Introduction
Pancreatic carcinoma is one of the most aggressive
human malignant tumors and a leading cause of cancer-related
mortality worldwide (1). Its
anatomical complexity and late diagnosis have led to a low
resectability rate of 10–20%. Due to the poor 5-year survival rate,
the incidence and mortality of the disease are approximately
equivalent (2). Although recent
significant advances in cancer therapy, including the introduction
of novel chemotherapeutic agents, have significantly impacted the
overall survival time of pancreatic cancer patients, complete
recovery is extremely rare (3).
Therefore, it is essential that therapeutic regimens be developed
to inhibit tumor growth and increase the chemosensitivity of
chemotherapeutics; new approaches, including gene therapy, are
required to improve treatment results (4,5).
B7-H3 is a novel member of the B7 family (6). B7-H3 is not expressed in quiescent
lymphocytes and may be induced in activated dendritic cells,
monocytes and T cells (7–9). The protein is overexpressed in several
human cancer types, including breast, renal cell, urothelial cell,
prostate, lung and pancreatic cancer. Previous studies have
demonstrated a correlation between high expression of B7-H3 and a
poor outcome in patients with these types of cancer (10–15).
However, the precise role of B7-H3 in tumors remains unclear. As
metastasis and proliferation are closely correlated with
chemoresistance, we aimed to investigate the role of B7-H3 in the
sensitivity of pancreatic carcinoma cells to gemcitabine, and the
possible underlying mechanisms.
Materials and methods
Reagents
Antiobdies, including anti-human B7-H3,
anti-survivin and an antibody against GAPDH, were purchased from
R&D Systems (Minneapolis, MN, USA). The horseradish
peroxidase-conjugated secondary anti-mouse, anti-rabbit and
anti-goat antibodies were purchased from Bio-Rad (Hercules, CA,
USA). Gemcitabine was purchased from Lilly France, Inc. (Neuilly,
France), while TRIzol reagent and Moloney murine leukemia virus
(MMLV) were purchased from Gibco-BRL (Carlsbad, CA, USA).
Taq DNA polymerase, dNTPs and DNA markers were obtained from
Takara Bio, Inc. (Shiga, Japan).
Clinical specimens from patients
This study was approved by the Ethics Committee of
The First Affiliated Hospital of Soochow University for Clinical
Investigation. Forty patients with pancreatic carcinoma who
underwent radical resection surgery were included in the study.
Patients were excluded from analysis if they had received
chemotherapy or radiation therapy prior to surgery, or if they had
undergone previous pancreatic surgery. Pancreatic carcinoma
specimens were obtained during surgery, following written consent.
Normal pancreatic tissue specimens (confirmed histopathologically
and distant to the tumor) were simultaneously obtained as controls.
Following dissection under sterile conditions, each tissue sample
was fixed in 10% buffered methanal for immunohistochemical
estimation of B7-H3 expression.
Cells and cell culture
The pancreatic carcinoma cell line Panc-1 was
purchased from the Chinese Academy of Science Cell Bank, and the
Patu8988 cell line was provided by Professor Chang-Geng Ruan from
the Jiangsu Provincial Institute of Hematology, China. Panc-1 cells
were cultured in Dulbecco’s modified Eagle’s medium (Sigma, St.
Louis, MO, USA) and Patu8988 cells were cultured in RPMI-1640
medium (Gibco-BRL), and all media were supplemented with 10% fetal
bovine serum (Atlanta Biologicals, Inc., Lawrenceville, GA, USA)
and 1% penicillin-streptomycin (Gibco-BRL) at 37°C and 5%
CO2.
Generation of stable cell lines
Small hairpin RNA (shRNA) of the human B7-H3
(GenBank, NM_001024736) lentivirus gene transfer vector encoding
the green fluorescent protein (GFP) sequence was constructed by
Shanghai Genechem Co. (Shanghai, China). The targeting sequence of
shB7-H3 was: 5′-GAGCAGGGCTTGTTTGATGTG-3′, and it was confirmed by
sequencing. The recombinant lentivirus of small interfering RNA
targeting B7-H3 (LV-shB7-H3) and the non-targeted control mock
lentivirus (LV-NC) were prepared and titered to 5×109
TU/ml (transfection unit). Cells were subcultured at
5×104 cells/well in 6-well tissue culture plates
overnight. The viral supernatant was then added to cells at a
multiplicity of infection (MOI) of 10 with Enhanced Infection
Solution (Shanghai Genechem Co., Shanghai, China) and 5
μg/ml polybrene. Real-time reverse transcription-polymerase
chain reaction (RT-PCR) and western blot analysis were performed to
confirm the knockdown of B7-H3 mRNA and protein, respectively, in
those transfectants.
Real time RT-PCR
RT-PCR was performed to confirm the knockdown of
B7-H3 mRNA in the transfectants. Total RNA was collected using
TRIzol reagent, according to the manufacturer’s instructions. The
concentration and purity of the total RNA were detected with an
ultraviolet spectrophotometer and the mRNA was reversely
transcribed into cDNA with MMLV. Quantitative real-time PCR assays
were conducted using SYBR Green realtime PCR Master Mix and
real-time PCR amplification equipment. GAPDH was used as an
internal control. The PCR conditions consisted of one cycle at 95°C
for 15 sec, followed by 45 cycles at 95°C for 5 sec and at 60°C for
30 sec. The primer sequences were as follows: Sense,
5′-CTCTGCCTTCTCACCTCTTTG-3′ and antisense,
5′-CCTTGAGGGAGGAACTTTATC-3′ for B7-H3 (134 bp); sense,
5′-TGACTTCAACAGCGACACCCA-3′ and antisense,
5′-CACCCTGTTGCTGTAGCCAAA-3′ for GAPDH (121 bp).
Flow cytometry
The infected positive clones were isolated by
sorting flow cytometry (FCM) according to GFP expression.
Furthermore, stable clones of each GFP expression rate group were
detected by FCM. The infected cells comprised the LV-shB7-H3 and
LV-NC groups, and the non-infected Patu8988 cells were the control
group. These three groups were used in the following
experiments.
In vitro growth inhibition
Cells (1×104 cells/well) were initially
plated in triplicate in 96-well culture plates. After 24 h, the
medium was replaced with fresh medium with or without gemcitabine
and cells were incubated for 72 h. Cell viability following
treatment with various concentrations of gemcitabine was assessed
by
3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium
(MTS) assay. Cell viability was determined using the Cell Titer 96
Aqueous One Solution Cell Proliferation Assay kit (Promega,
Madison, WI, USA).
Annexin V staining
Cells (3×105 cells/dish) were grown in
triplicate in 60-mm dishes with exposure to 5.00 μmol/l
gemcitabine for 0, 48 and 72 h. Cells were then collected and
processed as described in the Annexin V-FITC Apoptosis Detection
kit I manual (Invitrogen Life Technologies; Carlsbad, CA, USA).
However, we discovered that the GFP expression was capable of
interfering with the FITC analysis assessed by flow cytometry, as
both express a similar green fluorescence. Therefore, propidium
iodide (PI) reagent was used only to detect apoptosis, and the FITC
reagent was not used.
