Introduction
The salivary gland is a major exocrine gland of the
human body. Due to complexities in the occurrence sites, alveolar
types and structural origins, the underlying mechanisms of salivary
gland tumors may be significantly different. Moreover, salivary
gland tumors are prone to recur after treatment and malignant
transformation. For these reasons, identifying methods of diagnosis
and prevention of salivary gland tumors remains important work for
clinical physicians.
Heat shock proteins (HSPs) or stress proteins (SPs),
are a series of important molecular chaperones, which are expressed
in the cell membranes and cytoplasm as well as nuclei of both
prokaryotic and eukaryotic organisms, which function to regulate
the growth and proliferation of the cells (1,2). Based
on the molecular weight and homology, HSPs are classified into
HSP90, HSP70 (constitutive and inductive), small molecular weight
HSPs and ubiquitin. Recently, HSPs have been found to be
upregulated in numerous tumors varying with different causes,
tissues or distribution, and interacting with other client proteins
(1,3). Thus, it was confirmed that HSP
expression is involved in the occurrence and development of a
number of tumors as well as their biological behaviors, prognosis
and treatment effect (4–6).
However, to date, few studies on HSPs in oral
tumors, particularly in the salivary gland, have been conducted
(7), and knowledge of their exact
significances is limited.
In this study, the expression characteristics of the
HSP family, including HSP27, HSP60, HSP70 and two subtypes of
HSP90, HSP86 and HSP84, in salivary gland tumors, as well as their
variance with gender, age, location, size, neural invasion, local
metastasis and proliferation index (PI) in malignant tumor patients
was studied. Also, the possible role and pathological significance
of HSPs on the occurrence and development of salivary gland tumors
was assessed.
Materials and methods
Specimens
In total, 81 cases of formalin-fixed,
paraffin-embedded specimens of salivary gland tumors which had been
surgically removed were collected between 1991 and 2009. Complete
clinical and pathological records were investigated. Samples were
obtained from the First People’s Hospital of Nantong, People’s
Hospital of Haian County and Department of Pathology, Medical
College of Nantong University, Nantong, China. Of the 81 cases, 10
had 1.5-cm peritoneal non-tumor salivary gland tissues. Our study
was approved by the local medical Ethics Committee, and prior
written informed consent and approval was obtained from the
patients.
Reagents
Mouse anti-human HSP27, HSP60 and HSP70, and the
rabbit anti-human HSP86, HSP84 and HSP70 were all products from
NeoMarkers. Immunohistochemical (IHC) high-sensitivity S-P kits and
auxiliary reagents were purchased from Fuzhou Maixin Biotechnology
Development Co., Ltd. The mouse anti-human proliferating cell
nuclear antigen (PCNA) was purchased from Dako Co., Ltd. The
Picture double staining kits (kit95-9999 and kit87-9999) were
purchased from Zymed Co. The working concentrations of these
primary antibodies were all set at 1:50.
Methods
After the specimens were continuously sliced to 4 μm
thick, a double-blind method was used to pathologically diagnose
and classify tumors in line with the standards proposed by WHO
(2005)(8). The single factor
expression and co-expression of HSPs and PCNA were detected by the
IHC S-P method (DAB staining) and the IHC double-labeled double
staining method. For the procedure of IHC double-labeled double
staining, the HSPs were detected by the IHC three-step method, then
with the help of an enhancer, the PCNA was detected by the IHC
two-step method. The routine IHC S-P and double staining
experiments were all performed according to the reagent’s
instructions, and their positive-standards were consistent. During
the experiment, microwave antigen retrieval was used with pH 6.0
citrate buffer of 10 mM. Known positive slices of infiltrating
breast cancer were applied as the positive control group and
phosphate-buffered saline (PBS) was applied to substitute the
primary antibody as the negative control group. In addition, 10
specimens from the marginal normal mucosa of tumor were used as
normal controls.
