Introduction
Epithelial ovarian carcinoma is one of the most
common gynecological malignancies and the fifth most frequent cause
of cancer mortality in females (1).
Epithelial ovarian and peritoneal carcinomas and primary carcinoma
of the Fallopian tube are all classified as Müllerian carcinomas.
These diseases exhibit a number of similarities and are often
treated using similar therapeutic protocols. Advanced epithelial
ovarian cancer is highly chemosensitive and 70–80% of patients
initially respond to platinum-based chemotherapy. However, in
60–80% of cases these tumors increase in severity and treatment
with second-line agents becomes necessary (2,3).
Tumors which do not progress in severity until more than 6 months
following completion of first-line platinum-based treatment are
considered likely to remain platinum-sensitive and generally
receive a chemotherapy regimen containing a platinum agent.
However, tumors associated with severe progression in less than 6
months following completion of the first-line chemotherapy are
considered to be platinum-resistant (4). Secondary treatments currently induce a
response in 14–40% of patients and these responses are generally of
short duration (5). This results in
rapid disease recurrence following discontinuation of therapy. At
present, it is considered extremely difficult to cure recurrent
ovarian cancer and existing therapies are considered suitable for
increasing the duration of survival and palliative therapy only.
Consequently, suitable therapeutic agents for the treatment of this
disease are required to demonstrate efficacy and low toxicity.
Doxorubicin has a wide spectrum of activities in
human tumors and is considered to be suitable as an active agent
for the treatment of advanced ovarian cancer (6–8).
However, use of this anthracycline agent is limited by
side-effects, including gastrointestinal toxicity, myelosuppression
and cumulative cardiac damage (9).
Pegylated liposomal doxorubicin (PLD) is a unique formulation of
conventional doxorubicin that avoids phagocytosis. This results in
prolonged circulation time with drug retention in liposomes and
selective accumulation in the tumor vascular bed following
extravasation through the leaky tumor vasculature (10,11).
PLD was designed specifically to enhance the efficacy of
doxorubicin and reduce its toxicities, including myelosuppression,
alopecia and cardiotoxicity.
The aim of the present study was to evaluate the
effects of PLD in a non-trial setting to evaluate the efficacy and
safety of the treatment.
Materials and methods
Patients
The present study was retrospective and aimed to
evaluate the efficacy and safety of PLD in Japanese patients with
ovarian carcinoma previously treated with platinum-based
chemotherapy at our hospital. The primary endpoint of the study was
determination of best overall response. The secondary endpoint
included analysis of adverse events and drug reactions and duration
of response. The study included 18 patients with epithelial ovarian
carcinoma and 1 with peritoneal carcinoma and was approved by the
Ethics Committee of Osaka City University. All patients were
identified as platinum-resistant. Written informed consent was
provided by all patients prior to treatment with PLD at our
hospital between July 2009 and March 2011.
Treatment and clinical course
analysis
Patients were treated with PLD at a dose of 50
mg/m2 every 4 weeks, which was delivered in 250 ml of 5%
dextrose solution and administered as an intravenous infusion over
1 h. All patients received standard premedication of intravenous
dexamethasone (10 mg) and ramosetron hydrochloride (0.3 mg). Prior
to each PLD cycle, toxicity evaluations were performed. Tumor
response was reassessed by MRI following every 2–3 cycles of
treatment in all cases. Tumor response evaluation was performed
according to the RECIST guidelines. The severity of adverse events
was assessed according to the Common Terminology Criteria for
Adverse Events (v3.0). Subsequent courses of chemotherapy were
planned every 28 days pending an absolute neutrophil count (ANC) of
1,500 cells/ml and resolution of ≥grade II nonhematological
toxicity. In the event of persistent neutropenia (ANC <1,500
cells/ml) or ≥grade II nonhematological toxicity, the therapy was
delayed and the patients were reevaluated weekly using these
criteria for reinstitution of therapy. PLD dose reductions of 10
mg/m2 were instituted in the event of grade III or IV
nonhematological toxicity.
Statistical analysis
Probabilities of survival and progression-free
survival were analyzed using the Kaplan-Meier method (12). StatView 5.0 (Abacus Concepts,
Berkley, CA, USA) was used for statistical analysis. P<0.05 was
considered to indicate a statistically significant difference.
Results
Patient characteristics
The demographics and baseline characteristics of the
patients are presented in Table I.
