Introduction
Hepatocellular carcinoma (HCC) is the fifth most
prevalent and third most fatal type of cancer worldwide, and is
currently diagnosed in over half a million people each year
globally (1). Surgical resection is
often performed as the most effective treatment for early-stage
HCC. However, the 5-year risk of recurrence of HCC following
resection is as high as 50–70%, due to its high invasiveness and
the frequent occurrence of intra- and /or extrahepatic
metastases.
Epithelial-mesenchymal transition (EMT) has been
shown to be a pivotal mechanism contributing to cancer invasion and
metastasis, as epithelial cells lose their polarity and acquire the
migratory properties of mesenchymal cells. The characteristic
changes during EMT include the downregulation of epithelial
markers, such as E-cadherin and the upregulation of mesenchymal
markers such as vimentin (2). A
correlation between the expression profiles of EMT and EMT
inducers, and tumor recurrence and distant metastasis, has been
demonstrated in certain types of cancer, including breast and colon
cancer (3,4). With regards to HCC, several
EMT-related transcription factors such as Snail, Twist and Zinc
finger E-box binding protein 1 (ZEB1) have been demonstrated to be
involved in the process of EMT, and thus associated with a poor
prognosis (5,6).
Transforming growth factor (TGF)-β signaling plays a
central role in tumorigenesis and tumor progression by regulating
many critical cellular processes, including cell proliferation,
apoptosis and EMT. TGF-β binds to type I and type II
serine/threonine kinase receptors, resulting in the trans-location
of phosphorylated Smad proteins (phospho-Smad2 and 3) into the
nucleus where they regulate the expression of various target genes
(7). A previous study using HCC
cell lines demonstrated that TGF-β signaling triggered EMT, which
was characterized by
E-cadherinlow/vimentinhigh expression in
vitro(8). However, the
existence of a clinical association between the expression profiles
of EMT markers and TGF-β signaling, and its significance in HCC
patients remains to be elucidated.
In the present study, we investigated the expression
of the EMT markers E-cadherin and vimentin (epithelial and
mesenchymal markers, respectively) and phospho-Smad2 by
immunohistochemical (IHC) analyses. The clinical significance of
the expression profiles and TGF-β signaling in HCC patients were
further assessed.
Materials and methods
Patients and treatment
One-hundred and fifty primary HCC samples amongst
235 consecutive patients who underwent curative hepatic resection
in the Department of Gastroenterological Surgery, Graduate School
of Medical Sciences, Kumamoto University, between 2004 and 2007,
were analyzed in this study. None of the patients had received any
pre-operative anticancer treatment. The pathologic diagnoses and
clinicopathological features were established based on the general
guideline for primary liver cancer as defined by the Liver Cancer
Study Group of Japan (9,10) and the American Joint Committee on
Cancer (AJCC)/International Union Against Cancer (UICC) staging
system (11). The median follow-up
duration following surgery was 44 months. Informed consent was
obtained from all patients and the study was approved by the Human
Ethics Review Committee of the Graduate School of Medicine,
Kumamoto University (Kumamoto, Japan).
Immunohistochemistry and scoring
The sample processing and IHC procedures were
performed as described in a previous study by Okabe et
al(12). Endogenous peroxidase
activity was blocked using 3% hydrogen peroxide and the sections
were incubated with diluted antibodies. A subsequent reaction was
performed with a biotin-free horseradish peroxidase enzyme-labeled
polymer from the Envision Plus detection system (Dako Japan Inc.,
Tokyo, Japan). Phospho-Smad2 antibody binding was detected using
the Vectastain ABC Elite avidin/biotin/peroxidase kit (Vector
Laboratories Inc., Burlingame, CA, USA). A positive reaction was
visualized with the addition of diaminobenzidine solution, which
was followed by counterstaining with Mayer’s hematoxylin solution.
Primary antibodies for E-cadherin (1:100 dilution; Japan BD, Tokyo,
Japan), vimentin (1:50 dilution; Santa Cruz Biotechnology, Inc.,
Santa Cruz, CA, USA), and phospho-Smad2 (1:100 dilution; Cell
Signaling Technology Japan, Tokyo, Japan) were used for this study.
