Introduction
The incidence of testicular cancer has doubled in
the past 30 years and continues to increase (1–3).
Testicular tumors account for 1–2% of all malignancies but are
among the most common types of cancer in young males (4–7). It is
well known that cryptorchidism is a risk factor in the development
of germ cell tumors (8,9) and approximately 10% of all testicular
tumors arise in an undescended testicle (10). Seminoma is the most common histology
(4,11). In contrast to patients with scrotal
seminoma, clinical studies report a high proportion of advanced
stage disease in patients with abdominal or inguinal seminoma. Data
in the literature on cryptorchid seminomas are rare and the numbers
of patients in published series are small. Several studies have
suggested that early orchidopexy reduces the risk of seminoma de
velopment (11–13). Seminoma arising in an undescended
inguinal testicle is extremely rare in developed countries due to
the standard practice of orchidopexy in the early years of life and
orchiectomy in post-adolescent patients with undescended testicles
to prevent cancer and infertility. Despite these preventative
measures, cases of intra-abdominal testicular tumors occasionally
occur in adults. The origin of primary extragonadal gem cell tumors
is a matter of debate. Although they may arise virtually anywhere,
typically they are found in the midline where they present as
retroperitoneal, mediastinal or pineal masses. It remains
uncertain, however, whether such tumors develop primarily at
extragonadal sites or represent metastases of a primary testicular
tumor (14–19).
Case report
We report a 38-year-old male who was referred for
abdominal pain to our surgical department. The study was approved
by the Institutional Review Board of Catania University, Catania,
Italy. Written informed consent was obtained from the patient.
Physical examination revealed a well-nourished young male with a
palpable mass on the right iliac fossa and the absence of the right
testicle. The left spermatic cord and testis were palpable and
normal on clinical examination. An incisional scar on the right
groin was observed. The patient gave a history of having undergone
a right orchidectomy for an undescended testis via the inguinal
route 10 years previously. The patient, however, claimed that the
histology report described a ‘benign inflammatory mass’. He had
