Introduction
Inflammatory myofibroblastic tumors (IMTs) are
composed of myofibroblastic cells accompanied by an inflammatory
infiltrate of plasma cells, lymphocytes and eosinophils. IMT used
to be considered as an inflammatory pseudotumor, xanthogranuloma,
plasma-cell granuloma, plasma-cell pseudotumor or an inflammatory
myofibroblastic tumor. IMT commonly occurs in the lung, mesentery,
omentum and retroperitoneum, but it may also be observed in the
extremities, head and neck region, liver, spleen, thyroid,
gastrointestinal tract, genitourinary tract and other systems
(1–11). The tumors usually follow a benign
course, but recurrences have been documented in up to 25% of cases.
Recurrence rates are related to body site, multifocality and
completeness of resection (12–17).
Rare malignant transformation has been reported (18,19).
It is rare for IMT to occur in the breast, and only 19 cases have
been reported in the English literature (20–27).
Moreover, recurrence or metastasis of IMT is exceedingly rare.
Thus, we present a 56-year-old female patient with IMT of the
breast which recurred and metastasized 3, 7 and 10 months after
initial surgery. Our aim is to emphasize that IMT shows
occasionally malignant biological behavior although it is a
neoplasm of intermediate biological potential that frequently
recurs and rarely metastasizes. Thus, clinical physicians should
regularly follow up patients after focal resection for IMT.
Case report
A 56-year-old female was admitted to our hospital 5
days after finding a mass in the upper inner quadrant of her right
breast. The patient had no adenopathy. Mammogram and ultrasound
revealed a 4-cm mass at the 1 o’clock position that was highly
suspicious for malignancy. The rapid frozen section during surgery
revealed that the mass was a tumor with potential malignancy. Thus,
a resection of the mass without lymph nodules was performed after
informing the family of the patient. Grossly, the specimen was a
gray-yellow segment of fibroadipose tissue, measuring 6.8×5.2×3.5
cm; it contained a well-circumscribed gray-white mass measuring
4×4×3 cm. The cut surface was gray-white and the texture was soft.
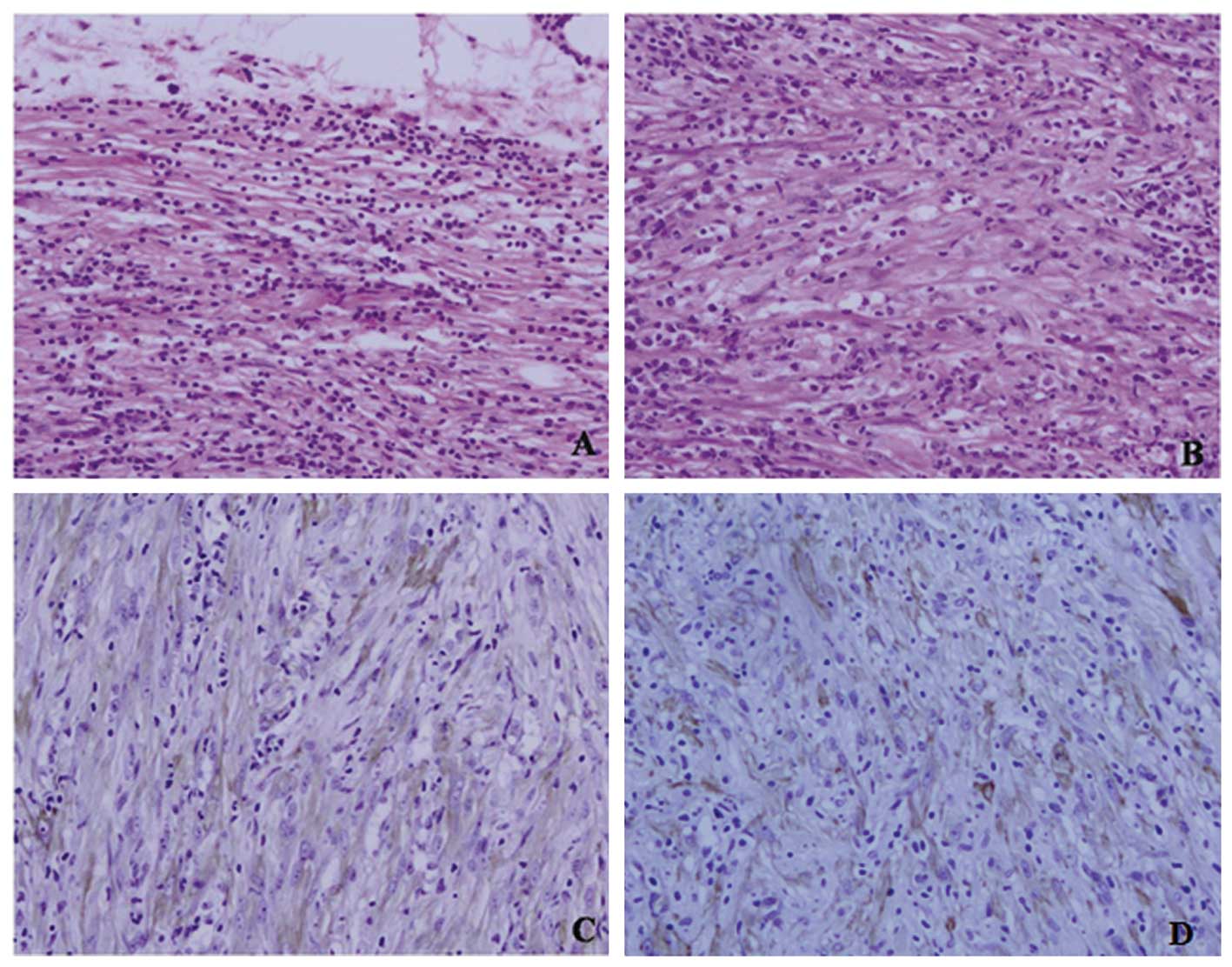
Microscopically, the tumor was mainly composed of spindle cells,
forming swirling storiform-like patterns, and inflammatory cells,
including plasma cells and lymphocytes. The spindle cells were