All IHC staining results were independently scored by two
pathologists. The membranous E-cadherin and cytoplasmic vimentin
expression was interpreted according to the guidelines published in
a previous study by Yang et al(13). For membranous E-cadherin,
cytoplasmic vimentin and phospho-Smad2-positive nuclei, the results
were graded into categories from 0–3+ as follows: 0, no staining;
1+, 1–25% staining; 2+, 26–50% staining and 3+, >50% of the
specimen was stained. For membranous E-cadherin and
phospho-Smad2-positive nuclei, the 2+ and 3+ samples were defined
as positive IHC results. For cytoplasmic vimentin, the 3+ specimens
were defined as positive IHC results.
Statistical analyses
All experiments were performed in triplicate and the
data shown are representative of the results. Categorical variables
were compared using a χ2 test. Overall survival and
disease-free survival were calculated using the Kaplan-Meier method
and compared using a log-rank test. Statistical analyses were
performed as indicated with a statistical analysis software program
(Excel Statistics, Social Survey Research Information Co. Ltd.,
Tokyo, Japan). P<0.05 was considered to indicate a statistically
significant difference.
Results
EMT expression profile in HCC patients
and its correlation with TGF-β signaling
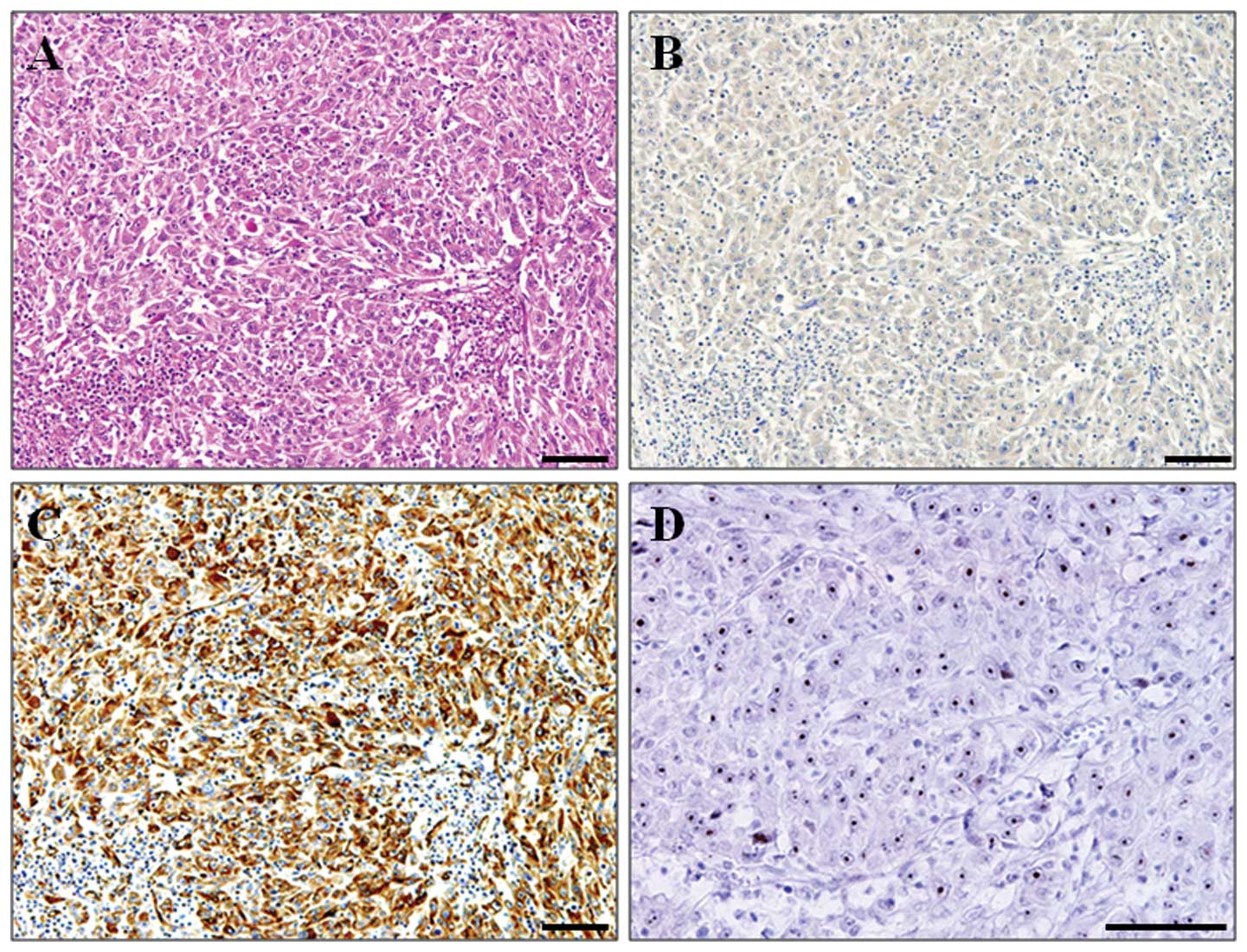
E-cadherin was mainly expressed in the tumor cell
membrane, whereas vimentin was expressed in the tumor cytoplasm
(Fig. 1). Among the 150 HCC
patients, low and high E-cadherin expression levels were found in
92 (61.3%) and 58 (38.7%) patients, respectively. On the other