remained well until 3 months prior to presentation when he noticed
the insidious onset of abdominal pain that progressively increased.
No lower urinary or constitutional symptoms were reported and there
was no history of trauma to the area. There was no palpable
inguinal, axillary or supraclavicular lymphadenopathy. Laboratory
studies upon admission revealed a normal blood cell count and
chemistry profile. Serum α-fetoprotein (AFP) and β-human chorionic
gonadotropin (HCG) were normal. The chest X-ray was also normal.
Scrotal ultrasonography revealed the absence of the right testis
and that the left testicle, kidney, spleen and liver were in normal
condition. Abdominal ultrasonography revealed a heterogeneous mass
suggestive of an encapsulated abscess. It was complemented by
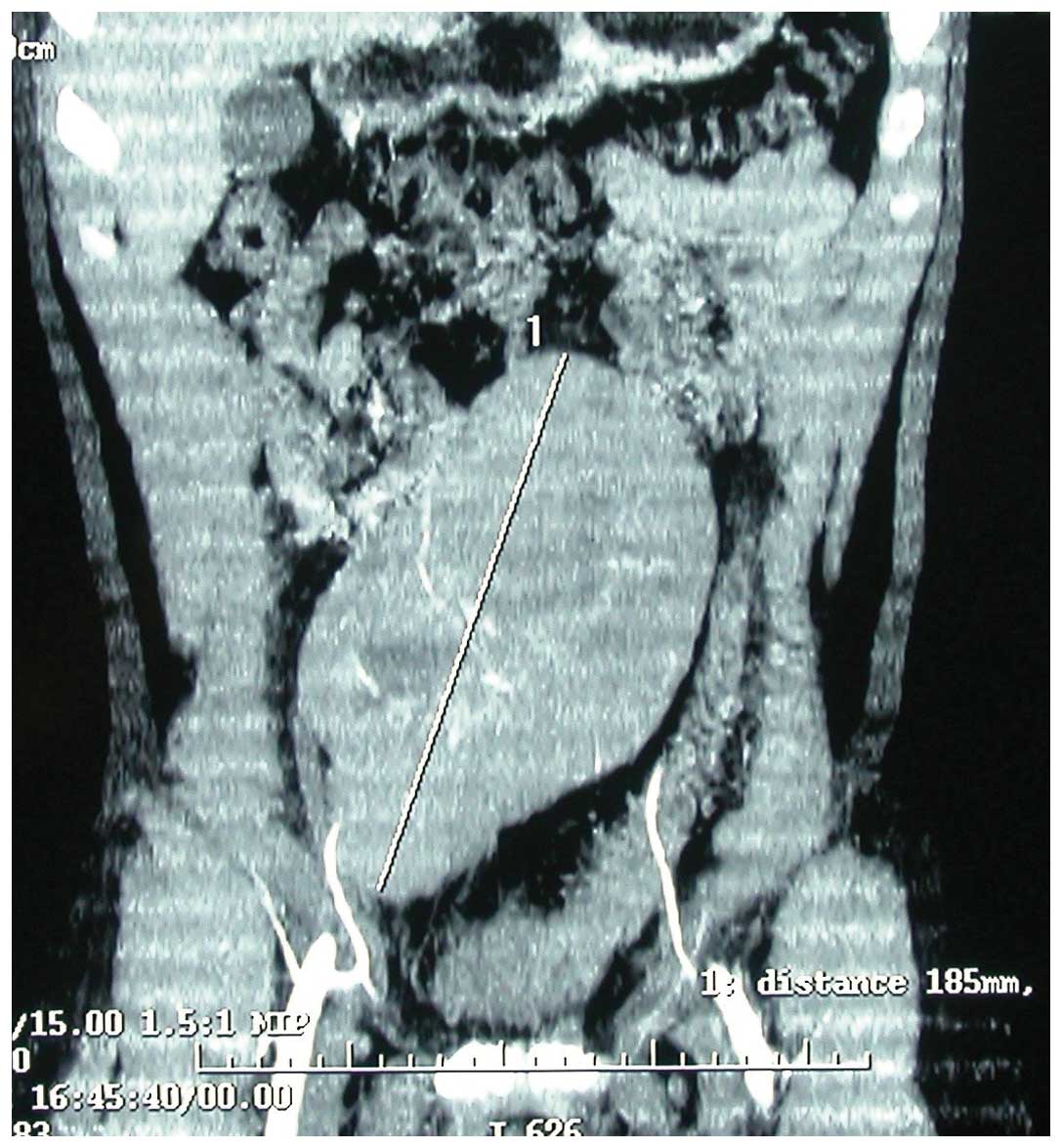
abdominal computerized tomography that revealed a solid mass
located in the right iliac fossa (Fig.
1). There was no presence of retroperitoneal or mesenteric
lymph node involvement. The patient underwent a laparotomy that
confirmed the diagnosis of a retroperitoneal tumor. At surgery the
tumor was almost encapsulated and the resection was performed
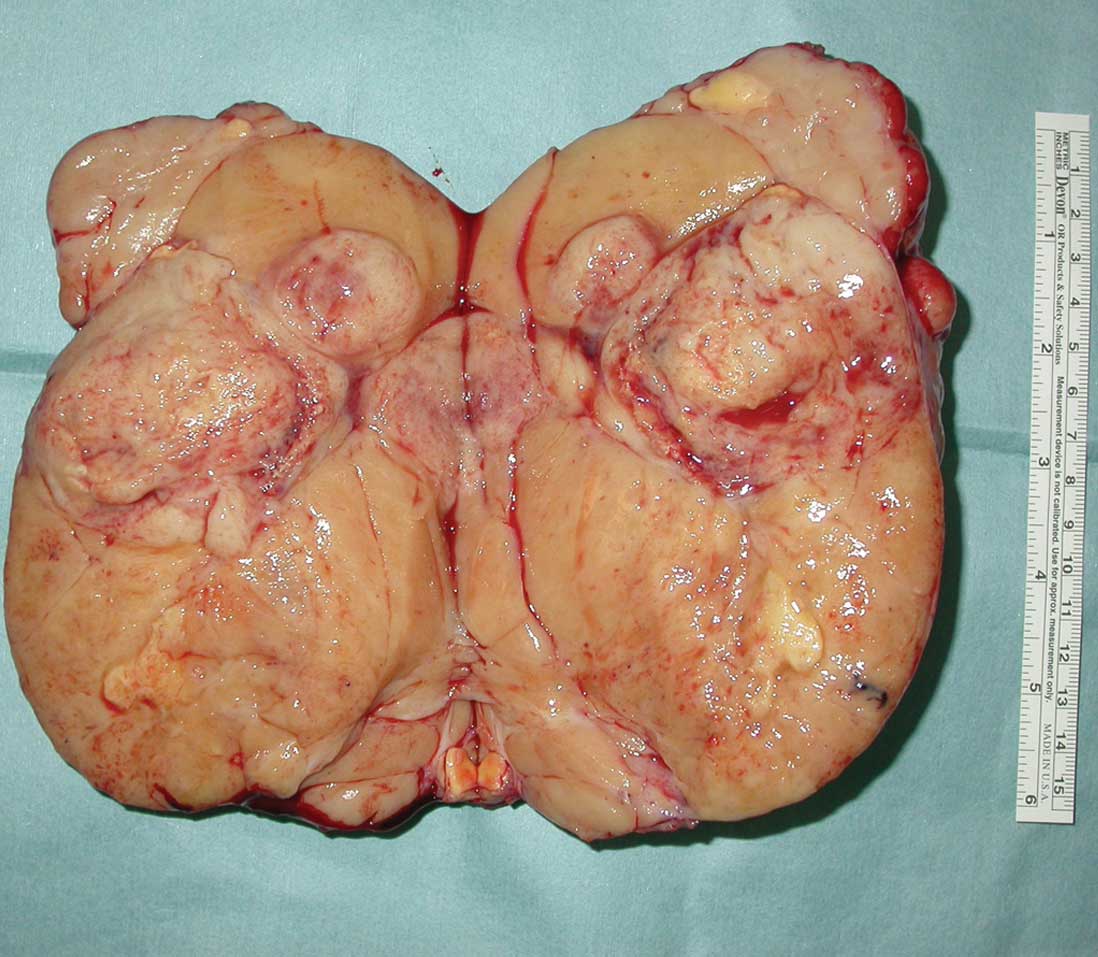
without complications. The tumor was removed totally. The excised
mass measured 18×10×8 cm. Its cut surface showed greyish white
tissue, cystic areas and a central fibrous core (Fig. 2). Frozen-section diagnosis indicated
that it may be a germ cell tumor. A permanent section revealed that
the tumor consisted of round and polygonal cells. The cells had a
clear or granular cytoplasm, with a large, centrally located
nucleus and coarse-clumped chromatin. There were no
non-seminomatous components in the tumor. The histopathology report
confirmed the mass to be a classical pure seminoma. Post-operative
recovery was good. The patient was referred to receive adjuvant
chemotherapy and external beam radiotherapy. The patient was alive
and well, without evidence of disease, 2 years and 6 months after
the surgery.
Discussion
Testicular cancer represents 1–2% of all male
malignant tumors and is the most frequent solid neoplasia in males
aged 20–35 years (5–7). Gonadal dysgenesis, testicular atrophy
and trauma have been proposed as etiological factors in the
development of testicular cancer but cryptorchidism and a history