Between July 2009 and March 2011, 19 patients were treated with
PLD. The median age of the patients was 54.8 years (range, 39–75
years). Among the 19 patients, 18 had epithelial ovarian carcinoma
and 1 had peritoneal carcinoma. Histologically, 11 patients had
serous adenocarcinoma, 3 had clear cell adenocarcinoma, 2 had
mucinous adenocarcinoma, 2 had poorly differentiated adenocarcinoma
and 1 had endometrioid adenocarcinoma. The most common FIGO stage
was III. Prior to treatment with PLD, 2 patients received one
chemotherapeutic regimen, 11 patients received two regimens, 4
patients received three regimens and 2 patients received four
regimens.
 | Table IPatient characteristics (n=19). |
Table I
Patient characteristics (n=19).
| Characteristics | Total |
|---|
| Age, years | |
| Median | 54.8 |
| Range | 39–75 |
| Primary cancer, n
(%) | |
| Epithelial ovarian
carcinoma | 18 (94.7) |
| Peritoneal
carcinoma | 1 (5.3) |
| Tumor histology, n
(%) | |
| Serous | 11 (57.9) |
| Mucinous | 2 (10.5) |
| Clear cell | 3 (15.8) |
| Endometrial | 1 (5.3) |
| Poorly
differentiated adenocarcinoma | 2 (10.5) |