hand, low and high vimentin expression levels were found in 126
(84.0%) and 24 (16.0%) patients, respectively. The EMT expression
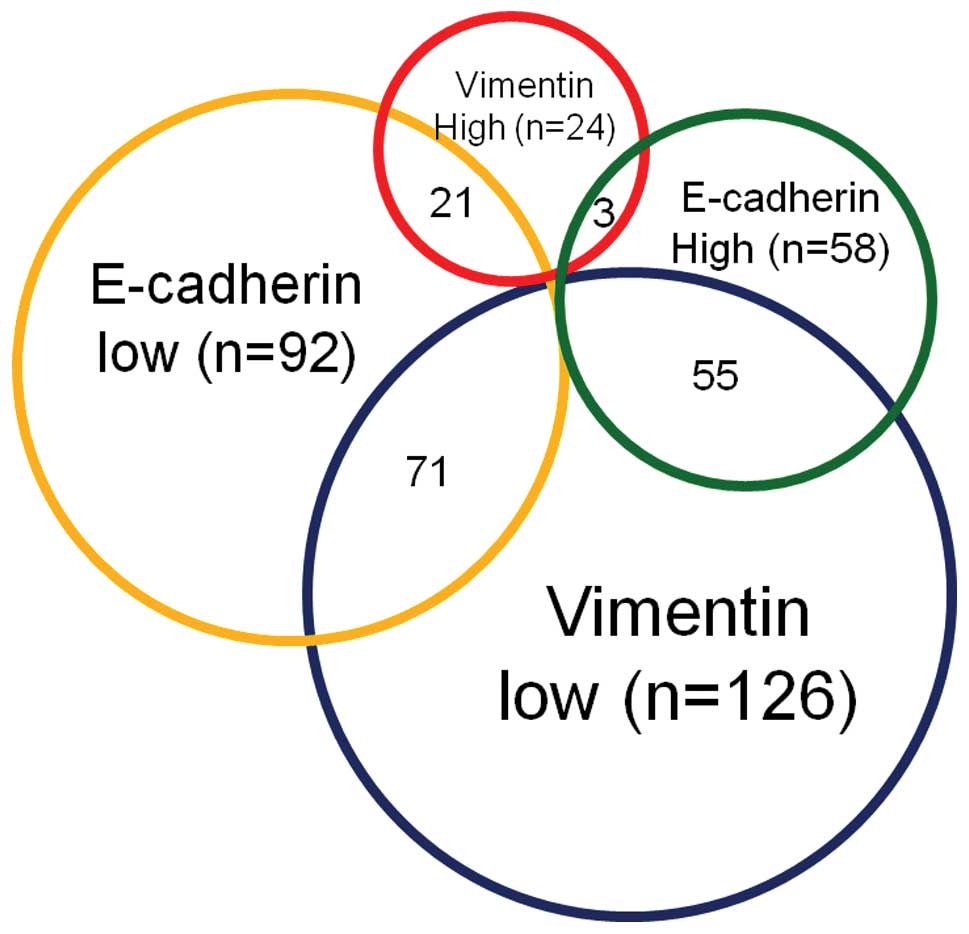
profiles in 150 HCC patients are summarized in Fig. 2. In patients with HCC, a majority
(47.3%) exhibited an
E-cadherinlow/vimentinlow expression profile,
whereas a minority (2%) had an
E-cadherinhigh/vimentinhigh expression
profile. Of the 150 HCC patients, 55 (36.7%) were revealed to have
an E-cadherinhigh/vimentinlow expression
profile (low ability of EMT), whereas 21 (14.0%) were diagnosed
with an E-cadherinlow/vimentinhigh profile
(high ability of EMT). TGF-β signaling plays a central role in EMT;
treatment of HCC cell lines with TGF-β1 induced the
E-cadherinlow/vimentinhigh expression profile
indicative of high ability of EMT (8). Therefore, we assessed the clinical
relevance of TGF-β signaling in patients with HCC by IHC analysis
of phospho-Smad2; 41 (27.3%) of 150 patients showed high
phospho-Smad2 nuclear positivity. To investigate the clinical
association between the EMT marker profiles and TGF-β signaling,
phospho-Smad2 nuclear positivity was compared between the subgroups
with an E-cadherinhigh/vimentinlow (low
ability of EMT) or E-cadherinlow/vimentinhigh
(high ability of EMT) expression profile. The
E-cadherinlow/vimentinhigh expression profile
was significantly correlated with high phospho-Smad2 nuclear
positivity, compared with the
E-cadherinhigh/vimentinlow expression profile
(P<0.001; Table I). These
findings suggest that TGF-β signaling is closely associated with
the EMT expression profile in HCC patients.
 | Table ICorrelation between