Terminal
deoxynucleotidyltransferase-mediated dUTP-biotin nick-end labeling
(TUNEL) assay for cells
A TUNEL assay was performed using recombinant
terminal transferase (TdT) and biotin-16-dUTP (Roche Diagnostics
GmbH, Mannheim, Germany). Cells were treated with 5.00
μmol/l gemcitabine for 0, 48 and 72 h. Cells were processed
according to the manufacturer’s instructions and were analyzed by
flow cytometery (BD Biosciences, Franklin Lakes, NJ, USA). Each
experiment was repeated three times.
Western blot analysis
Cells were washed twice and lysed on ice. Following
centrifugation, the supernatants were collected. Protein
concentrations were determined by the Bio-Rad Dc Protein Assay
system. Samples were then separated by 10% SDS-PAGE and transferred
onto a polyvinylidene fluoride (PVDF) membrane. Membranes were
blocked and incubated with primary antibodies, such as anti-B7-H3
(1:50 dilution), anti-survivin (1:50 dilution) or anti-GAPDH (1:100
dilution) antibodies, at 4°C overnight. After three washes, the
membranes underwent hybridization with a goat-anti-mouse IgG
conjugated with horseradish peroxidase (1:5,000 dilution) for 2 h
at room temperature. Following further washing, reactive bands were
visualized using ECLTM Western Blot Detection Reagents with
exposure to X-ray film for 30–120 sec. The intensities of the bands
were calculated by densitometric analysis using Image J
software.
In vivo studies
Six groups of six male Balb/c nude mice, 5–6 weeks
old and 20 g in weight, were bred in aseptic specific pathogen-free
(SPF) conditions and maintained at a constant humidity and
temperature (25–28°C). Animal experiments were conducted according
to protocols approved by the Animal Care and Use Committee of
Soochow University and were in compliance with the guidelines
regarding animal welfare of the China National Committee for Animal
Experiments. Cells (2×107; LV-shB7-H3, LV-NC or Patu8988
cells) in 0.2 ml normal sodium were injected subcutaneously into
the right inguinal region of nude mice. For therapy experiments,
gemcitabine was dissolved in normal sodium (0.02 mmol/ml) and a
single dose of 2.5 ml/kg of the solution was injected intravenously
into the tail vein when the mean tumor diameter was 5–6 mm (day 0).
The untreated mice groups received only the solvent. The tumor size
was measured twice a week with calipers, and the volume was
determined using the simplified formula of a rotational ellipsoid
(L×W2×0.5). Growth curves were constructed, and the data
are presented as mean ± standard deviation. Tumors were harvested
from mice seven weeks after treatment. B7-H3 and survivin
expression were detected by immunohistochemistry, and a TUNEL assay
was performed in the tumor xenografts.
Immunohistochemistry
Clinical specimens and the tumor xenografts were
used for immunohistochemical studies. Specimens were fixed in
formalin overnight and embedded in paraffin. Series of 4-μm
sections were prepared for immunohistological staining. Tissue
sections were quenched for endogenous peroxidase with freshly
prepared 3% H2O2 with 0.1% sodium azide and
then placed in an antigen retrieval solution for 15 min. Following
incubation in the casein block, primary antibodies, including
anti-B7-H3 (1:50 dilution) or anti-survivin (1:50 dilution), were
applied to the sections for 1 h at room temperature. This was