cytologically bland and most had wispy pink cytoplasm. No mitotic
figures were found (Fig. 1A and B).
Immunohistochemically, the tumor cells were diffusely positive for
SM-actin, anaplastic lymphoma kinase (ALK) and vimentin (Fig. 1C and D) and negative for CK, CD117,
CD34, CD21, CD35, NSE, S-100 and NF. The margins of the resection
were negative for tumor cells. Thus, we made a diagnosis of IMT and
advised regular follow-up. However, three months later, a new mass
measuring 3×3×3 cm was observed at the same site. Ultrasound
revealed a mixed mass, and the site was considered to be a
recurrent focus. A second excision was performed. The
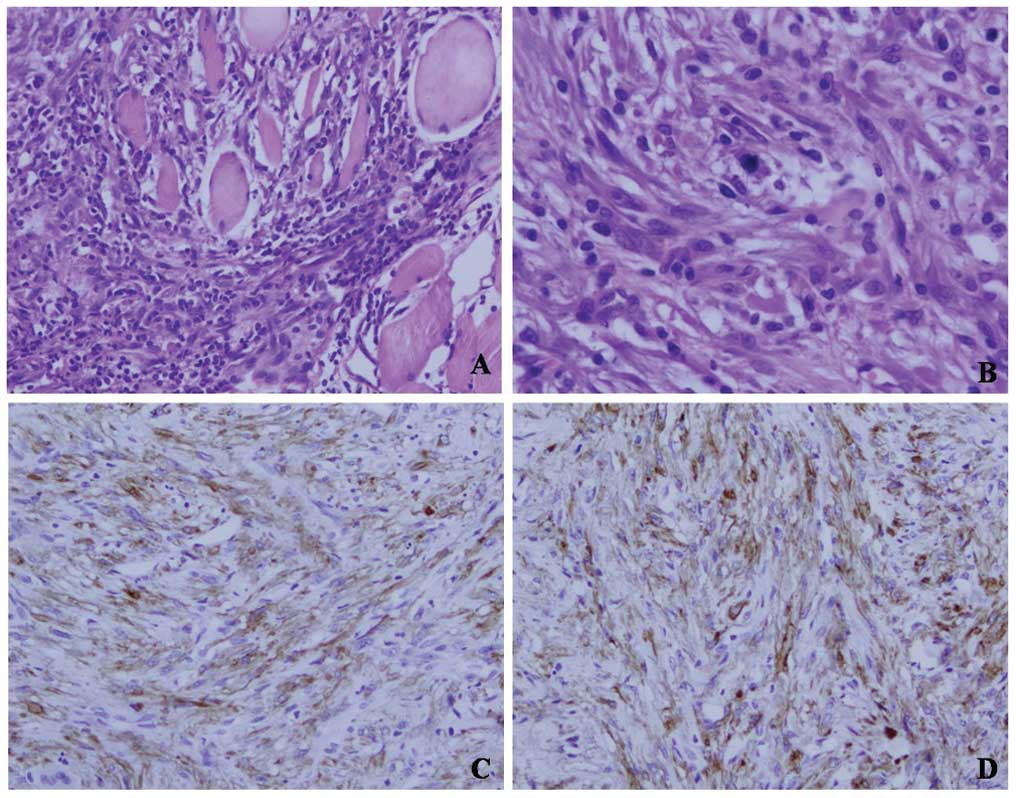
histopathological examination revealed that the tumor cells had
invaded into the surrounding striated muscle. Moreover, mitotic
figures were found (Fig. 2A and B).
Immohistochemically, the tumor cells also expressed SM-actin and
ALK (Fig. 2A and B). Thus, the
diagnosis of malignant IMT was confirmed. Regular follow-up was
again advised. Seven months after the initial surgery, two new
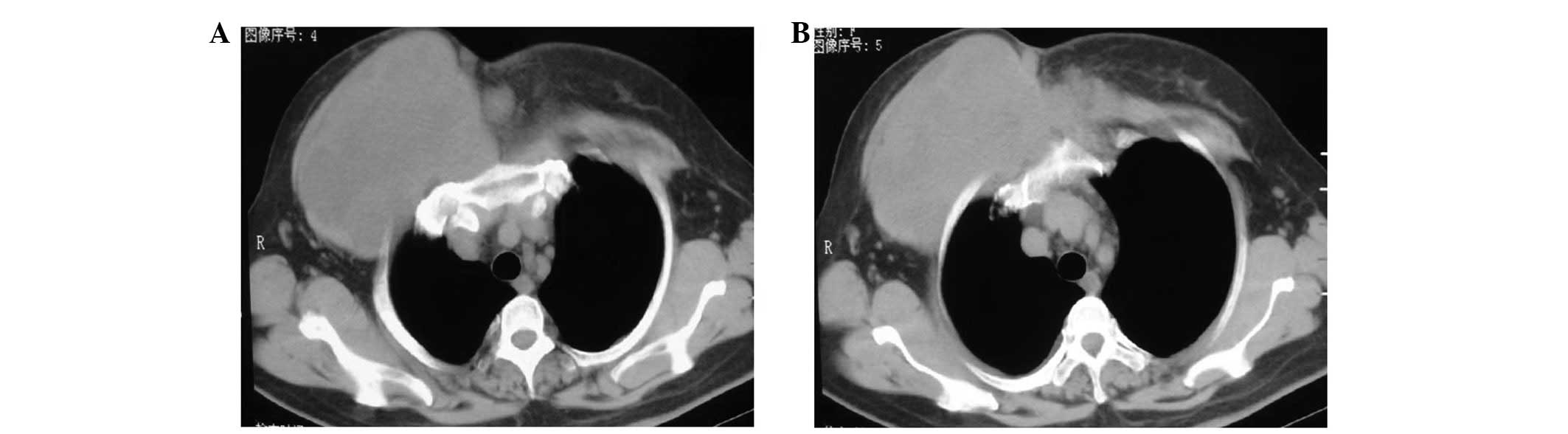
masses measuring 10×8×7 and 4×3×3 cm were found at the same site
and the chest wall, respectively (Fig.