E-cadherin/vimentin expression and clinicopathological features in
HCC patients. |
Table I
Correlation between
E-cadherin/vimentin expression and clinicopathological features in
HCC patients.
|
E-cadherinhigh/vimentinlow
(n=55) |
E-cadherinlow/vimentinhigh
(n=21) | P-value |
|---|
| Age (years) | | | |
| ≤60 | 20 | 8 | |
| >60 | 35 | 13 | 0.889 |
| Gender | | | |
| Male | 46 | 18 | |
| Female | 9 | 3 | 0.824 |
| HBs-Ag | | | |
| Negative | 38 | 15 | |
| Positive | 17 | 6 | 0.842 |
| HCV-Ab | | | |
| Negative | 32 | 11 | |
| Positive | 23 | 10 | 0.648 |
| Child-Pugh
classification | | | |
| A | 53 | 19 | |
| B | 2 | 2 | 0.304 |
| AFP (ng/ml) | | | |
| ≤20 | 27 | 9 | |
| >20 | 28 | 12 | 0.627 |
| PIVKA-II
(mAU/ml) | | | |
| ≤107 | 29 | 12 | |
| >107 | 26 | 9 | 0.730 |
| Tumor size
(cm) | | | |
| ≤3 | 20 | 6 | |
| >3 | 35 | 15 | 0.522 |
| Number of
tumors | | | |
| 1 | 43 | 12 | |
| ≥2 | 12 | 9 | 0.067 |
| Tumor
differentiation | | | |
|
Moderate/well | 50 | 11 | |
| Poor | 5 | 10 |
<0.001 |
| LCSGJ TNM
stage | | | |
| 1 and 2 | 38 | 10 | |
| 3 and 4 | 17 | 11 | 0.083 |
| AJCC/UICC TNM
stage | | | |
| 1 and 2 | 47 | 16 | |
| 3 and 4 | 8 | 5 | 0.338 |
| Vascular
invasiona | | | |
| Absent | 54 | 17 | |
| Present | 1 | 4 | 0.007 |
| Extrahepatic
recurrenceb | | | |
| Absent | 53 | 17 | |
| Present | 2 | 4 | 0.026 |
| Phospho-Smad2
nuclear positivity | | | |
| Negative | 42 | 7 | |
| Positive | 13 | 14 |
<0.001 |
E-cadherinlow/vimentinhigh expression profile
is associated with tumor invasion and metastasis in HCC
patients
The correlation between the
E-cadherinlow/vimentinhigh expression profile
