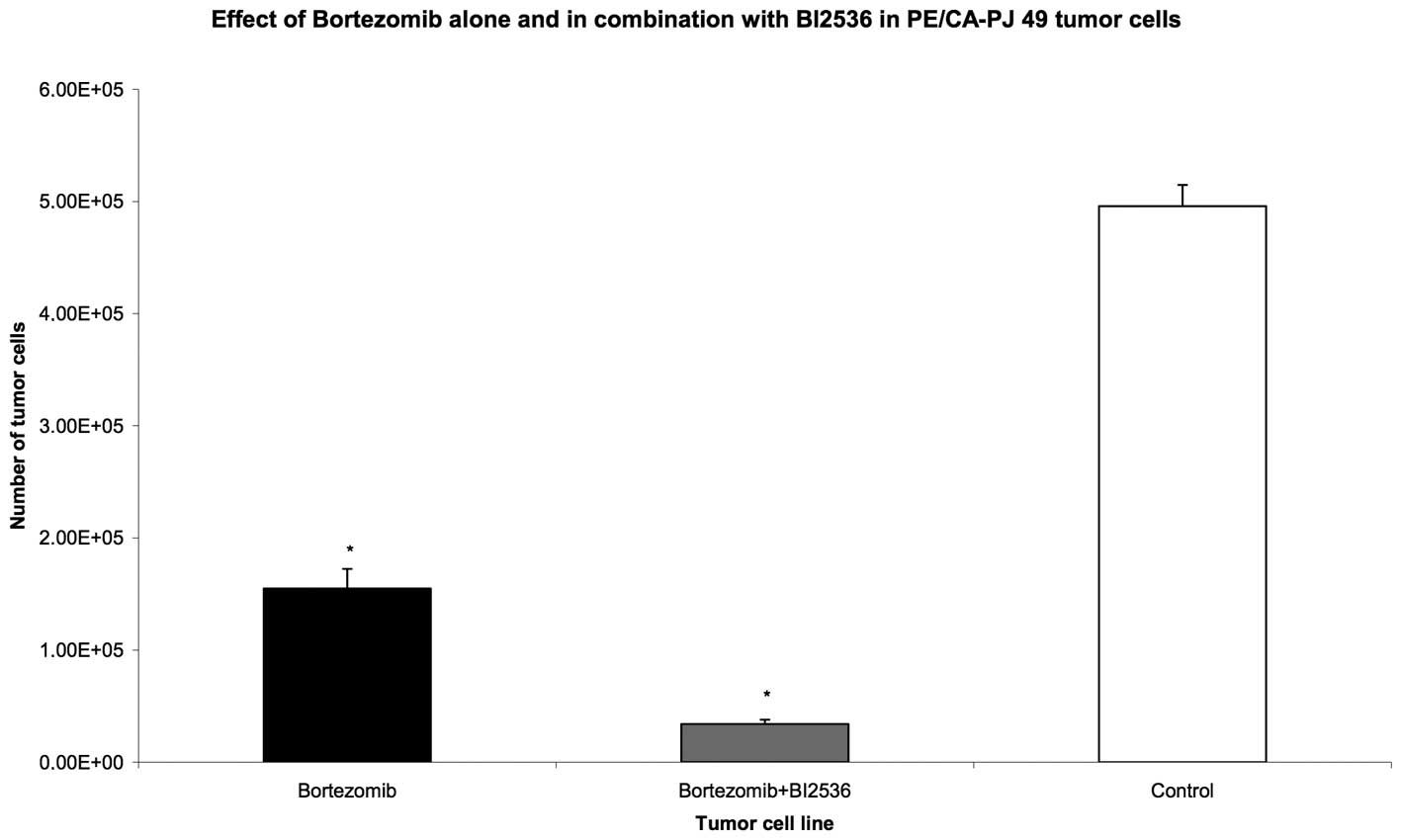
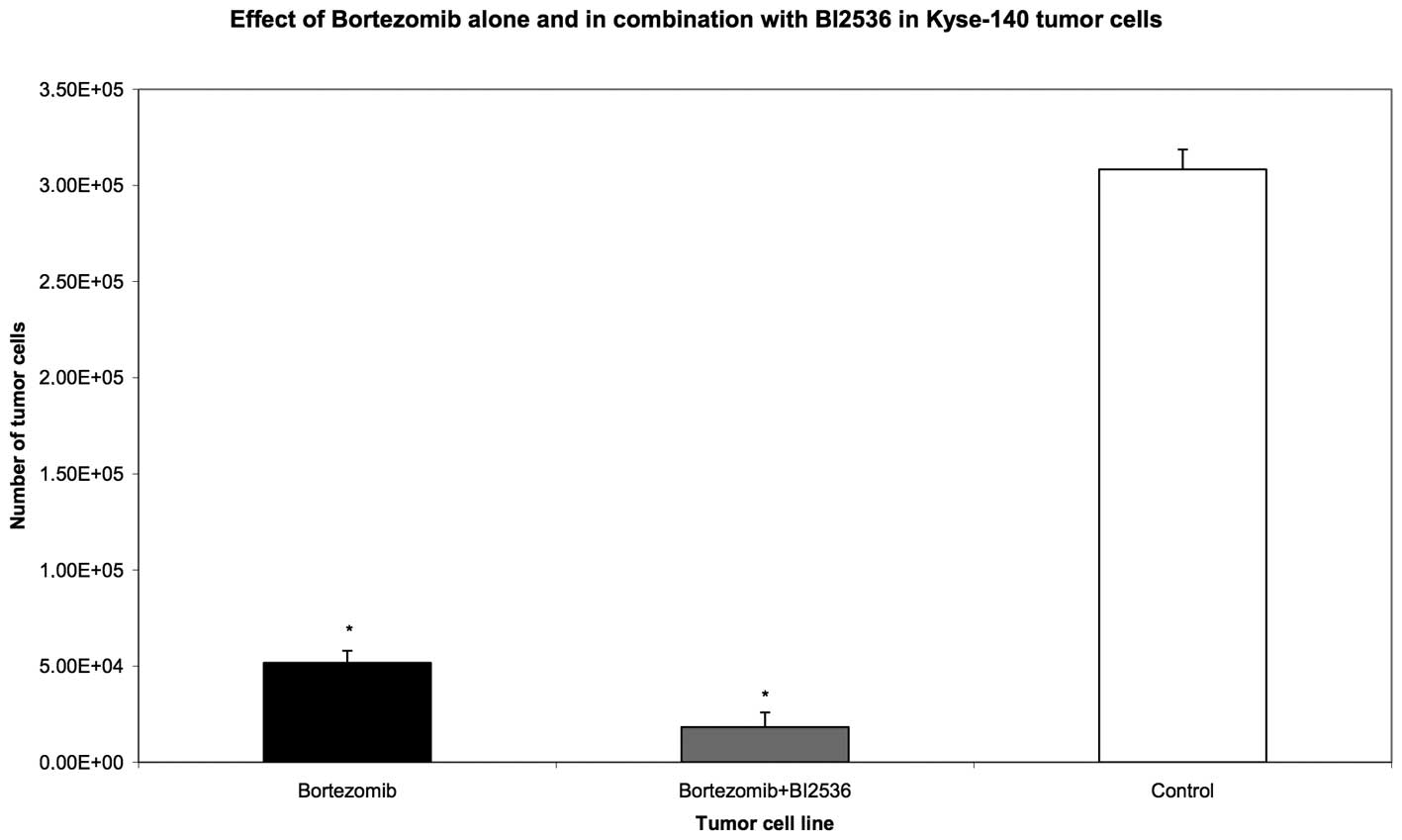
and 72 h, respectively. Compared with the untreated control group,
the proteasome inhibitor, Bortezomib, as well as the PLK-1
inhibitor, BI2536, had highly significant anti-proliferative and
apoptotic activity when used as single agent treatments in all nine
tumour cell lines (P=0.008). The drug combination of Bortezomib and
BI2536 showed a significantly higher antiproliferative activity
compared with Bortezomib alone (P=0.008) (Figs. 1–3).