| Initial FIGO stage, n
(%) | |
| I | 2 (10.5) |
| II | 2 (10.5) |
| III | 14 (73.7) |
| IV | 1 (5.3) |
| Number of prior
chemotherapy cycles, n (%) | |
| 1 | 2 (10.5) |
| 2 | 11 (57.9) |
| 3 | 4 (21.1) |
| 4 | 2 (10.5) |
Tumor characteristics
The antitumor effects (best overall responses) and
response rates are presented in Table
II. The best overall responses in the 19 patients were 5
partial responses (PR), 6 stable diseases (SD) and 8 progressive
diseases (PD). The response rate was 26.3%. The proportion of
patients with complete response (CR), PR or SD was 57.9% (11/19
patients).
 | Table IIResponse rates. |
Table II
Response rates.
| Response | n (%) |
|---|
| Best overall
response | |
| CR | 0 (0) |
| PR | 5 (26.3) |
| SD | 6 (31.6) |
| PD | 8 (42.1) |
| Response rate | 5 (26.3) |
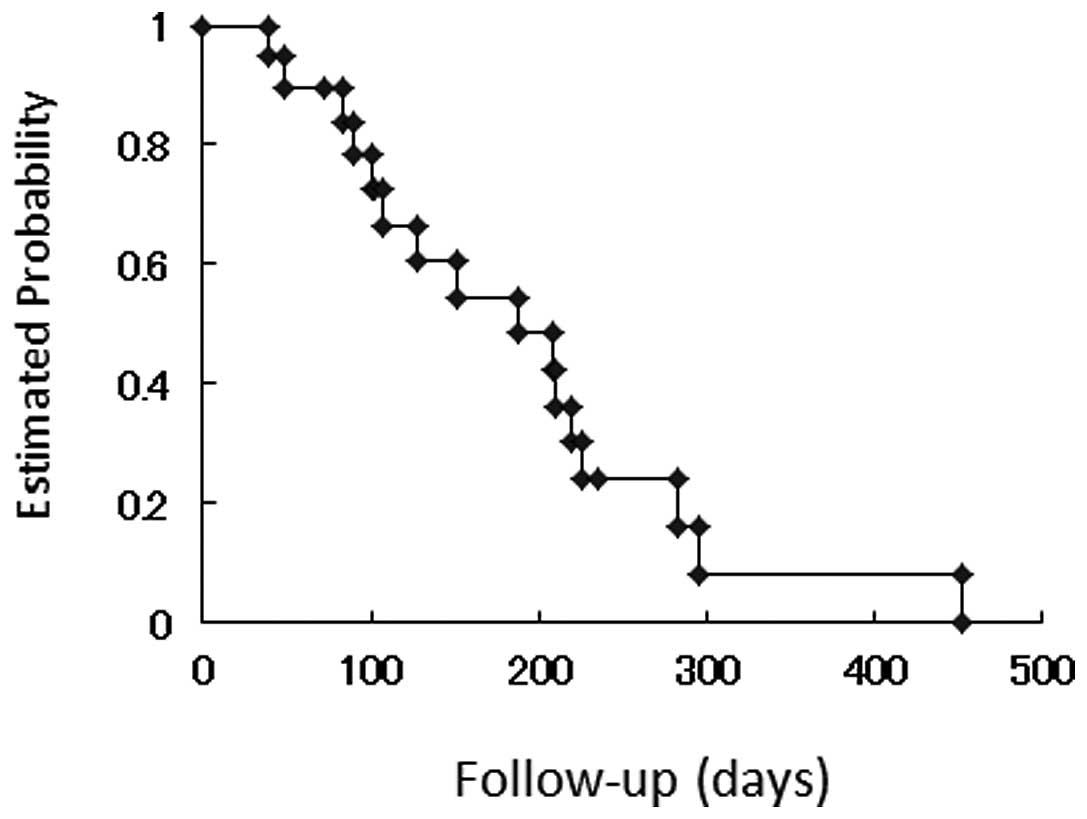
The Kaplan-Meier curve for time to progression is
demonstrated in Fig. 1. Median time
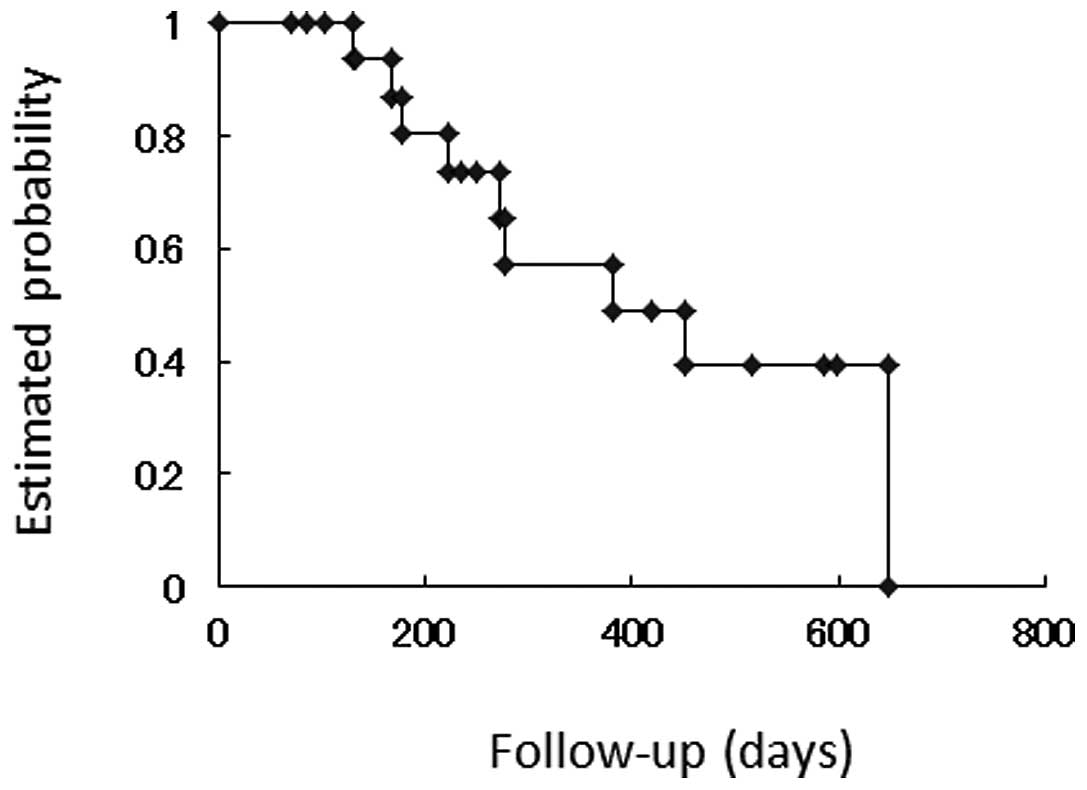
to progression was 188.0 days. The Kaplan-Meier curve for overall
survival is shown in Fig. 2. Median
survival time was 381.0 days.