of tumor in the contralateral testicle are the most significant
(11). In cryptorchidic testicles
the incidence of testicular cancer is greater than that in the
general population (10).
Cryptorchidism is a frequent pathology that affects
2–5% of children. In only in 20% of the cases of cryptorchidism are
the testicles non-palpable at clinical examination, in the
remaining cases they are in the abdomen or the inguinal canal
(9).
Abdominal testicles present a higher rate of
malignancy and develop cancer in 30% of cases. The high
temperatures in the abdomen and inguinal canal and maternal risk
factors appear to be responsible for the malignant degeneration
(20). The testicle should be
located in patients with non-palpable testicles and orchidectomy is
recommended in post-adolescent patients. An exploratory laparoscopy
that is, in most cases, simultaneously diagnostic and therapeutic
may be performed (9,21).
The histopathology of undescended testicle tumors in
the adult depends on location; it is pure seminoma in more than 90%
of the cases when it is intra-abdominal (8,21–23).
Moreover the prognosis depends on the initial stage and tumor
histology (24–26). Orchidopexy does not eliminate the
cancer risk but allows an early diagnosis as a result of the
testicle being accessible to clinical examination (11). Numerous studies have underlined the
association of cancer and cryptorchidism but there is no long
series of studies concerning the intra-abdominal localization of
testicular tumors in the literature, with the exception of sporadic
case reports. Despite the use of chemotherapy, approximately 4% of
patients with extragonadal germ cell tumors develop a metachronous
testicular cancer (27,28). However, there is disagreement over
whether the extragonadal germ cell tumor is a primary disease or
metastatic from the burned-out primary testicular lesion (29,30).
No cases of abdominal seminoma following
orchidectomy have been reported in the surgical literature. As to
whether the resected tumor in the present report was primary or a
local recurrence, it would appear that the latter is the case as
there are no known structures that may give rise to such a tumor
following inguinal orchidectomy. It is possible that a microscopic
deposit survived and gave rise to the mass in question, thus
calling into question the accuracy of the first pathology report.