and clinical invasiveness in HCC was further investigated by
comparing the clinicopathological features between patients with
E-cadherinlow/vimentinhigh (high ability of
EMT) and E-cadherinhigh/vimentinlow (low
ability of EMT) expression profiles (Table I). The
E-cadherinlow/vimentinhigh expression profile
was significantly correlated with poor tumor differentiation
(P<0.001), vascular invasion (P=0.007) and extrahepatic
recurrence following curative surgery (P=0.026), compared with the
E-cadherinhigh/vimentinlow expression
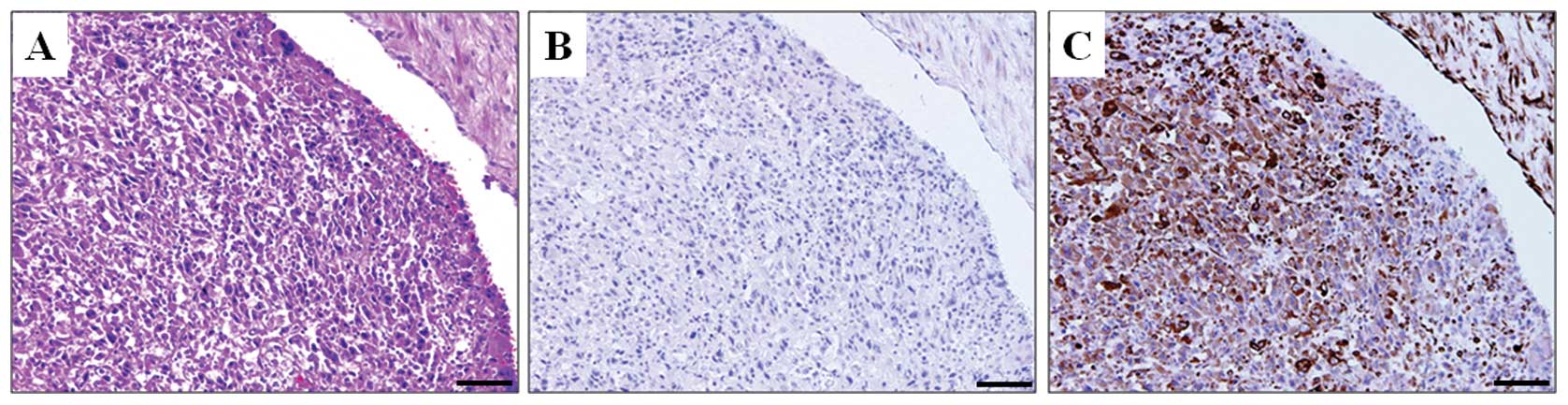
profile. Cancer cells invading the portal vein, which is a
distinctive characteristic of HCC, were negative for E-cadherin and
positive for vimentin expression (Fig.
3). These findings suggest that EMT plays an important role in
the invasive and metastatic phenotype of human HCC. In addition, we
compared the clinicopathological features between patients with low
and high phospho-Smad2 nuclear positivity. High phospho-Smad2
nuclear positivity was significantly correlated with the
E-cadherinlow/vimentinhigh expression profile
(P<0.001). Although high phospho-Smad2 nuclear positivity was
positively correlated with large tumor size (P=0.154), multiple
tumors (P=0.110) and poor tumor differentiation (P=0.154) compared
with low phospho-Smad2 nuclear positivity, the correlations were
not statistically significant (Table
II).
 | Table IICorrelation between phospho-Smad2
nuclear positivity and clinicopathological features in HCC
patients. |
Table II
Correlation between phospho-Smad2
nuclear positivity and clinicopathological features in HCC
patients.