followed by incubation with the second antibody and
extravidin-conjugated horseradish peroxidase. The immune reaction
was counterstained with hematoxylin, then dehydrated and mounted.
Sections were subsequently evaluated for the presence of brown
diaminobenzidine precipitates indicative of positive reactivity by
microscopy. The brown staining around the nucleus was interpreted
as positive reactivity for B7-H3 and survivin.
TUNEL assay for tumor xenografts
Apoptotic tumor cells were detected in vivo
with the TUNEL method, using an In Situ Cell Death Detection
kit (Roche Diagnostics GmbH). The assay was performed according to
the manufacturer’s instructions. Briefly, following routine
deparaffinization and treatment with H2O2
(3%), sections were digested with proteinase K (20 μg/ml;
pH, 7.4; 12 min) at 25°C and incubated with the reaction mixture
(1:40; 60 min) at 37°C. Incorporated fluorescein was detected with
horseradish peroxidase following a 30 min incubation at 37°C, and
subsequently dyed with 3,3′-diaminobenzidine (DAB). A brown nucleus
was considered to indicate a positive apoptotic cell.
Statistical analysis
Pancreatic carcinoma and normal pancreas tissue
B7-H3 expression in the immunohistochemical staining was compared
and assessed using the χ2 test. The remainder of the
data are presented as mean ± standard deviation. Statistical
comparisons were performed using a Student’s t-test. All P-values
were determined by two-sided tests, and P<0.05 was considered to
indicate a statistically significant difference. Analyses were
performed using SPSS 13.0 software (SPSS, Inc., Chicago, IL,
USA).
Results
B7-H3 expression in pancreatic carcinoma
cell lines and clinical specimens
The expression of B7-H3 in human pancreatic
carcinoma tissues from primary tumors has been demonstrated
(15,16). In the present study, we investigated
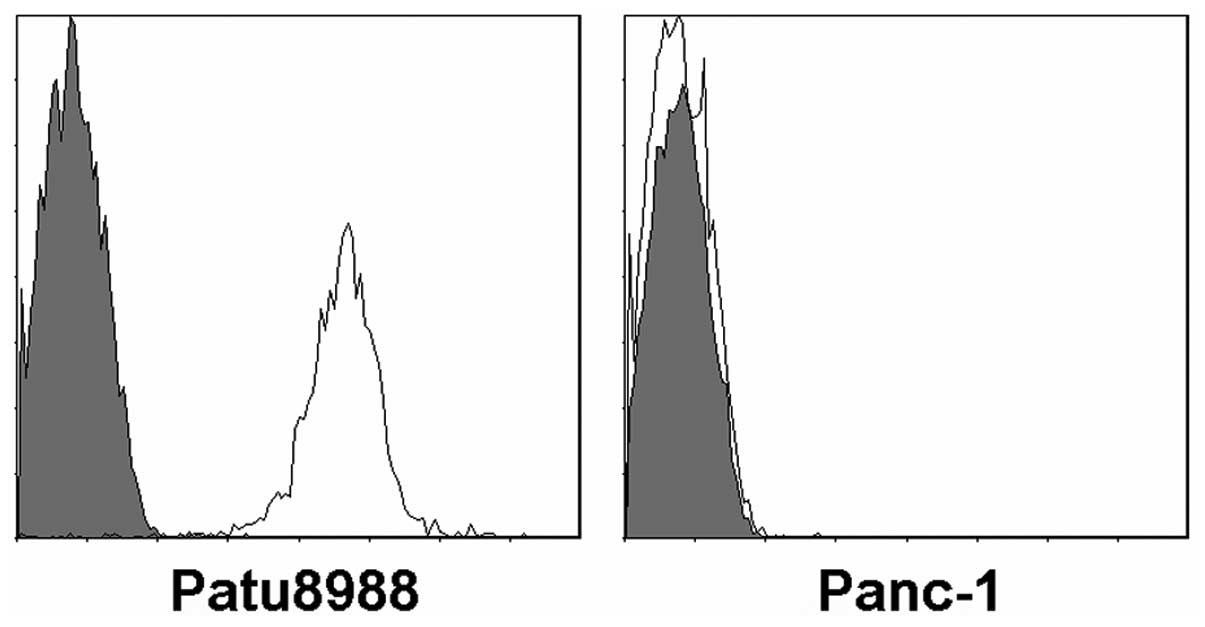
two pancreatic carcinoma cell lines, Panc-1 and Patu8988 (Fig. 1). As assessed by FCM, the B7-H3
protein was present in Patu8988 cell lines but not in Panc-1 cell
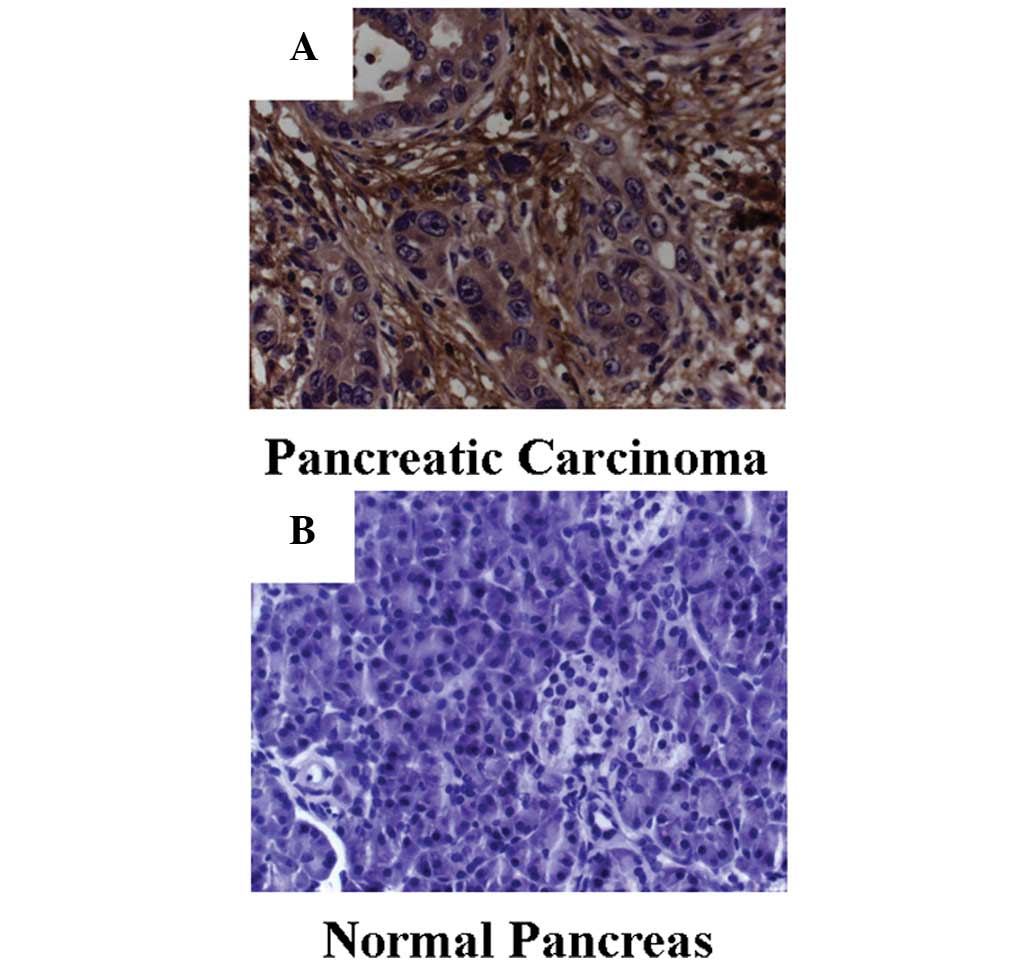
lines. Samples from pancreatic cancer patients were also included,
and immunohistochemical staining revealed that B7-H3 was
significantly overexpressed in the tumor tissue (χ2,
57.313; P<0.001). B7-H3 expression was detected in >50% of
cells in 31 tumor specimens, but not in the normal pancreas tissue
specimens. In six of the tumor specimens and five of the normal
pancreas tissue specimens, 25–50% of cells stained positively. In
three of the tumor specimens and 35 of the normal pancreas tissue
specimens, <25% positive staining was observed (Fig. 2).
Silencing of B7-H3 enhances
gemcitabine-induced cytotoxicity in pancreatic carcinoma
To study the possible involvement of B7-H3 in the
sensitivity of pancreatic carcinoma cells to gemcitabine, shRNA was
used to create a stable B7-H3 knockdown cell variant derived from
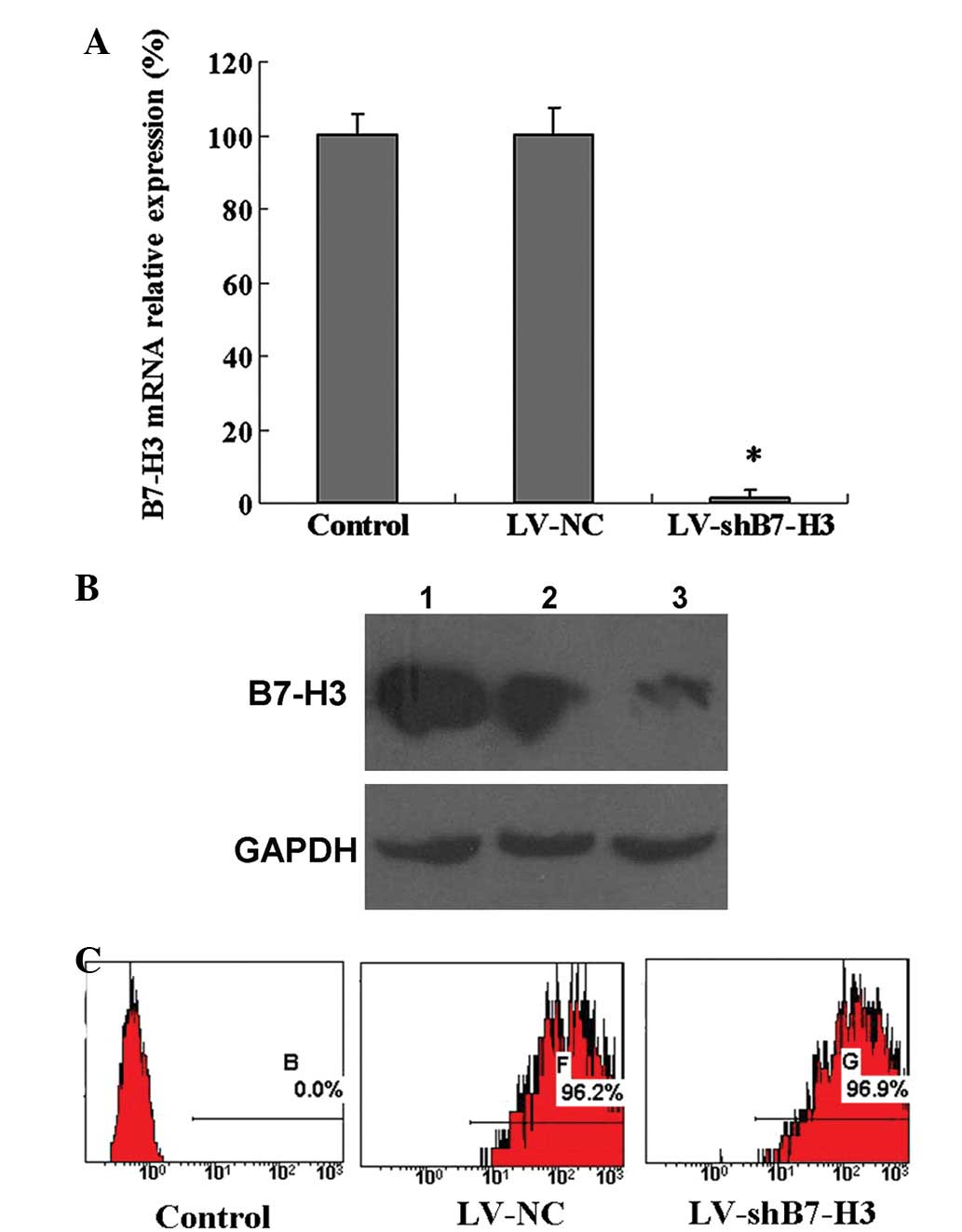
the Patu8988 cell line. As compared with the control and the LV-NC
cell variants, the corresponding B7-H3 knockdown cell variant,
LV-shB7-H3, expressed a low level of B7-H3 mRNA and protein
(Fig. 3A and B). Following
isolation by sorting FCM, stable Patu8988 cell lines in which B7-H3
had been knocked down (LV-shB7-H3) and non-targeted control mock
lentivirus infected Patu8988 cells (LV-NC) were established
(Fig. 3C).