Table III provides a
summary of the grade III and IV toxicities. The toxicities were
grade III hand-foot syndrome (HFS) in 1 patient (5.3%), grade IV
HFS in 1 (5.3%), grade IV stomatitis in 1 (5.3%), grade III
neutropenia in 2 (10.5%) and grade IV neutropenia in 1 (5.3%). One
case of grade IV stomatitis required a delay of therapy and a dose
reduction.
 | Table IIIGrade III and IV adverse drug
reactions. |
Table III
Grade III and IV adverse drug
reactions.
| Disease | Grade III
n (%) | Grade IV
n (%) |
|---|
| Hand-foot
syndrome | 1 (5.3) | 1 (5.3) |
| Stomatitis | 0 (0) | 1 (5.3) |
| Neutropenia | 2 (10.5) | 1 (5.3) |
Discussion
PLD is an active drug in patients with persistent or
recurrent platinum-pretreated epithelial ovarian carcinoma. The
clinical applications of PLD have expanded considerably in recent
years and the drug has been introduced into the management of the
majority of protocols for epithelial ovarian carcinoma treatment
alone and in combination with non-platinum and platinum agents
(13). The recent CALYPSO trial
demonstrated the efficacy of PLD/carboplatin combination treatments
for recurrent platinum-sensitive disease. This combination is
currently associated with a more favorable risk-benefit profile
than paclitaxel/carboplatin in patients with partially
platinum-sensitive recurrent ovarian carcinoma (14). A previous study in elderly patients
revealed that PLD demonstrated an improved therapeutic index with
less toxicity than paclitaxel/carboplatin (15).
In the present study, the response rate was 26.3%.
Clinical studies conducted in the United States and Europe
identified response rates to PLD of 28.4% in the platinum-sensitive
group and 6.5–18.3% in the platinum-resistant group (16–18). A
phase II clinical trial in Japan reported response rates of 27.3
and 21.0% in the platinum-sensitive and platinum-resistant groups,
respectively (19). These response
rates are consistent with those obtained in the present study.
PLD is an attractive second-line agent due to the
absence of treatment-associated mortality reports and low rates of
febrile neutropenia even in heavily pretreated patients (16,20,21).
The common toxicities identified in the present study were
hematological toxicities, HFS and stomatitis. Grade III–IV HFS was
observed in 2 patients (10.5%), grade IV stomatitis was observed in
1 (5.3%) and grade III–IV neutropenia was observed in 3 (15.8%). A
previous phase II clinical trial in Japan identified that 16.2, 8.1
and 67.6% of patients experienced grade III–IV HFS, stomatitis and
neutropenia, respectively (19).
Only 1 case of grade IV stomatitis required therapy delay and a
dose reduction. However, all patients were able to receive PLD
continually. Side-effects previously associated with doxorubicin,
including allergic reactions and cardiotoxicity, were not
identified, indicating that PLD is a well-tolerated agent.
In conclusion, recurrent ovarian cancer is extremely
difficult to cure, with current therapies functioning only to
lengthen the duration of survival and palliative therapy. At
present, the main aim of recurrent epithelial ovarian carcinoma
management is to reduce harm. Additional aims of therapy must
include the alleviation of cancer-related symptoms and extension of
symptom-free, progression-free and overall survival where possible.
Suitable agents must not only be effective but should also be
associated with low toxicities. The present study confirms that PLD
is suitable for use in salvage therapy of Müllerian carcinoma and
has a reduced toxicity profile compared with current therapeutic
options.
References
|
1
|
Jemal A, Siegel R, Ward E, Hao Y, Xu J and
Thun MJ: Cancer statistics, 2009. CA Cancer J Clin. 59:225–249.
2009. View Article : Google Scholar
|
|
2
|
Seewaldt VL, Greer BE, Cain JM, et al:
Paclitaxel (Taxol) treatment for refractory ovarian cancer: phase
II clinical trial. Am J Obstet Gynecol. 170:1666–1670. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Neijt IP, ten Bokkel Huiniuk WW, van der
Berg ME, et al: Long-term survival in ovarian cancer. Mature data
from the Netherlands Joint Study Group for Ovarian Cancer. Eur J
Cancer. 27:1367–1372. 1991.PubMed/NCBI
|
|
4
|
Dear RF, Gao B and Harnett P: Recurrent
ovarian cancer: Treat ment with pegylated liposomal doxorubicin; a
Westmead Cancer Care Centre Experience. Asia Pac J Clin Oncol.
6:66–73. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Safra T, Groshen S, Jeffers S, Tsao-Wei
DD, Zhou L, Muderspach L, Roman L, Morrow CP, Burnett A and Muggia
FM: Treatment of patients with ovarian carcinoma with pegylated
liposomal doxorubicin: analysis of toxicities and predictors of
outcome. Cancer. 91:90–100. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
No authors listed. Cyclophosphamide plus
cisplatin versus cyclophosphamide, doxorubicin and cisplatin
chemotherapy of ovarian carcinoma: a meta-analysis. The Ovarian
Cancer Meta Analysis Project. J Clin Oncol. 9:1668–1674.