Apoptosis was detected by microscopic cytohistology as well as by
Human Apoptose Array kit detecting pro caspase 3 as a typical
molecular apoptosis marker.
These results demonstrate that the combination of
proteasome inhibitor, Bortezomib, with the PLK-1 inhibitor, BI2536,
enhances the apoptotic effects of Bortezomib alone and thus leads
to a significantly higher anti-proliferative effect.
Discussion
Head and neck cancer is one of the most common
cancer types worldwide and is associated with many different sites
in the upper aerodigestive tract. Although surgical techniques and
pharmacological regimens have been improved in the past decades,
there has been little improvement in the 5-year survival rate for
patients suffering from these types of malignant tumours (2). Often there is a tumour relapse, which
is typically resistant to conventional antineoplastic drugs
(3). This fact among others,
underlines the great need to develop new therapeutic regimens which
are potent on one hand and well tolerated on the other. Therefore,
identifying new targets with special regard to the various
signalling pathways is one of the greatest challenges at present.
Several in vitro and in vivo studies showed the
proteasome inhibitor Bortezomib to effectively inhibit tumour
growth, induce apoptosis and lower vessel density in SCCHN
(7,16–19).
Furthermore, Bortezomib led to a sustained but limited response
rate in SCCHN patients suffering from tumour recurrence, who
underwent radiation therapy combined with Bortezomib (20,21).
With regard to these findings, it may be possible that optimisation
of Bortezomib efficacy may yield substantial improvement in the
therapy of head and neck cancer although the use of Bortezomib in
SCCHN is discussed controversially in the literature (22,23).
PLK-1 is the most thoroughly characterised member of the polo
family and is known to control critical steps in the passage of
cells through the M phase of the cell cycle, initiating the entry
into mitosis, centrosome separation necessary for the formation of
a bipolar mitotic spindle, metaphase to anaphase transition,
mitotic exit and finally, the onset of cell division (12). BI2536 is a highly selective and
potent small-molecule PLK-1 inhibitor.
In a previous study we demonstrated that BI2536,
when applied as a single agent, showed strong inhibition of cell
proliferation accompanied by enhanced apoptosis in nine different
squamous carcinoma cell lines (unpublished results). In the present
study, we examined the effects of combination therapy of Bortezomib
and BI2536 together in SCCHN cell lines, for the first time. Our
results showed that compared with the untreated control group, the
proteasome inhibitor Bortezomib, as well as the PLK-1 inhibitor
BI2536, had a highly significant anti-proliferative and apoptotic
activity when used as single agent treatment in all nine tumour
cell lines (P=0.008). Notably, the drug combination of Bortezomib
and BI2536 showed significant higher anti-proliferative activity
compared with Bortezomib alone (P=0.008). These findings indicate
that the antiproliferative effect of proteasome inhibitior
Bortezomib is enhanced by adding BI2536.
With regard to the complex mechanism of tumour
biology, and with special regard to the different signalling
pathways that play an important role in tumour genesis, this study
introduces the concept that a cancer may be treated successfully by
targeting several pathways at the same time.
Although Bortezomib showed a notable anticancer
activity in previous in vitro and in vivo set-ups,
its clinical activity in solid tumours is still limited (24–26).
Therefore, new combination regimens targeting the tumour from
different signalling pathways may be one key to success. Due to our
favourable results presented, the addition of BI2536 to Bortezomib
in SCCHN treatment may be subject to further pre-clinical and
clinical phase I investigations.
Acknowledgements
The authors thank Boehringer Ingelheim
GmbH for the donation of BI2536. The authors thank Erika Weith for
her excellent technical support.