Positive standards
The positive staining for HSP27 and HSP60 was
located in the cytoplasm of the tumor cells, the positive staining
for HSP86 and HSP84 in the cytoplasm with nuclear staining and the
positive staining for HSP70 was located both in the nucleus and the
cytoplasm of tumor cells. The staining degree was scored as 0, 1, 2
and 3 corresponding to no staining, mild yellow, brownish yellow
and tawny, respectively. Then, 5 visual fields (magnificaion, ×200)
were selected at random in all slices, and the positive ratio of
the tumor cells among the total tumor parenchymal cells in the
field was calculated, based on the following criteria; <5%
scored as 0, 5–30% as 1, 30–60% as 2 and ≥60% as 3. The products of
the above 2 indices were used to determine the expression strength
of the tumor tissues, which were ranked as: 0, negative (−); 1–3,
weak expression (+); 4–6, medium expression (++); and 7–9, strong
expression (+++).
The PCNA was located in nucleus. Five visual fields
(magnification, ×200) were detected in each of the slice at random,
and the expression rate of the tumor cells is presented as PI
inferred from the ratio between the positive cells and the whole
cells in the visual field.
Statistical analysis
All data were analyzed using the Statistical Package
for Social Science (SPSS, Inc., Chicago, IL, USA) version 17.0. The
Chi-square test and the Spearman’s rank correlation test was used
in data of the categorical variables, and the t-test was used for
comparison of the mean. When the sample size (n) was ≥40 and the
expected count (T) was between 1 and 5, or when the sample size was
<40 and T was <1, the likelihood-ratio Chi-square test was
used. Fisher’s exact test (two-sided) was only used in a four-fold
table. In all tests, the significance level was set at 0.05.
Results
Clinical data
In total, 81 cases aged from between 13 and 83 years