| Low phospho-Smad2
(n=109) | High phospho-Smad2
(n=41) | P-value |
|---|
| Age (years) | | | |
| ≤60 | 34 | 10 | |
| >60 | 75 | 31 | 0.415 |
| Gender | | | |
| Male | 87 | 36 | |
| Female | 22 | 5 | 0.256 |
| HBs-Ag | | | |
| Negative | 77 | 30 | |
| Positive | 32 | 11 | 0.760 |
| HCV-Ab | | | |
| Negative | 62 | 19 | |
| Positive | 47 | 22 | 0.248 |
| Child-Pugh
classification | | | |
| A | 97 | 38 | |
| B | 12 | 3 | 0.502 |
| AFP (ng/ml) | | | |
| ≤20 | 58 | 18 | |
| >20 | 51 | 23 | 0.310 |
| PIVKA-II
(mAU/ml) | | | |
| ≤107 | 56 | 19 | |
| >107 | 53 | 22 | 0.583 |
| Tumor size
(cm) | | | |
| ≤3 | 40 | 10 | |
| >3 | 69 | 31 | 0.154 |
| Number of
tumors | | | |
| 1 | 81 | 25 | |
| ≥2 | 28 | 16 | 0.110 |
| Tumor
differentiation | | | |
|
Moderate/well | 91 | 30 | |
| Poor | 18 | 11 | 0.154 |
| LCSGJ TNM
stage | | | |
| 1 and 2 | 67 | 22 | |
| 3 and 4 | 42 | 19 | 0.386 |
| AJCC/UICC TNM
stage | | | |
| 1 and 2 | 87 | 34 | |
| 3 and 4 | 22 | 7 | 0.667 |
| Vascular
invasiona | | | |
| Absent | 101 | 37 | |
| Present | 8 | 4 | 0.627 |
| Extrahepatic
recurrenceb | | | |
| Absent | 102 | 36 | |
| Present | 7 | 5 | 0.245 |
|
E-cadherinlow/vimentinhigh | | | |
| Negative | 102 | 27 | |
| Positive | 7 | 14 |
<0.001 |
E-cadherinlow/vimentinhigh expression profile
is associated with poor disease-free survival in HCC patients
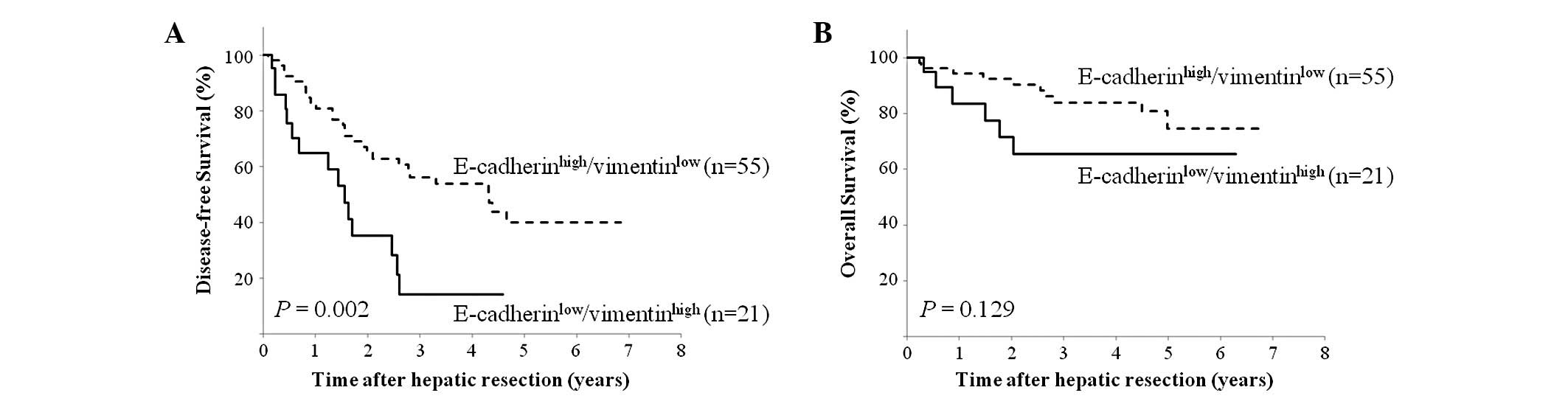
We investigated the prognostic implications of the
E-cadherinlow/vimentin-high expression profile in HCC
patients. The E-cadherinlow/vimentinhigh
expression profile was significantly correlated with shorter
disease-free survival, compared to the
E-cadherinhigh/vimentinlow expression profile
(P= 0.002; Fig. 4A).
E-cadherinlow/vimentinhigh was also
correlated with poorer overall survival than
E-cadherinhigh/vimentinlow; however, the
difference was not statistically significant (P=0.129; Fig. 4B).
Discussion
This study demonstrated that a high ability of EMT
(an E-cadherinlow/vimentinhigh expression
profile) was closely correlated with high-grade malignant behavior,
such as vascular invasion, extrahepatic recurrence and poor
disease-free survival, in HCC patients. In addition, the
overexpression of vimentin, but not E-cadherin, was implicated in
poorer disease-free survival in HCC patients. Vimentin expression
has also been demonstrated to be correlated with the invasive
phenotype in patients with gastric and breast cancer (14,15).