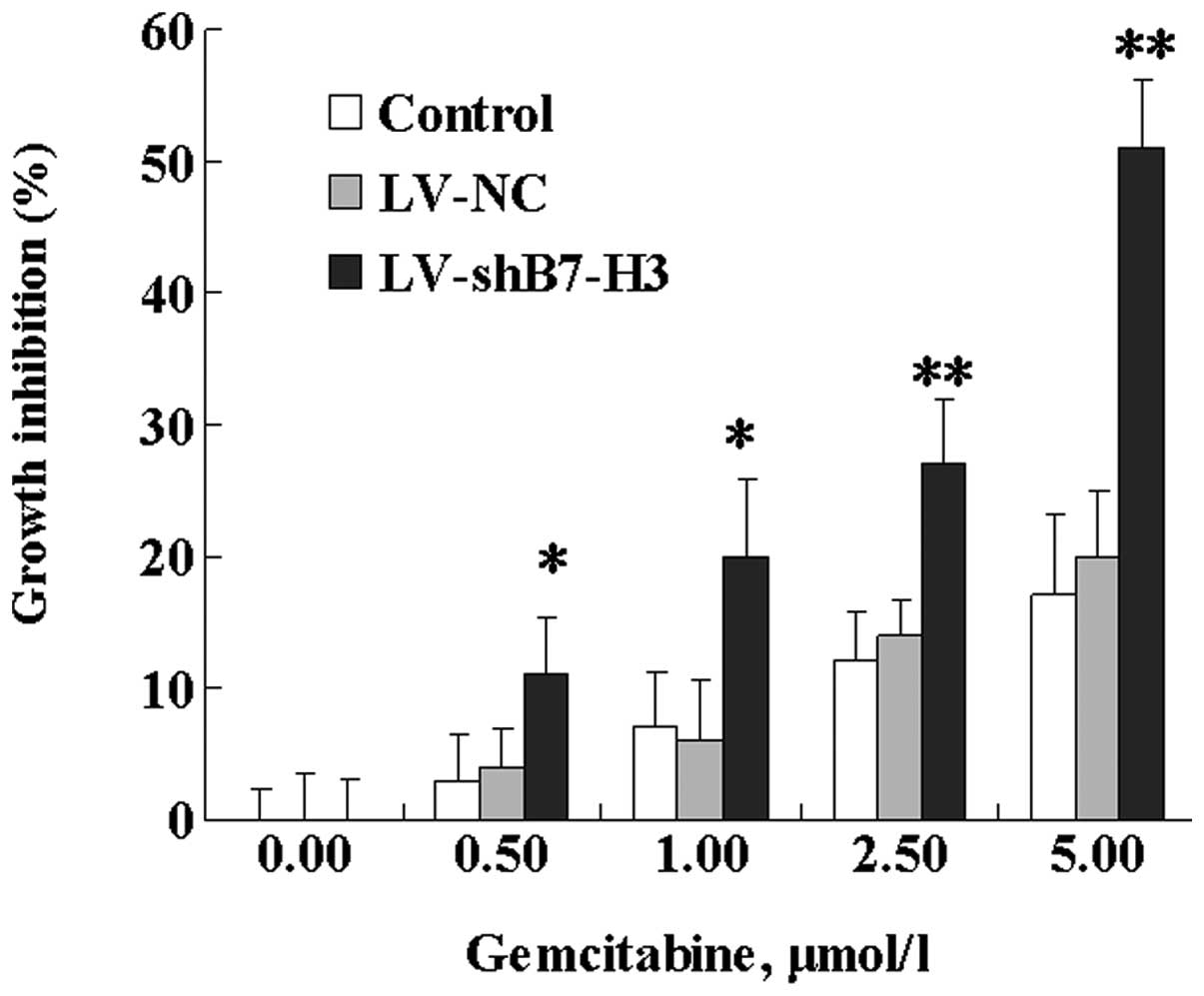
Following treatment with various concentrations of
gemcitabine for 72 h, a dose-dependent inhibition of cell growth
was observed in Patu8988 cells (Fig.
4). In Patu8988 cell variants, the inhibition of cell growth
was 51% following exposure to 5.00 μmol/l gemcitabine in the
LV-shB7-H3 cells, compared with 17 and 20% in the control and LV-NC
cells, respectively. Statistical analysis revealed that the
difference in growth inhibition between LV-shB7-H3 and LV-NC cells
was significant. These results indicate that B7-H3 is involved in
tumor cell resistance to gemcitabine. No marked differences were
observed between the LV-NC and control cells with respect to
gemcitabine responsiveness.