1991.PubMed/NCBI
|
|
7
|
A’Hern RP and Gore ME: Impact of
doxorubicin on survival in advanced ovarian cancer. J Clin Oncol.
13:726–732. 1995.PubMed/NCBI
|
|
8
|
Fanning J, Bennett TZ and Hilgers RD:
Meta-analysis of cisplatin, doxorubicin and cyclophosphamide versus
cisplatin and cyclophosphamide chemotherapy of ovarian carcinoma.
Obstet Gynecol. 80:954–960. 1992.PubMed/NCBI
|
|
9
|
Singal PK and Iliskovic N:
Doxorubicin-induced cardiomyopathy. N Engl J Med. 339:900–905.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Gabizon A and Martin F: Polyethylene
glycol-coated (pegylated) liposomal doxorubicin: rationale for use
in solid tumors. Drugs. 54(Suppl 4): 15–21. 1997.PubMed/NCBI
|
|
11
|
Collins Y and Lele S: Long-term pegylated
liposomal doxorubicin use in recurrent ovarian carcinoma. J Natl
Med Assoc. 97:1414–1416. 2005.PubMed/NCBI
|
|
12
|
Kaplan EL and Meier P: Nonparametric
estimation from incomplete observations. J Am Stat Assoc.
53:4571958. View Article : Google Scholar
|
|
13
|
Ferrandina G, Corrado G, Licameli A,
Lorusso D, Fuoco G, Pisconti S and Scambia G: Pegylated liposomal
doxorubicin in the management of ovarian cancer. Ther Clin Risk
Manag. 6:463–483. 2010.PubMed/NCBI
|
|
14
|
Gladieff L, Ferrero A, De Rauglaudre G, et
al: Carboplatin and pegylated liposomal doxorubicin versus
carboplatin and paclitaxel in partially platinum-sensitive ovarian
cancer patients: results from a subset analysis of the CALYPSO
phase III trial. Ann Oncol. 5:1185–1189. 2011.
|
|
15
|
Kurtz JE, Kaminsky MC, Floquet A, et al:
Ovarian cancer in elderly patients: carboplatin and pegylated
liposomal doxorubicin versus carboplatin and paclitaxel in late
relapse: a Gynecologic Cancer Intergroup (GCGI) CALYPSO sub-study.
Ann Oncol. 22:2417–2423. 2011. View Article : Google Scholar
|
|
16
|
Gordon AN, Granai CO, Rose PG, et al:
Phase II study of stealth liposomal doxorubicin in platinum- and
paclitaxel-refractory epithelial ovarian cancer. J Clin Oncol.
18:3093–3100. 2000.
|
|
17
|
Johnston SRD and Gore ME: Caelyx: phase II
studies in ovarian cancer. Eur J Cancer. 37(Suppl 9): S8–S14.
2001.
|
|
18
|
Gordon AN, Fleagle JT, Guthrie D, Parkin
DE, Gore ME and Lacave AJ: Recurrent epithelial ovarian carcinoma:
a randomized phase II study of pegylated liposomal doxorubicin
versus topotecan. J Clin Oncol. 19:3312–3322. 2001.PubMed/NCBI
|
|
19
|
Katsumata N, Fujiwara Y, Kamura T, et al:
Phase II clinical trial of pegylated liposomal doxorubicin (JNS002)
in Japanese patients with Müllerian carcinoma (epithelial ovarian
carcinoma, primary carcinoma of fallopian tube, peritoneal
carcinoma) having a therapeutic history of platinum-based
chemotherapy: A phase II study of the Japanese Gynecologic Oncology
Group. Jpn J Clin Oncol. 38:777–785. 2008.PubMed/NCBI
|
|
20
|
Muggia FM, Hainsworth JD, Jeffers S, et
al: Phase II study of liposomal doxorubicin in refractory ovarian
cancer: antitumor activity and toxicity modification by liposomal
encapsulation. J Clin Oncol. 15:987–993. 1997.PubMed/NCBI
|
|
21
|
Gordon AN, Fleagle JT, Guthrie D, Parkin
DE, Gore M, Lacave AJ and Mutch D: Interim analysis of a phase III
randomized trial of doxil/caelyx (D) versus topotecan (T) in the
treatment of patients with relapsed ovarian cancer. Proc Am Soc
Clin Oncol. 9:3802000.
|