3A). CT scan revealed that the tumor involved the ribs and
vessels (Fig. 3B). It was
impossible to excise the mass. Thus, the patient received
radiotherapy. However, the patient found a mass measuring
1.5×1.0×1.0 cm in her left groin area 10 months after the initial
surgery. Needle biopsy revealed a tumor with spindle cells similar
to the original tumor in the right breast. In addition, the tumor
cells were positive for ALK, SM-actin and vimentin, but negative
for desmin, CD117, CD34, NSE, S-100, CK, ALK, EMA, CD21 and CD35.
Thus, we concluded that the site was a metastatic focus of IMT.
The study protocol was approved by the Medical
Ethics Committee of the Fourth Military Medical University in
Xi’an, China. Written informed consent was obtained from the
patient.
Discussion
IMT was first described in the lungs in 1939
(28). It is an uncommon
mesenchymal tumor, and it has been gradually recognized by
pathologists and clinical physicians. IMT is composed of a spectrum
of fibroblastic or myofibrotic proliferations with a varying
infiltrate of inflammatory cells, including lymphocytes, plasma
cells and histiocytes. Most IMTs occur in the lungs and airways of
young patients. However, other organs, including mesentery,
omentum, stomach, small intestine, large intestine, mediastinum,
retroperitoneum, liver and bladder, have been documented (1–11).
Among these extrapulmonary IMTs, 43% arise in the mesentery and
omentum (29). Cases of IMT of the
breast are scarce. To our knowledge, only 19 cases have been
described in the English literature (20–27).
Moreover, all the IMTs were unilateral and surgically excised.
However, three showed recurrence following surgery, with two of the
three patients having bilateral recurrence (30). With regard to our case, the tumor
showed local recurrence 3 and 7 months after surgery. Notably, a
metastatic focus was confirmed 10 months after the initialsurgical
resection. IMT presents with recurrence, metastasis or malignant
transformation in certain cases, although most tumors behave in a
benign manner after surgical resection, and IMT is classified as an
intermediate neoplasm in the World Health Organization histological
typing. Patients diagnosed with IMT should be regularly followed up
even if surgical resection is performed.
The pathogenesis of IMT is unknown. Some consider
IMT to be an immunological response to an infectious or
non-infectious insult (31,32). Other researchers found that there
was ectopic chromosomal rearrangements in the long arm of
chromosome 2 and the short arm of chromosome 9, and confirmed that
IMT was a monoclonal proliferation by genetic and molecular
techniques (33–36). In addition, approximately half of
IMTs harbor a clonal cytogenetic aberration that activates the
ALK-receptor tyrosine kinase gene at 2p23 (15,37).
Thus, IMT should be considered as a true neoplasm, rather than
inflammatory pseudotumor as at present. These aggressive features,
such as local recurrence, metastasis and malignant transformation,
suggested a neoplastic process. In our case, the tumor cells were
positive for ALK besides SM-actin and vimentin and supported the
diagnosis of IMT.
Similar to most soft-tissue sarcomas, IMTs are
traditionally insensitive to chemotherapy and radiotherapy. In
addition, nonsteroidal anti-inflammatory drugs (NSAIDs), steroids
and cyclosporin-A have been used as treatment modalities, but
surgical resection is considered to be the treatment of choice.
In conclusion, IMT shows occasionally malignant
biological behavior although it is a neoplasm of intermediate
biological potential that frequently recurs and rarely
metastasizes. Thus, clinical physicians should regularly follow up
patients after focal resection for IMT.
Acknowledgements
This study was supported by The
National Natural Science Foundation of China (nos. 30800417 and
30801121).
References
|
1
|
Kim EY, Lee IK, Lee YS, et al:
Inflammatory myofibroblastic tumor in colon. J Korean Surg Soc.
82:45–49. 2012. View Article : Google Scholar
|
|
2
|
Pannain VL, Passos JV, Rocha Filho A,
Villela-Nogueira C and Caroli-Bottino A: Agressive inflammatory
myofibroblastic tumor of the liver with underlying schistosomiasis:
a case report. World J Gastroenterol. 16:4233–4236. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Faraj W, Ajouz H, Mukherji D, Kealy G,
Shamseddine A and Khalife M: Inflammatory pseudo-tumor of the
liver: a rare pathological entity. World J Surg Oncol. 9:52011.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Singh S, Chhabra S, Modi S, Marwah N,
Rawal A and Arora B: Inflammatory pseudo-tumor of the spleen.