B7-H3 plays a critical role in cancer
cell resistance to gemcitabine-induced apoptosis
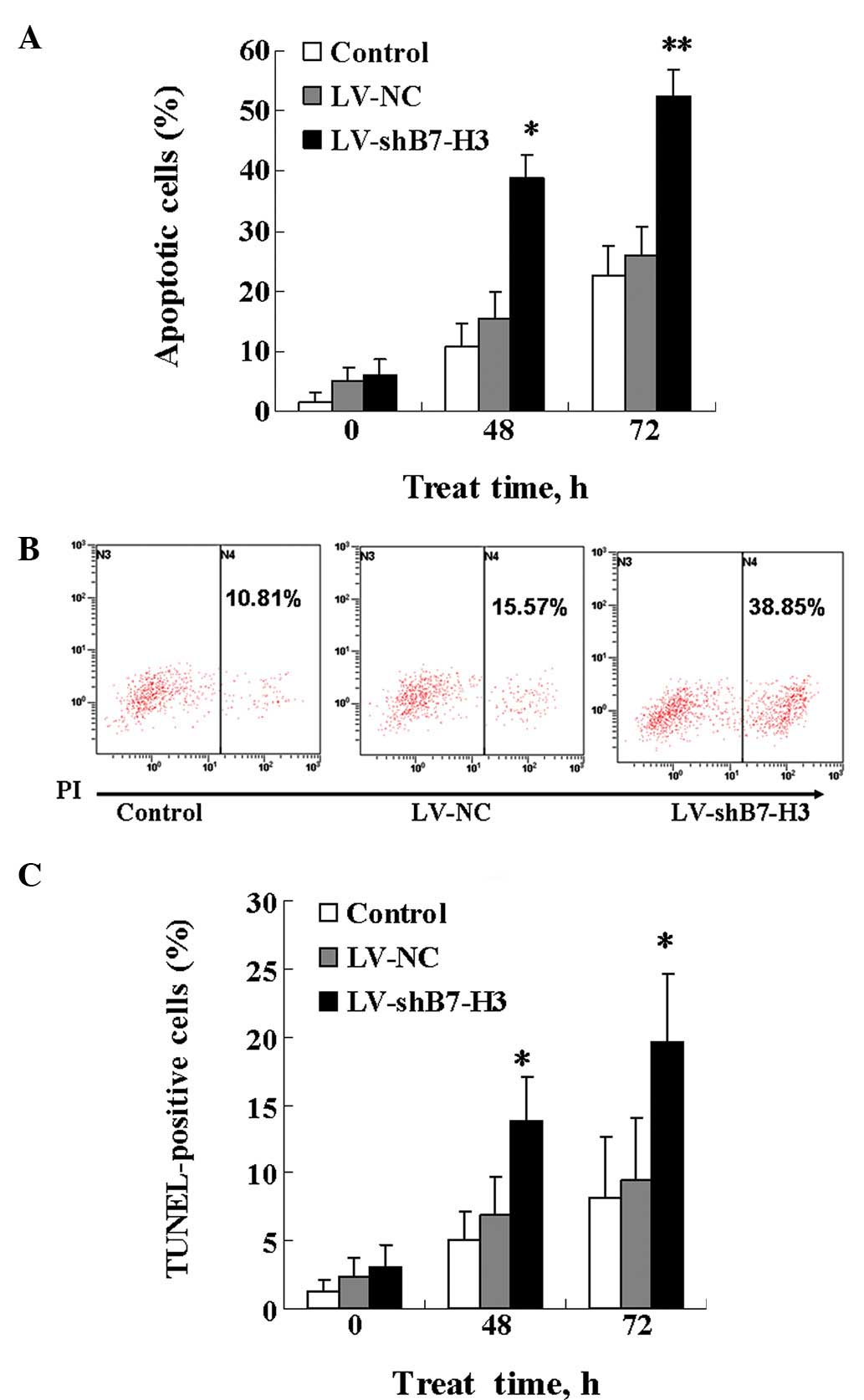
Gemcitabine is known to exert its cytotoxic effect
through induction of apoptosis. Therefore, we investigated whether
the increased gemcitabine cytotoxicity observed in cells in which
B7-H3 has been knocked down was correlated with effects on
apoptosis. The extent of apoptosis in Patu8988 cells was
investigated by measuring the percentage of Annexin V (PI)-stained
cells, a marker for early stage apoptosis, and by measuring the
percentage of TUNEL-positive cells, which reflects late-stage
apoptosis. In the Annexin V assay, the response to 5.00
μmol/l gemcitabine was time-dependent, with an increase in
the percentage of Annexin V-positive cells detected at 48 and 72 h.
The LV-shB7-H3 cells were more sensitive to gemcitabine-induced
apoptosis than the control and LV-NC cells (Fig. 5A and B). Similar results were
observed for the TUNEL assay. As demonstrated in Fig. 5C, the LV-shB7-H3 cells were
significantly more susceptible to gemcitabine-induced apoptosis
than the control and LV-NC cells; the percentage of TUNEL-positive
cells was 13.76% vs. 5.07% (control) and 6.84% (LV-NC) at 48 h, and
19.64% vs. 8.21% (control) and 9.43% (LV-NC) at 72 h. Overall,
these results demonstrate that silencing of B7-H3 expression by
shB7-H3 causes the cells to become more prone to
gemcitabine-induced apoptosis.
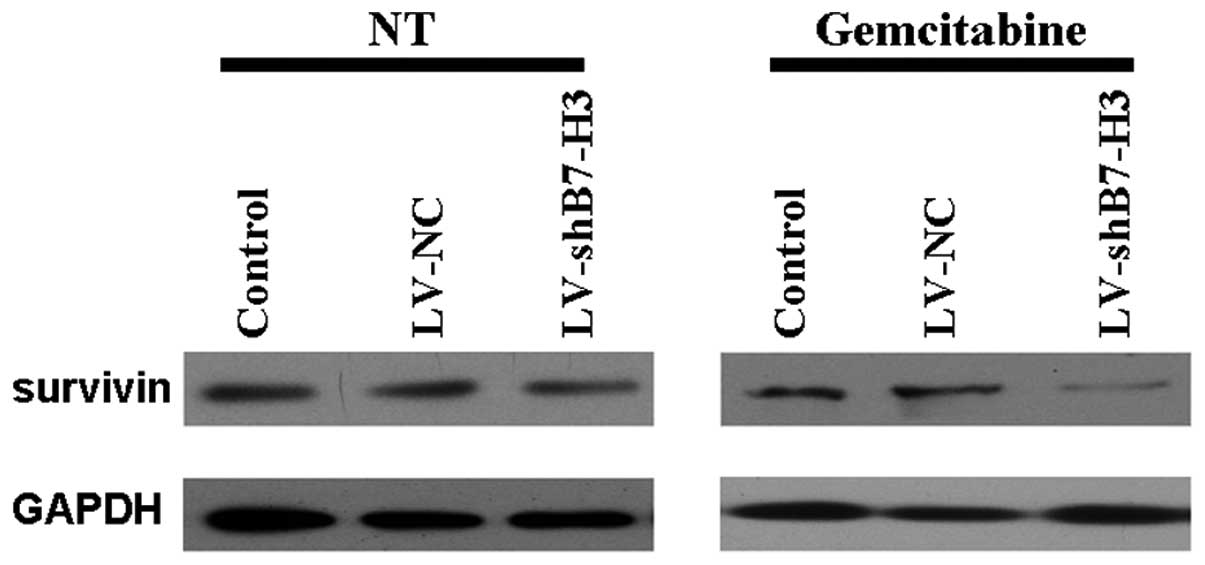
B7-H3 regulates the activation of the
anti-apoptotic molecule survivin
As chemosensitization accompanied with an increase
in apoptosis was observed in gemcitabine-treated cells in which
BH-73 had been knocked down, we subsequently studied whether the
effects of B7-H3 may be associated with molecules known to be
involved in the apoptotic response. As demonstrated in Fig. 6, the silencing of B7-H3 induced a
marked reduction in the level of survivin, an anti-apoptotic
factor, both in untreated and gemcitabine-treated cells. This
indicates that the effect of silencing B7-H3 on increasing
gemcitabine cytotoxicity was through decreased expression of the
anti-apoptotic protein, survivin. This may be the result of B7-H3
regulating a certain signaling pathway.