Cancer cell invasion of the portal vein is a clinically defined
characteristic of HCC. Notably, the cancer cells invading the
portal vein revealed a high ability of EMT, reflected in their
E-cadherinlow/vimentinhigh expression profile
(Fig. 3). In contrast, analysis of
another cohort of 123 HCC samples revealed no correlation between
either Snail/Twist overexpression or E-cadherin downregulation and
vascular invasion (5). This study
suggests that the loss of E-cadherin followed by the overexpression
of vimentin may play a pivotal role in the invasive and metastatic
phenotype and in the process of EMT, leading to unfavorable
outcomes in patients with HCC.
TGF-β signaling is known to be a potent EMT inducer
and is strongly associated with cancer progression; EMT primes
cancer cells for pulmonary metastasis and metastatic colonization
in the bones (16,17). In the present study, activated TGF-β
signaling indicated by phospho-Smad2 nuclear positivity was found
in 27.3% (41/150) of HCC patients. Furthermore, phospho-Smad2
nuclear positivity was correlated with a high ability of EMT
(E-cadherinlow/vimentinhigh) in HCC patients.
It has been demonstrated that TGF-β1 is overexpressed in tumor
cells, which is associated with a poor prognosis in patients with
HCC (18,19). These findings suggest that the
activation of TGF-β signaling is closely associated with EMT
expression profiles in HCC patients. However, TGF-β signaling was
not activated in one-third of patients with an
E-cadherinlow/vimentinhigh expression
profile. Although the precise mechanism of EMT induction in these
patients remains unclear, it is possible that several transcription
factors (such as Snail, Twist and ZEB1) as well as other molecules
are also involved in the process of EMT and are therefore
associated with a poor prognosis in HCC (5,6,20–27).
Thus, several mechanisms, including TGF-β signaling, may be
involved in the process of EMT in HCC patients.
In conclusion, EMT expression profiles are useful
prognostic markers for disease-free survival in HCC patients, and
an E-cadherinlow/vimentinhigh expression
profile is closely associated with high-grade malignant behavior,
such as tumoral vascular invasion and metastasis in HCC.
TGF-β-mediated EMT may play a significant role in the
aggressiveness of HCC.
Acknowledgements
The authors thank Keisuke Miyake and
Naoko Yokoyama for their valuable technical assistance.
References
|
1
|
El-Serag HB: Hepatocellular carcinoma. N
Engl J Med. 365:1118–1127. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Thiery JP, Acloque H, Huang RY and Nieto
MA: Epithelialmesenchymal transitions in development and disease.
Cell. 139:871–890. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Moody SE, Perez D, Pan TC, et al: The
transcriptional repressor Snail promotes mammary tumor recurrence.
Cancer Cell. 8:197–209. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Shioiri M, Shida T, Koda K, et al: Slug
expression is an independent prognostic parameter for poor survival
in colorectal carcinoma patients. Br J Cancer. 94:1816–1822. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Yang MH, Chen CL, Chau GY, et al:
Comprehensive analysis of the independent effect of twist and snail
in promoting metastasis of hepatocellular carcinoma. Hepatology.
50:1464–1474. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zhou YM, Cao L, Li B, et al:
Clinicopathological significance of ZEB1 protein in patients with
hepatocellular carcinoma. Ann Surg Oncol. 19:1700–1706. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Siegel PM and Massagué J: Cytostatic and
apoptotic actions of TGF-beta in homeostasis and cancer. Nat Rev
Cancer. 3:807–821. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
8
|
Mima K, Okabe H, Ishimoto T, et al: CD44s
regulates the TGF-β-mediated mesenchymal phenotype and is
associated with poor prognosis in patients with hepatocellular
carcinoma. Cancer Res. 72:3414–3423. 2012.PubMed/NCBI
|
|
9
|
Liver Cancer Study Group of Japan: The
General Rules for the Clinical and Pathological Study of Primary
Liver Cancer. Kanehara, Tokyo: 2009
|
|
10
|
Minagawa M, Ikai I, Matsuyama Y, Yamaoka Y
and Makuuchi M: Staging of hepatocellular carcinoma: assessment of
the Japanese TNM and AJCC/UICC TNM systems in a cohort of 13,772
patients in Japan. Ann Surg. 245:909–922. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Vauthey JN, Lauwers GY, Esnaola NF, et al:
Simplified staging for hepatocellular carcinoma. J Clin Oncol.