Indian J Pathol Microbiol. 52:564–565. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Bruyeer E and Ramboer K: Multiple
inflammatory pseudotumors of the liver and spleen. JBR-BTR.
93:122–123. 2010.PubMed/NCBI
|
|
6
|
Trimeche M, Ziadi S, Mestiri S, et al:
Inflammatory myofibroblastic tumor of the thyroid in its sclerosing
subtype: the first case report. Eur Arch Otorhinolaryngol.
266:763–766. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Choi AH, Bohn OL, Beddow TD and McHenry
CR: Inflammatory myofibroblastic tumor of the small bowel
mesentery: an unusual cause of abdominal pain and uveitis. J
Gastrointest Surg. 15:584–588. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hirschburger M, Enders J, Alzen G, Padberg
W and Wagner HJ: An inflammatory myofibroblastic tumor of the
stomach as a rare cause of gastric outlet obstruction in an
8-month-old infant. Klin Padiatr. 222:192–193. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Chow SC, Nahal A, Mayrand S and Ferri LE:
Pulmonary inflammatory myofibroblastic tumor invading the
gastroesophageal junction. Ann Thorac Surg. 89:1659–1661. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Chen Y, Tang Y, Li H, et al: Inflammatory
myofibroblastic tumor of the esophagus. Ann Thorac Surg.
89:607–610. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lecuona AT, Van Wyk AC, Smit SG, Zarrabi
AD and Heyns CF: Inflammatory myofibroblastic tumor of the bladder
in a 3-year-old boy. Urology. 79:215–218. 2012.PubMed/NCBI
|
|
12
|
Dehner LP: Inflammatory myofibroblastic
tumor: the continued definition of one type of so-called
inflammatory pseudotumor. Am J Surg Pathol. 28:1652–1654. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Coffin CM and Fletcher JA: Inflammatory
myofibroblastic tumour. Pathology and Genetics of Tumours of Soft
Tissue and Bone. Fletcher CDM, Unni KK and Mertens F: World Health
Organization Classification of Tumours, IARC Press; Lyon: pp.
91–93. 2002
|
|
14
|
Coffin CM, Watterson J, Priest JR and
Dehner LP: Extrapulmonary inflammatory myofibroblastic tumor
(inflammatory pseudotumor): A clinicopathologic and
immunohistochemical study of 84 cases. Am J Surg Pathol.
19:859–872. 1995. View Article : Google Scholar
|
|
15
|
Coffin CM, Hornick JL and Fletcher CDM:
Inflammatory myofibroblastic tumor: comparison of
clinicopathologic, histologic, and immunohistochemical features
including ALK expression in atypical and aggressive cases. Am J
Surg Pathol. 31:509–520. 2007. View Article : Google Scholar
|
|
16
|
Makhlouf HR and Sobin LH: Inflammatory
myofibroblastic tumors (inflammatory pseudotumors) of the
gastrointestinal tract: how closely are they related to
inflammatory fibroid polyps? Hum Pathol. 33:307–315. 2002.
View Article : Google Scholar
|
|
17
|
Montgomery EA, Shuster DD, Burkart AL, et
al: Inflammatory myofibroblastic tumors of the urinary tract: a
clinicopathologic study of 46 cases, including a malignant example
inflammatory fibrosarcoma and a subset associated with high-grade
urothelial carcinoma. Am J Surg Pathol. 30:1502–1512. 2006.
View Article : Google Scholar
|
|
18
|
Ernst CW, Van Der Werff Ten Bosch J,
Desprechins B, De Mey J and De Maeseneer M: Malignant
transformation of an abdominal inflammatory myofibroblastic tumor
with distant metastases in a child. JBR-BTR. 94:78–80.
2011.PubMed/NCBI
|
|
19
|
Lu CH, Huang HY, Chen HK, et al: Huge
pelvi-abdominal malignant inflammatory myofibroblastic tumor with
rapid recurrence in a 14-year-old boy. World J Gastroenterol.
16:2698–2701. 2010.PubMed/NCBI
|
|
20
|
Park SB, Kim HH, Shin HJ and Gong G:
Inflammatory pseudotumor (myoblastic tumor) of the breast: a case
report and review of the literature. J Clin Ultrasound. 38:52–55.