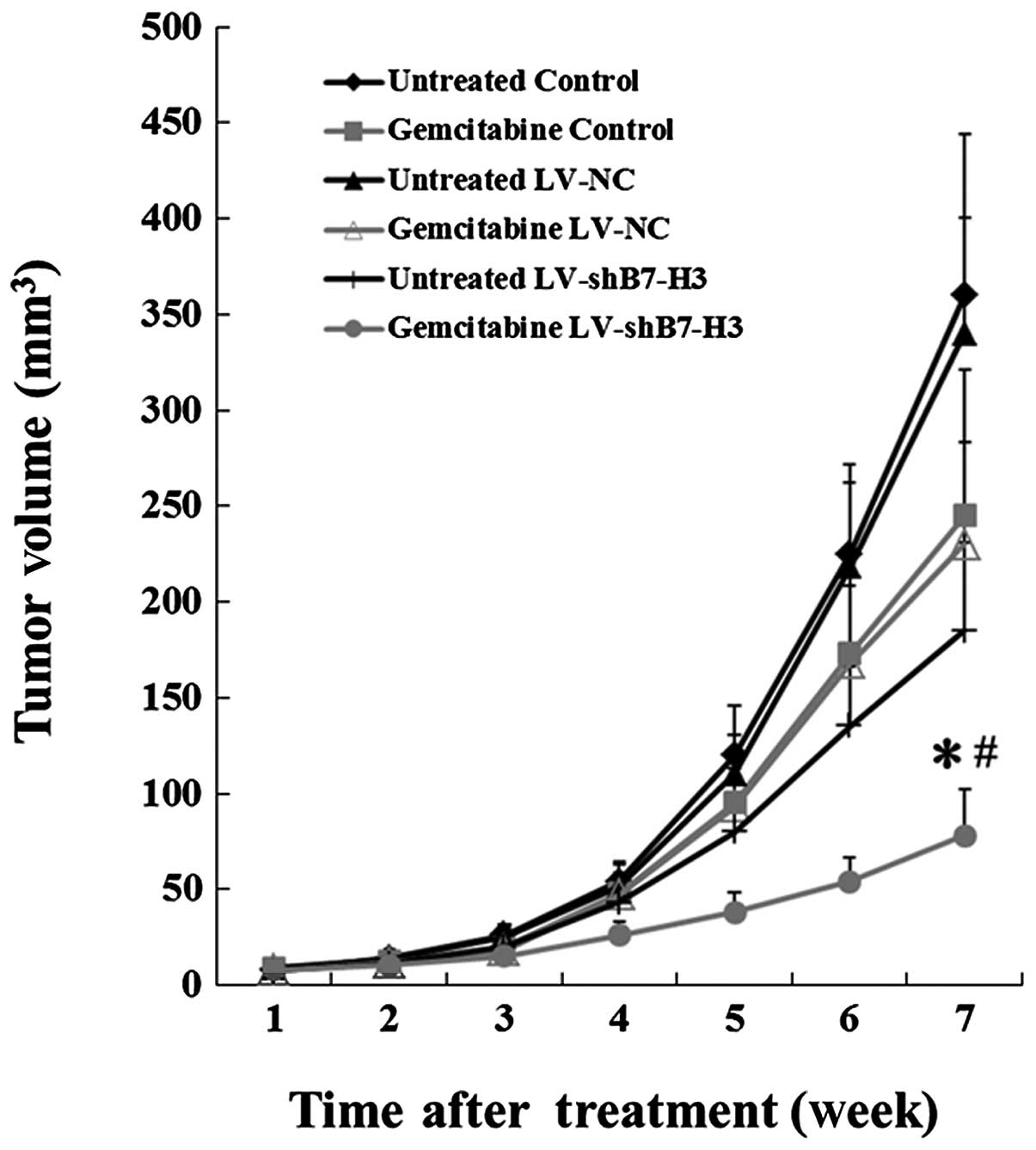
Silencing of B7-H3 enhances cancer cell
sensitivity to gemcitabine in a xenograft mouse model
The in vitro experiments with the Patu8988
cells demonstrated that the cytotoxic effect of gemcitabine was
enhanced in cells with silenced B7-H3. Hence, we examined whether
this effect also occurred in vivo. LV-shB7-H3, LV-NC and
control cells were injected subcutaneously into nude mice, and the
animals were treated with gemcitabine when the tumors had reached a
mean diameter of 5–6 mm. As demonstrated in Fig. 7, the growth rate was reduced by
knocking down B7-H3 alone. Additionally, although gemcitabine had
an effect on the growth of control group tumors, it demonstrated a
strong antitumor effect in the mice carrying LV-shB7-H3 xenografts.
All 36 mice developed detectable tumors at the initiation of this
experiment. Inhibition of growth was observed in the
gemcitabine-treated LV-shB7-H3 group for seven weeks; the average
tumor volume at seven weeks was 78±24 mm3, which was
significantly lower than that of the gemcitabine-treated LV-NC and
gemcitabine-treated control groups (230±53 and 245±61
mm3, respectively; P<0.01). No significant
differences were observed between the gemcitabine-treated LV-NC and
control groups. In addition, the average tumor volume of the
gemcitabine-treated LV-shB7-H3 group (78±24 mm3) was
significantly lower than that of the untreated LV-shB7-H3 group
(185±46 mm3; P<0.05; Fig.
7).