20:1527–1536. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Okabe H, Beppu T, Hayashi H, et al:
Hepatic stellate cells accelerate the malignant behavior of
cholangiocarcinoma cells. Ann Surg Oncol. 18:1175–1184. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yang MH, Hsu DS, Wang HW, et al: Bmi1 is
essential in Twist1-induced epithelial-mesenchymal transition. Nat
Cell Biol. 12:982–992. 2010. View
Article : Google Scholar : PubMed/NCBI
|
|
14
|
Iwatsuki M, Mimori K, Fukagawa T, et al:
The clinical significance of vimentin-expressing gastric cancer
cells in bone marrow. Ann Surg Oncol. 17:2526–2533. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Vora HH, Patel NA, Rajvik KN, et al:
Cytokeratin and vimentin expression in breast cancer. Int J Biol
Markers. 24:38–46. 2009.PubMed/NCBI
|
|
16
|
Padua D, Zhang XH, Wang Q, et al: TGFbeta
primes breast tumors for lung metastasis seeding through
angiopoietin-like 4. Cell. 133:66–77. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yin JJ, Selander K, Chirgwin JM, et al:
TGF-beta signaling blockade inhibits PTHrP secretion by breast
cancer cells and bone metastases development. J Clin Invest.
103:197–206. 1999. View
Article : Google Scholar : PubMed/NCBI
|
|
18
|
Bedossa P, Peltier E, Terris B, Franco D
and Poynard T: Transforming growth factor-beta 1 (TGF-beta 1) and
TGF-beta 1 receptors in normal, cirrhotic, and neoplastic human
livers. Hepatology. 21:760–766. 1995.PubMed/NCBI
|
|
19
|
Abou-Shady M, Baer HU, Friess H, et al:
Transforming growth factor betas and their signaling receptors in
human hepatocellular carcinoma. Am J Surg. 177:209–215. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Lee TK, Man K, Poon RT, et al: Signal
transducers and activators of transcription 5b activation enhances
hepatocellular carcinoma aggressiveness through induction of
epithelial-mesenchymal transition. Cancer Res. 66:9948–9956. 2006.
View Article : Google Scholar
|
|
21
|
Chen L, Chan TH, Yuan YF, et al: CHD1L
promotes hepatocellular carcinoma progression and metastasis in
mice and is associated with these processes in human patients. J
Clin Invest. 120:1178–1191. 2010. View
Article : Google Scholar : PubMed/NCBI
|
|
22
|
Chung KY, Cheng IK, Ching AK, Chu JH, Lai
PB and Wong N: Block of proliferation 1 (BOP1) plays an oncogenic
role in hepatocellular carcinoma by promoting
epithelial-to-mesenchymal transition. Hepatology. 54:307–318. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Fu J, Chen Y, Cao J, et al: p28GANK
overexpression accelerates hepatocellular carcinoma invasiveness
and metastasis via phosphoinositol 3-kinase/AKT/hypoxia-inducible
factor-1alpha pathways. Hepatology. 53:181–192. 2011. View Article : Google Scholar
|
|
24
|
Kim H, Choi GH, Na DC, et al: Human
hepatocellular carcinomas with “stemness”-related marker
expression: keratin 19 expression and a poor prognosis. Hepatology.
54:1707–1717. 2011.
|
|
25
|
Wang J, Chen L, Li Y and Guan XY:
Overexpression of cathepsin Z contributes to tumor metastasis by
inducing epithelial-mesenchymal transition in hepatocellular
carcinoma. PLoS One. 6:e249672011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Yokomizo C, Yamaguchi K, Itoh Y, et al:
High expression of p300 in HCC predicts shortened overall survival
in association with enhanced epithelial mesenchymal transition of
HCC cells. Cancer Lett. 310:140–147. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zhu K, Dai Z, Pan Q, et al: Metadherin
promotes hepatocellular carcinoma metastasis through induction of
epithelial-mesenchymal transition. Clin Cancer Res. 17:7294–7302.
2011. View Article : Google Scholar : PubMed/NCBI
|