References
|
1.
|
A JemalR SiegelE WardY HaoJ XuMJ
ThunCancer statistics, 2009CA Cancer J
Clin59225249200910.3322/caac.20006
|
|
2.
|
A ForastiereW KochA TrottiD SidranskyHead
and neck cancerN Engl J Med34518901900200110.1056/NEJMra001375
|
|
3.
|
MK GibsonAA ForastiereReassessment of the
role of induction chemotherapy for head and neck cancerLancet
Oncol7565574200610.1016/S1470-2045(06)70757-416814208
|
|
4.
|
P SpecenierJB VermorkenCetuximab in the
treatment of squamous cell carcinoma of the head and neckExpert Rev
Anticancer Ther11511524201110.1586/era.11.2021504318
|
|
5.
|
MK GibsonY LiB MurphyMH HussainRC DeContiJ
EnsleyRandomized phase III evaluation of cisplatin plus
fluorouracil versus cisplatin plus paclitaxel in advanced head and
neck cancer (E1395): an intergroup trial of the Eastern Cooperative
Oncology GroupJ Clin
Oncol2335623567200510.1200/JCO.2005.01.05715908667
|
|
6.
|
B BurtnessMA GoldwasserW FloodB MattarAA
ForastierePhase III randomized trial of cisplatin plus placebo
compared with cisplatin plus cetuximab in metastatic/recurrent head
and neck cancer: an Eastern Cooperative Oncology Group studyJ Clin
Oncol2386468654200510.1200/JCO.2005.02.4646
|
|
7.
|
J WagenblastM HambekM BaghiW GstottnerK
StrebhardtH AckermannAntiproliferative activity of bortezomib alone
and in combination with cisplatin or docetaxel in head and neck
squamous cell carcinoma cell linesJ Cancer Res Clin
Oncol134323330200810.1007/s00432-007-0287-917701215
|
|
8.
|
J WagenblastM BaghiC ArnoldnerS BisdasW
GstottnerH AckermannCetuximab enhances the efficacy of bortezomib
in squamous cell carcinoma cell linesJ Cancer Res Clin
Oncol135387393200910.1007/s00432-008-0477-018830627
|
|
9.
|
PL ZhangPK PellitteriA LawPA GilroyGC
WoodTL KennedyOverexpression of phosphorylated nuclear factor-kappa
B in tonsillar squamous cell carcinoma and high-grade dysplasia is
associated with poor prognosisMod
Pathol18924932200510.1038/modpathol.380037215920558
|
|
10.
|
CH ChungJS ParkerK ElyJ CarterY YiBA
MurphyGene expression profiles identify epithelial-to-mesenchymal
transition and activation of nuclear factor-κB signaling as
characteristics of a high-risk head and neck squamous cell
carcinomaCancer Res6682108218200616912200
|
|
11.
|
J AdamsVJ PalombellaEA SausvilleJ JohnsonA
DestreeDD LazarusProteasome inhibitors: a novel class of potent and
effective antitumor agentsCancer Res5926152622199910363983
|
|
12.
|
P SchoffskiPolo-like kinase (PLK)
inhibitors in preclinical and early clinical development in
oncologyOncologist14559570200910.1634/theoncologist.2009-001019474163
|
|
13.
|
P SchoffskiJY BlayJ De GreveE BrainJP
MachielsJC SoriaMulticentric parallel phase II trial of the
polo-like kinase 1 inhibitor BI 2536 in patients with advanced head
and neck cancer, breast cancer, ovarian cancer, soft tissue sarcoma
and melanoma. The first protocol of the European Organization for
Research and Treatment of Cancer (EORTC) Network Of Core Institutes
(NOCI)Eur J Cancer46220622152010
|
|
14.
|
D DattaP BanerjeeM GasserAM Waaga-GasserS
PalCXCR3-B can mediate growth-inhibitory signals in human renal
cancer cells by down-regulating the expression of heme oxygenase-1J
Biol Chem2853684236848201010.1074/jbc.M110.17032420855888
|
|
15.