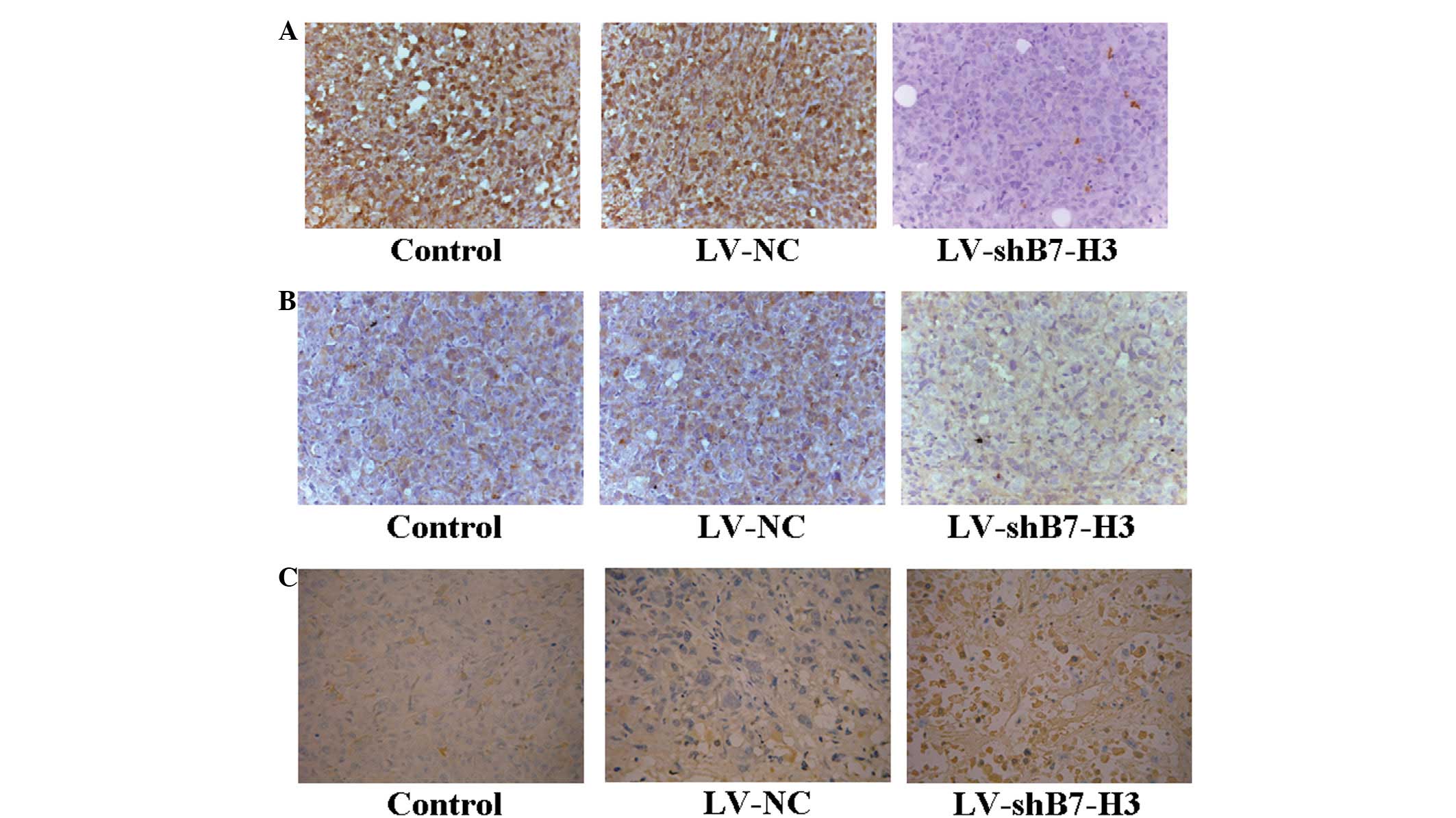
Knockdown in the shB7-H3 xenografts was confirmed by
immunochemical staining, and while the level of B7-H3 expression
remained low in the LV-shB7-H3 tumors, the LV-NC and control tumors
demonstrated strong staining (Fig.
8A). In order to demonstrate the mechanism of the
anti-apoptotic effect by targeting B7-H3 gene RNAi, the expression
of survivin was analyzed by immunohistochemistry in nude mice
transplanted tumors. The LV-shB7-H3 group (Fig. 8B) was demonstrated to have
downregulated survivin expression compared with the LV-NC group. No
differences between the LV-NC and control groups were observed. The
TUNEL staining revealed that an increased number of apoptotic cells
was evident in LV-shB7-H3 tumors treated with gemcitabine. When
compared with the LV-NC group or the control group, the number of
apoptotic cells in the LV-shB7-H3 group was significantly higher
than that in the former two groups (Fig. 8C). This indicated that with
gemcitabine treatment, inhibition of B7-H3 expression caused
apoptotic cell death in pancreatic carcinoma cells in vivo.
These in vivo results strongly support the effects observed
in vitro; B7-H3 plays a critical role in responses to
gemcitabine in pancreatic carcinoma cells.
Discussion
In the present study, we examined the role of B7-H3
in gemcitabine resistance in the human pancreatic carcinoma cell
line, Patu8988. Lentivirus-mediated shRNA targeting B7-H3 induced
knockdown of the B7-H3 protein in the cells and resulted in
increased sensitivity to the chemotherapeutic agent gemcitabine by
promoting apoptosis. Furthermore, in order to demonstrate the
mechanisms underlying the observed effects, we obtained the
evidence for B7-H3 regulation of the key anti-apoptotic gene,
survivin.
B7-H3, a member of the B7-family of molecules, is
important in adaptive immune responses, and has been demonstrated
to either promote or inhibit T-cell responses in various
experimental systems. B7-H3 has been observed to be expressed in
certain human cancer types and to be correlated with a poor outcome
of cancer patients. Numerous studies have supported a role of B7-H3
in cancer progression. It was demonstrated that B7-H3 was highly
expressed in human non-small cell lung cancer, and was
significantly correlated with an increased risk of lymph node
metastases (14). Roth et
al(17) evaluated B7-H3
immunoreactivity in >300 patients who suffered from prostate
cancer and underwent radical prostatectomy, and indicated that
increased levels of B7-H3 intensity correlated with worsened
clinicopathological features as well as a poorer postoperative
prognosis. This result was further confirmed by an expanded sample
in a tissue microarray (13). B7-H3
immunostaining represents an additional tool for the differential
diagnosis of small round cell tumors and may be useful in
identifying neuroblastoma patients at risk of relapse, who may take
advantage of more careful follow-up (18). A previous study demonstrated that
B7-H3 expression in clear cell renal cell carcinoma was present in
both the tumor cells and the tumor vasculature, and represented
prognostic implications (11).
Thus, based on studies of previous literature, it was concluded
that B7-H3 expression may play physiological and pathological roles
in the oncogenesis and development of pancreatic carcinoma.
However, the exact role of B7-H3 in cancer
progression remains elusive. Notably, in the present study we
demonstrated that downregulation of B7-H3 reduced survivin
expression. This may explain why the B7-H3 knockdown cells became
more prone to gemcitabine-induced apoptosis. Survivin is a member
of the family of inhibitors of apoptosis proteins (IAPs) (19–22),
and is preferentially and highly expressed in cancer cells,
including those of pancreatic cancer, while it is expressed at low
levels in the majority of normal non-dividing adult tissues
(23). The integral role of
survivin in cancer cell division and survival causes it to be an
attractive therapeutic target for the inhibition of cancer cell
growth (19,20). It has been suggested that survivin
inhibits cell death induced via the extrinsic and intrinsic
apoptotic pathways, and that it confers resistance to apoptosis by
directly suppressing caspase activity (24). Overexpression of survivin is
correlated with resistance to gemcitabine-induced apoptosis in
cancer cells. In a previous study by Yoon et al(25), it was demonstrated that the survivin
suppressant YM155 increased human pancreatic cancer cell
chemosensitivity to gemcitabine. Concomitant treatment with YM155
enhanced the chemosensitivity to gemcitabine, which was accompanied
by a decrease in the expression of survivin. Knockdown of
endogenous survivin via RNA interference also enhanced the
sensitivity to gemcitabine. Moreover, YM155 potentiated the
antitumor effect of gemcitabine in xenograft tumors of MiaPaCa-2.
In a study by Hung et al(26), knockdown of survivin expression in a
hepatocellular carcinoma cell line via short interfering RNA,
increased the apoptotic cell population in such cells that had been
treated with gemcitabine in comparison with scrambled control
cells. Survivin knockdown resulted in a reduction of
glucose-regulated protein 78 (GRP78), which may be responsible for
the observed increased cell sensitivity to gemcitabine.