Post-orchidectomy evaluation of the surgical pathology specimen and
the definitive removal of the residual testicle tissue is
established by an awareness of the histological spectrum exhibited
by testicular remnants (31).
Although the exact extragonadal germ cell tumor histogenesis is not
known, several theories relating to the possibility that all germ
cell tumors originated from extragonadal, potentially biphasic germ
cells have been proposed. It has been suggested that germ cells are
present in apparently ectopic sites in all healthy individuals,
having been distributed widely during normal embryogenesis,
conveying genetic information or providing regulatory functions at
somatic sites (16,32–34).
As a conclusion, and despite the elevated risk of
testicular cancer in patients with intra-abdominal testicles, we
considered retroperitoneal seminoma to be a disease of low
incidence. This is due to the standard practice of orchidopexy in
pre-adolescent patients and orchiectomy in post-adolescents with
cryptorchidism. Nevertheless, tumors of intra-abdominal testicles
occasionally appear that could be avoided by the adoption of
adequate prevention strategies and a careful follow-up. If a
patient presents with an abdominal mass following orchidectomy, the
differential diagnosis of seminoma should be considered even if the
prior histopathology report stated it to be benign. Extirpation of
such tumors may be carried out safely by laparotomy. This will
obviate the possible rupture and local seeding of tumor cells. We
also wish to highlight the fact that a greater number of sections
should be taken when preparing histopathological slides of
post-orchidectomy testicles to reduce the risk of missing areas of
the tumor.
References
|
1
|
Chia VM, Quraishi SM, Devesa SS, Purdue
MP, Cook MB and McGlynn KA: International trends in the incidence
of testicular cancer, 1973–2002. Cancer Epidemiol Biomarkers Prev.
19:1151–1159. 2010.
|
|
2
|
McGlynn KA, Devesa SS, Sigurdson AJ, Brown
LM, Tsao L and Tarone RE: Trends in the incidence of testicular
germ cell tumors in the United States. Cancer. 97:63–70. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Garner MJ, Turner MC, Ghadirian P and
Krewski D: Epidemiology of testicular cancer: an overview. Int J
Cancer. 116:331–339. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Michels J, Van Der Westhuizen N and Ross
A: Synchronous metastatic seminoma and primary retroperitoneal
ganglioneuroma: case report and literature review. Can Urol Assoc
J. 5:E109–E112. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Jemal A, Siegel R, Ward E, Murray T, Xu J
and Thun MJ: Cancer statistics. CA Cancer J Clin. 57:43–66.
2007.
|
|
6
|
dos Santos Silva I, Swerdlow AJ, Stiller
CA and Reid A: Incidence of testicular germ-cell malignancies in
England and Wales: trends in children compared with adults. Int J
Cancer. 83:630–634. 1999.PubMed/NCBI
|
|
7
|
Taskinen S, Fagerholm R, Aronniemi J,
Rintala R and Taskinen M: Testicular tumors in children and
adolescents. J Pediatr Urol. 4:134–137. 2008. View Article : Google Scholar
|
|
8
|
Carmona Campos E, Regueiro López JC,
Prieto Castro R, Leva Vallejo M, Moreno Arcas P and Requena Tapia
MJ: Cryptorchism and testicular cancer. Actas Urol Esp. 24:49–51.
2000.(In Spanish).
|
|
9
|
Taran I and Elder JS: Results of
orchiopexy for the undescended testis. World J Urol. 4:2231–2239.
2006.
|
|
10
|
Kulkarni JN and Kamat MR: Tumors in
undescended testis. J Surg Oncol. 46:257–260. 1991. View Article : Google Scholar
|
|
11
|
Abratt RP, Reddi VB and Sarembock LA:
Testicular cancer and cryptorchidism. Br J Urol. 70:656–659. 1992.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Gauwitz MD and Zagars GK: Treatment of
seminoma arising in cryptorchid testes. Int J Radiat Oncol Biol
Phys. 24:153–159. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Jones BJ, Thornhill JA, O’Donnell B, Kelly
DG, Walsh A, Fennelly JJ and Fitzpatrick JM: Influence of prior
orchiopexy on stage and prognosis of testicular cancer. Eur Urol.
19:201–203. 1991.PubMed/NCBI
|
|
14
|
Goss PE, Schwertfeger L, Blackstein ME,
Iscoe NA, Ginsberg RJ, Simpson WJ, Jones DP and Shepherd FA:
Extragonadal germ cell tumors. A 14-year Toronto experience.