|
RB RayA RaychoudhuriR SteeleP
NerurkarBitter melon (Momordica charantia) extract inhibits breast
cancer cell proliferation by modulating cell cycle regulatory genes
and promotes apoptosisCancer
Res7019251931201010.1158/0008-5472.CAN-09-343820179194
|
|
16.
|
M LunPL ZhangPK PellitteriA LawTL
KennedyRE BrownNuclear factor-κB pathway as a therapeutic target in
head and neck squamous cell carcinoma: pharmaceutical and
validation in human cell lines using Velcade and siRNA/N molecular
F-κBAnn Clin Lab Sci352512582005
|
|
17.
|
JB SunwooZ ChenG DongN YehC Crowl
BancroftE SausvilleNovel proteasome inhibitor PS-341 inhibits
activation of nuclear factor-κB, cell survival, tumor growth, and
angiogenesis in squamous cell carcinomaClin Cancer
Res7141914282001
|
|
18.
|
A FribleyQ ZengCY WangProteasome inhibitor
PS-341 induces apoptosis through induction of endoplasmic reticulum
stress-reactive oxygen species in head and neck squamous cell
carcinoma cellsMol Cell
Biol2496959704200410.1128/MCB.24.22.9695-9704.2004
|
|
19.
|
C LiR LiJR GrandisDE JohnsonBortezomib
induces apoptosis via Bim and Bik up-regulation and synergizes with
cisplatin in the killing of head and neck squamous cell carcinoma
cellsMol Cancer
Ther716471655200810.1158/1535-7163.MCT-07-244418566236
|
|
20.
|
C AllenK SaigalL NottinghamP ArunZ ChenC
Van WaesBortezomib-induced apoptosis with limited clinical response
is accompanied by inhibition of canonical but not alter-native
nuclear factor-κB subunits in head and neck cancerClin Cancer
Res1441754185200818593997
|
|
21.
|
C Van WaesAA ChangPF LebowitzCH DruzgalZ
ChenYA ElsayedInhibition of nuclear factor-κB and target genes
during combined therapy with proteasome inhibitor bortezomib and
reirradiation in patients with recurrent head-and-neck squamous
cell carcinomaInt J Radiat Oncol Biol Phys63140014122005
|
|
22.
|
A ArgirisAG DuffyS KummarNL SimoneY AraiSW
KimEarly tumor progression associated with enhanced EGFR signaling
with bortezomib, cetuximab, and radiotherapy for head and neck
cancerClin Cancer
Res1757555764201110.1158/1078-0432.CCR-11-086121750205
|
|
23.
|
C LiY ZangM SenRJ Leeman-NeillDS ManJR
GrandisBortezomib up-regulates activated signal transducer and
activator of transcription-3 and synergizes with inhibitors of
signal transducer and activator of transcription-3 to promote head
and neck squamous cell carcinoma cell deathMol Cancer
Ther822112220200910.1158/1535-7163.MCT-09-032719638453
|
|
24.
|
NB DavisDA TaberRH AnsariCW RyanC GeorgeEE
VokesPhase II trial of PS-341 in patients with renal cell cancer: a
University of Chicago phase II consortium studyJ Clin
Oncol22115119200410.1200/JCO.2004.07.16514701773
|
|
25.
|
CN PapandreouDD DalianiD NixH YangT
MaddenX WangPhase I trial of the proteasome inhibitor bortezomib in
patients with advanced solid tumors with observations in
androgen-independent prostate cancerJ Clin
Oncol2221082121200410.1200/JCO.2004.02.10615169797
|
|
26.
|
MH ShahD YoungHL KindlerI WebbB KleiberJ
WrightPhase II study of the proteasome inhibitor bortezomib
(PS-341) in patients with metastatic neuroendocrine tumorsClin
Cancer Res1061116118200410.1158/1078-0432.CCR-04-042215447997
|