Notably, the effects on gemcitabine sensitivity
in vitro were confirmed in our animal model. The growth rate
of established B7-H3 knockdown xenografts was slower than that of
LV-NC and the control tumors; however, the growth of these tumors
was significantly inhibited by gemcitabine treatment, whereas that
of LV-NC tumors was only marginally affected. Immunohistochemical
analysis of the xenograft tissue confirmed that the tumors
originating from shB7-H3 cells retained low expression levels of
the protein, whereas the LV-NC and control tumors demonstrated
strong B7-H3 staining. The expression of survivin was also
downregulated in the B7-H3 knockdown xenografts. The TUNEL assay
data indicated that silencing of B7-H3 induces, in parallel,
reduced proliferation, and it enhances the apoptosis induced by
gemcitabine.
In summary, our study investigating the role of
B7-H3 in drug resistance has demonstrated that the protein confers
resistance to gemcitabine in vitro and in vivo by
reducing the sensitivity of pancreatic carcinoma cells to
apoptosis, which is mediated via survivin expression. Furthermore,
in contrast to previous studies focusing on the immunoregulatory
effects of B7-H3, which is involved in the suppression of tumor
immune surveillance, our data demonstrated that B7-H3 is important
in determining the resistance to gemcitabine via
nonimmunomechanisms, increasing gemcitabine sensitivity by
promoting apoptosis. These findings provide novel insights into the
role of B7-H3 in cancer and may have important implications in the
development of targeted therapeutics for overcoming gemcitabine
resistance. However, whether B7-H3 regulates survivin expression
directly or via certain important intracellular pathways, requires
further investigation.
B7-H3 is more highly expressed in pancreatic
carcinoma than in normal pancreatic tissue. B7-H3 silencing in
pancreatic carcinoma increases tumor cell sensitivity to
gemcitabine by promoting apoptosis. Furthermore, the mechanisms
underlying these effects may include that silencing B7-H3
downregulates the anti-apoptotic molecule, survivin.
Acknowledgements
This study was supported by a grant
from the Post-graduate Scientific Research Innovation Project of
the Education Department of Jiangsu (No. CXZZ11_0125) and the
Science and Technology Research Project of the Science and
Technology Bureau of Suzhou City (No. SYS201120), China.
References
|
1
|
Li D, Xie K, Wolff R and Abbruzzese JL:
Pancreatic cancer. Lancet. 363:1049–1057. 2004. View Article : Google Scholar
|
|
2
|
Jemal A, Murray T, Samuels A, et al:
Cancer statistics, 2003. CA Cancer J Clin. 53:5–26. 2003.
View Article : Google Scholar
|
|
3
|
Laheru D and Jaffee EM: Immunotherapy for
pancreatic cancer-science driving clinical progress. Nat Rev
Cancer. 5:459–467. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ghaneh P, Costello E and Neoptolemos JP:
Biology and management of pancreatic cancer. Postgrad Med J.
84:478–497. 2008. View Article : Google Scholar
|
|
5
|
Pan X, Sheng WH, Zhu QY, et al: Inhibition
of pancreatic carcinoma growth by adenovirus-mediated human
Interleukin-24 expression in animal model. Cancer Biother
Radiopharm. 23:425–434. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Chapoval AI, Ni J, Lau JS, et al: B7-H3: a
costimulatory molecule for T cell activation and IFN-gamma
production. Nat Immunol. 2:269–274. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Sun M, Richards S, Prasad DV, et al:
Characterization of mouse and human B7-H3 genes. J Immunol.
168:6294–6297. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Steinberger P, Majdic O, Derdak SV, et al:
Molecular characterization of human 4Ig-B7-H3, a member of the B7
family with four Ig-like domains. J Immunol. 172:2352–2359. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhang GB, Zhou H, Chen YJ, et al:
Characterization and application of two novel monoclonal antibodies
against 2IgB7-H3: expression analysis of 2IgB7-H3 on dendritic
cells and tumor cells. Tissue Antigens. 66:83–92. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Arigami T, Narita N, Mizuno R, et al:
B7-h3 ligand expression by primary breast cancer and associated
with regional nodal metastasis. Ann Surg. 252:1044–1051. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Crispen PL, Sheinin Y, Roth TJ, et al:
Tumor cell and tumor vasculature expression of B7-H3 predict
survival in clear cell renal cell carcinoma. Clin Cancer Res.
14:5150–5157. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Boorjian SA, Sheinin Y, Crispen PL, et al:
T-cell coregulatory molecule expression in urothelial cell
carcinoma: clinicopathologic correlations and association with
survival. Clin Cancer Res. 14:4800–4808. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zang X, Thompson RH, Al-Ahmadie HA, et al:
B7-H3 and B7x are highly expressed in human prostate cancer and
associated with disease spread and poor outcome. Proc Natl Acad Sci
USA. 104:19458–19463. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Sun Y, Wang Y, Zhao J, et al: B7-H3 and
B7-H4 expression in non-small-cell lung cancer. Lung Cancer.
53:143–151. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Yamato I, Sho M, Nomi T, et al: Clinical
importance of B7-H3 expression in human pancreatic cancer. Br J
Cancer. 101:1709–1716. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Loos M, Hedderich DM, Ottenhausen M, et
al: Expression of the costimulatory molecule B7-H3 is associated
with prolonged survival in human pancreatic cancer. BMC Cancer.
26:4632009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Roth TJ, Sheinin Y, Lohse CM, et al: B7-h3
ligand expression by prostate cancer: a novel marker of prognosis
and potential target for therapy. Cancer Res. 67:7893–7900. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Gregorio A, Corrias MV, Castriconi R, et
al: Small round blue cell tumours: diagnostic and prognostic
usefulness of the expression of B7-H3 surface molecule.
Histopathology. 53:73–80. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Altieri DC: Survivin, cancer networks and
pathway-directed drug discovery. Nat Rev Cancer. 8:61–70. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Li F, Ambrosini G, Chu EY, et al: Control
of apoptosis and mitotic spindle checkpoint by survivin. Nature.
396:580–584. 1998. View
Article : Google Scholar : PubMed/NCBI
|
|
21
|
Hunter AM, LaCasse EC and Korneluk RG: The
inhibitors of apoptosis (IAPs) as cancer targets. Apoptosis.
12:1543–1568. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Schimmer AD: Inhibitor of apoptosis
proteins: translating basic knowledge into clinical practice.
Cancer Res. 64:7183–7190. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ambrosini G, Adida C and Altieri DC: A
novel anti-apoptosis gene, survivin, expressed in cancer and
lymphoma. Nat Med. 3:917–921. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Tamm I, Wang Y, Sausville E, et al:
IAP-family protein survivin inhibits caspase activity and apoptosis
induced by Fas (CD95), Bax, caspases, and anticancer drugs. Cancer
Res. 58:5315–5320. 1998.PubMed/NCBI
|
|
25
|
Yoon DH, Shin JS, Jin DH, et al: The
survivin suppressant YM155 potentiates chemosensitivity to
gemcitabine in the human pancreatic cancer cell line MiaPaCa-2.
Anticancer Res. 32:1681–1618. 2012.PubMed/NCBI
|
|
26
|
Hung CS, Lin SF, Liu HH, et al:
Survivin-mediated therapeutic efficacy of gemcitabine through
glucose-regulated protein 78 in hepatocellular carcinoma. Ann Surg
Oncol. 19:2744–2752. 2012. View Article : Google Scholar : PubMed/NCBI
|