2010.PubMed/NCBI
|
|
21
|
Kim SJ, Moon WK, Kim JH, Cho N and Chang
CM: Inflammatory pseudotumor of the breast: a case report with
imaging findings. Korean J Radiol. 10:515–518. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Hill PA: Inflammatory pseudotumor of the
breast: a mimic of breast carcinoma. Breast J. 16:549–550. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Akbulut M, Gunhan-Bilgen I, Zekioglu O,
Duygulu G, Oktay A and Ozdemir N: Fine needle aspiration cytology
of inflammatory myofibroblastic tumour (inflammatory pseudotumour)
of the breast: a case report and review of the literature.
Cytopathology. 18:384–387. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Khanafshar E, Phillipson J, Schammel DP,
Minobe L, Cymerman J and Weidner N: Inflammatory myofibroblastic
tumor of the breast. Ann Diagn Pathol. 9:123–129. 2005. View Article : Google Scholar
|
|
25
|
Ilvan S, Celik V, Paksoy M, Cetinaslan I
and Calay Z: Inflammatory myofibroblastic tumor (inflammatory
pseudotumor) of the breast. APMIS. 113:66–69. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zardawi IM, Clark D and Williamsz G:
Inflammatory myofibroblastic tumor of the breast. A case report
Acta Cytol. 47:1077–1081. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Sastre-Garau X, Couturier J, Derré J,
Aurias A, Klijanienko J and Lagacé R: Inflammatory myofibroblastic
tumour (inflammatory pseudotumour) of the breast:
Clinicopathological and genetic analysis of a case with evidence
for clonality. J Pathol. 196:97–102. 2002. View Article : Google Scholar
|
|
28
|
Brunn H: Two interesting benign lung
tumors of contradictory histopathology: remarks on the necessity
for. J Thorac Surg. 9:119–131. 1939.
|
|
29
|
Khoddami M, Sanae S and Nikkhoo B: Rectal
and appendiceal inflammatory myofibroblastic tumors. Arch Iran Med.
9:277–281. 2006.PubMed/NCBI
|
|
30
|
Vecchio GM, Amico P, Grasso G, Vasquez E,
La Greca G and Magro G: Post-traumatic inflammatory pseudotumor of
the breast with atypical morphological features: A potential
diagnostic pitfall. Report of a case and a critical review of the
literature. Pathol Res Pract. 207:322–326. 2011. View Article : Google Scholar
|
|
31
|
Coffin CM, Humphrey PA and Dehner LP:
Extrapulmonary inflammatory myofibroblastic tumor: a clinical and
pathological survey. Semin Diagn Pathol. 15:85–101. 1998.PubMed/NCBI
|
|
32
|
Hojo H, Newton W, Hamoudi A, et al:
Pseudosarcomatous myofibroblastic tumor or the urinary bladder in
children: a study of 11 cases with review of the literature. Am J
Surg Pathol. 19:1224–1236. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Griffin C, Hawkins A, Dvorak C, et al:
Recurrent involvement of 2p23 in inflammatory myofibroblastic
tumors. Cancer Res. 59:2276–2280. 1999.
|
|
34
|
Su L, Atayde-Perez A, Sheldon S, et al:
Inflammatory myofibroblastic tumor: cytogenetic evidence supporting
clonal origin. Mod Pathol. 11:364–368. 1998.PubMed/NCBI
|
|
35
|
Snyder C, Dell’Aquila M, Haghighi P, et
al: Clonal changes in inflammatory pseudotumor of the lung. A case
report Cancer. 76:1545–1549. 1995.PubMed/NCBI
|
|
36
|
Sastre-Garau X, Couturier J, Derre J, et
al: Inflammatory myofibroblastictumor (inflammatory pseudotumor) of
the breast. Clinicopathological and genetic analysis of a case with
evidence for clonality. J Pathol. 216:97–102. 2002. View Article : Google Scholar
|
|
37
|
Gleason BC and Hornick JL: Inflammatory
myofibroblastic tumours: where are we now? J Clin Pathol.
61:428–437. 2008. View Article : Google Scholar : PubMed/NCBI
|