Cancer. 73:1971–1979. 1994.PubMed/NCBI
|
|
15
|
Böhle A, Studer UE, Sonntag RW and
Scheidegger JR: Primary or secondary extragonadal germ cell tumors?
J Urol. 135:939–943. 1986.
|
|
16
|
Oosterhuis JW, Stoop H, Honecker F and
Looijenga LH: Why human extragonadal germ cell tumours occur in the
midline of the body: old concepts, new perspectives. Int J Androl.
30:256–263. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yang CJ, Cheng MS, Chou SH, Tsai KB and
Huang MS: Primary germ cell tumors of the mediastinum: 10 years of
experience in a tertiary teaching hospital. Kaohsiung J Med Sci.
21:395–400. 2005.PubMed/NCBI
|
|
18
|
Brown RS, Hayne D, Burcombe RJ, Harbin LJ
and Coulter CA: Massive mediastinal seminoma post-orchidectomylate
relapse with skip-metastases or new primary? Scand J Urol Nephrol.
35:422–424. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Hosono TY, Kuratsukuri K, Nitta Y,
Sugimura K, Harada T and Nakatani T: A case of primary extragonadal
seminoma arising in the perineum. Urol Int. 76:364–7. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Winter C and Albers P: Testicular germ
cell tumors: pathogenesis, diagnosis and treatment. Nat Rev
Endocrinol. 7:43–53. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Cortes D, Thorup JM, Lenz K, Beck BL and
Nielsen OH: Laparoscopy in 100 consecutive patientes with 128
impalpable testis. Br J Urol. 75:281–287. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Stang A, Rusner C, Eisinger B, Stegmaier C
and Kaatsch P: Subtype-specific incidence of testicular cancer in
Germany: a pooled analysis of nine population-based cancer
registries. Int J Androl. 32:306–316. 2009. View Article : Google Scholar
|
|
23
|
Marulaiah M, Gilhotra A, Moore L, Boucaut
H and Goh DW: Testicular and paratesticular pathology in children:
a 12-year histopathological review. World J Surg. 34:969–974.
2010.PubMed/NCBI
|
|
24
|
Pohl HG, Shukla AR, Metcalf PD, Cilento
BG, Retik AB, Bagli DJ, Huff DS and Rushton HG: Prepubertal testis
tumors: actual prevalence rate of histological types. J Urol.
72:12370–12372. 2004.PubMed/NCBI
|
|
25
|
Warde P, Specht L, Horwich A, Oliver T,
Panzarella T, Gospodarowicz M and von der Maase H: Prognostic
factors for relapse in stage I seminoma managed by surveillance: a
pooled analysis. J Clin Oncol. 20:4448–4452. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Choo R, Thomas G, Woo T, Lee D, Kong B,
Iscoe N, Danjoux C, Klotz L, Morton G and Chander S: Long-term
outcome of post-orchiectomy surveillance for stage I testicular
seminoma. Int J Radiat Oncol Biol Phys. 61:736–740. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Fosså SD, Cvancarova M, Chen L, Allan AL,
Oldenburg J, Peterson DR and Travis LB: Adverse prognostic factors
for testicular cancer-specific survival: a population-based study
of 27,948 patients. J Clin Oncol. 29:963–970. 2011.PubMed/NCBI
|
|
28
|
de Wit R and Fizazi K: Controversies in
the management of clinical stage I testis cancer. J Clin Oncol.
24:5482–5492. 2006.
|
|
29
|
Bokemeyer C, Hartmann JT, Fossa SD, Droz
JP, Schmol HJ, Horwich A, Gerl A, Beyer J, Pont J, Kanz L, Nichols
CR and Einhorn L: Extragonadal germ cell tumors: relation to
testicular neoplasia and management options. APMIS. 111:49–59.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Carver BS, Motzer RJ, Kondagunta GV,
Sogani PG and Sheinfeld J: Late relapse of testicular germ cell
tumors. Urol Oncol. 23:441–445. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Oldenburg J, Martin JM and Fosså SD: Late
relapses of germ cell malignancies: incidence, management, and
prognosis. J Clin Oncol. 24:5503–5511. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Antic T, Hyjek EM and Taxy JB: The
vanishing testis: a histomorphologic and clinical assessment. Am J
Clin Pathol. 136:872–880. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Friedman NB: The comparative morphogenesis
of extragenital and gonadal teratoid tumors. Cancer. 4:265–276.
1951. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Schlumberger HG: Teratoma of the anterior
mediastinum in the group of military age: a study of 16 cases and
review of theories of genesis. Arch Pathol (Chicago). 41:398–444.
1946.PubMed/NCBI
|