(mean, 49.3) were included in the study, of which 42 were male and
39 were female. The occurrence sites included the parotid gland
(n=64), the submandibular gland (n=7) and other small glands
(n=10). For the tumors, 41 were benign (25 mixed tumors, 13
adenolymphoma and 3 others) and 40 were malignant with diameters of
the tumor tissue ranging from 1.5 to 6 cm [7 malignant mixed tumor
and 18 adenoid cystic carcinoma (ACC), 9 mucus epidermoid cancer
and 6 other malignant tumors].
Expression of 5 HSPs in benign or
malignant salivary gland tumors
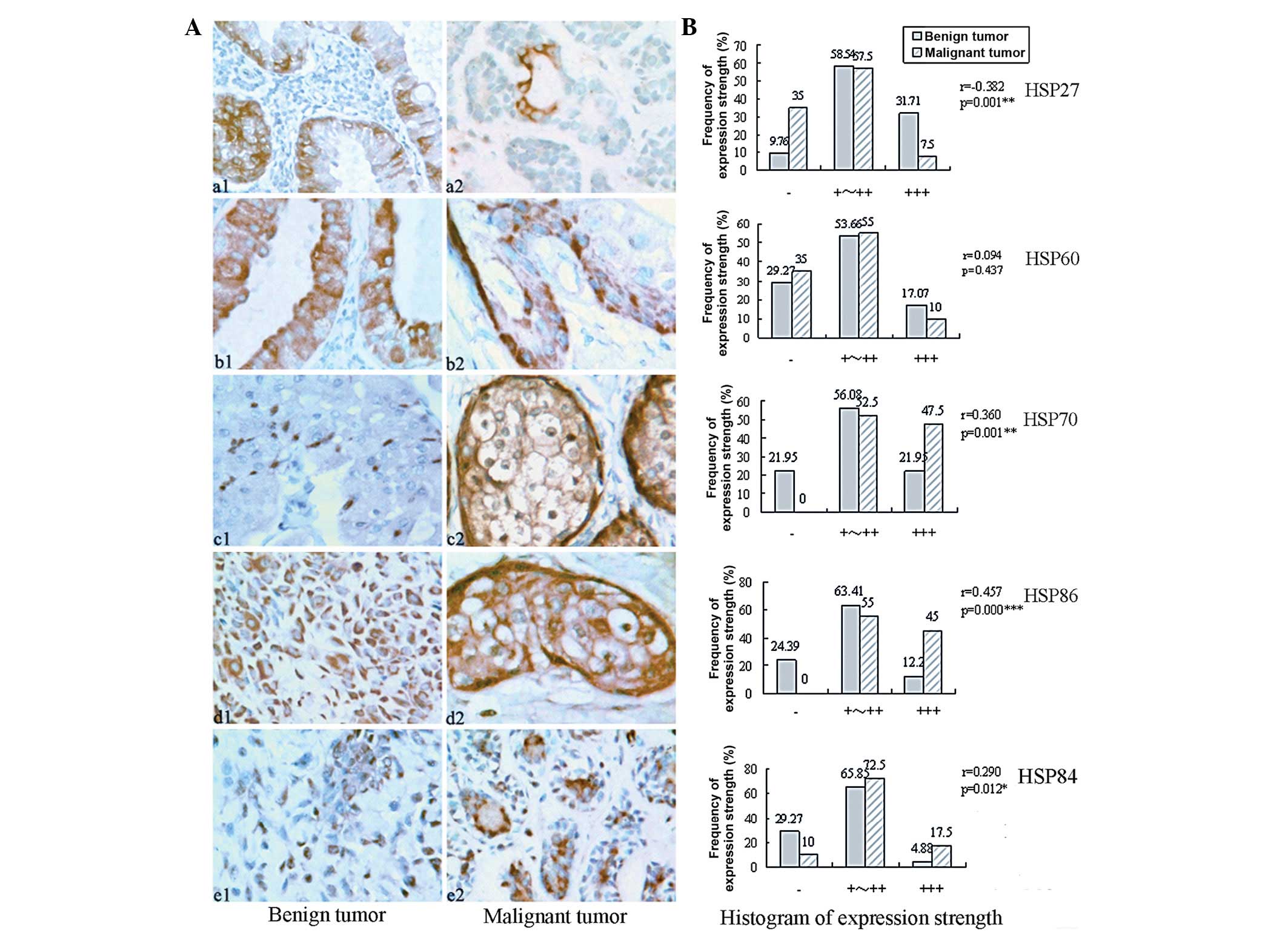
Five of the HSPs were positively expressed in normal
salivary ducts, but negative in normal glands. Positive HSP27 and
HSP60 were expressed in the cytoplasm of the tumor epithelium
(Fig. 1Aa and b), but for
adenolymphoma, HSP27 was mainly expressed in the lower epithelium
(Fig. 1Aa1) and HSP60 in the upper
(Fig. 1Ab1). HSP70 was expressed in
the nucleus and cytoplasm (Fig.
1Ac). HSP86 and HSP84 were mainly expressed in the cytoplasm,
with some in the nucleus (Fig. 1Ad and
Ae). However, for the mixed tumors, they were mainly located in
the cytoplasm of the gland ductal epithelium and the nucleus of
myoepithelium.
The expression rates of HSP27, HSP60, HSP70, HSP86
and HSP84 were 90.24% (37/41), 70.73% (29/41), 78.05% (32/41),
75.61% (31/41) and 70.73% (29/41) in the benign tumor group,
respectively. On the contrary, in the malignant tumor group, that
of HSP27 and HSP60 both decreased to 65.00% (26/40), and those of
HSP70, HSP86 and HSP84 increased to 100% (40/40), 100% (40/40) and
90% (36/40), respectively (P=0.006, 0.581, 0.002, 0.001 and 0.029,
respectively). Moreover, compared with those in the benign tumors,
the expression strength of HSP27 was lower in malignant tumor
(r=−0.382, pr=0.001), but those of HSP70, HSP86 and HSP84 were
higher (r= 0.360, 0.457 and 0.290, respectively; each P<0.01;
Fig. 1B).
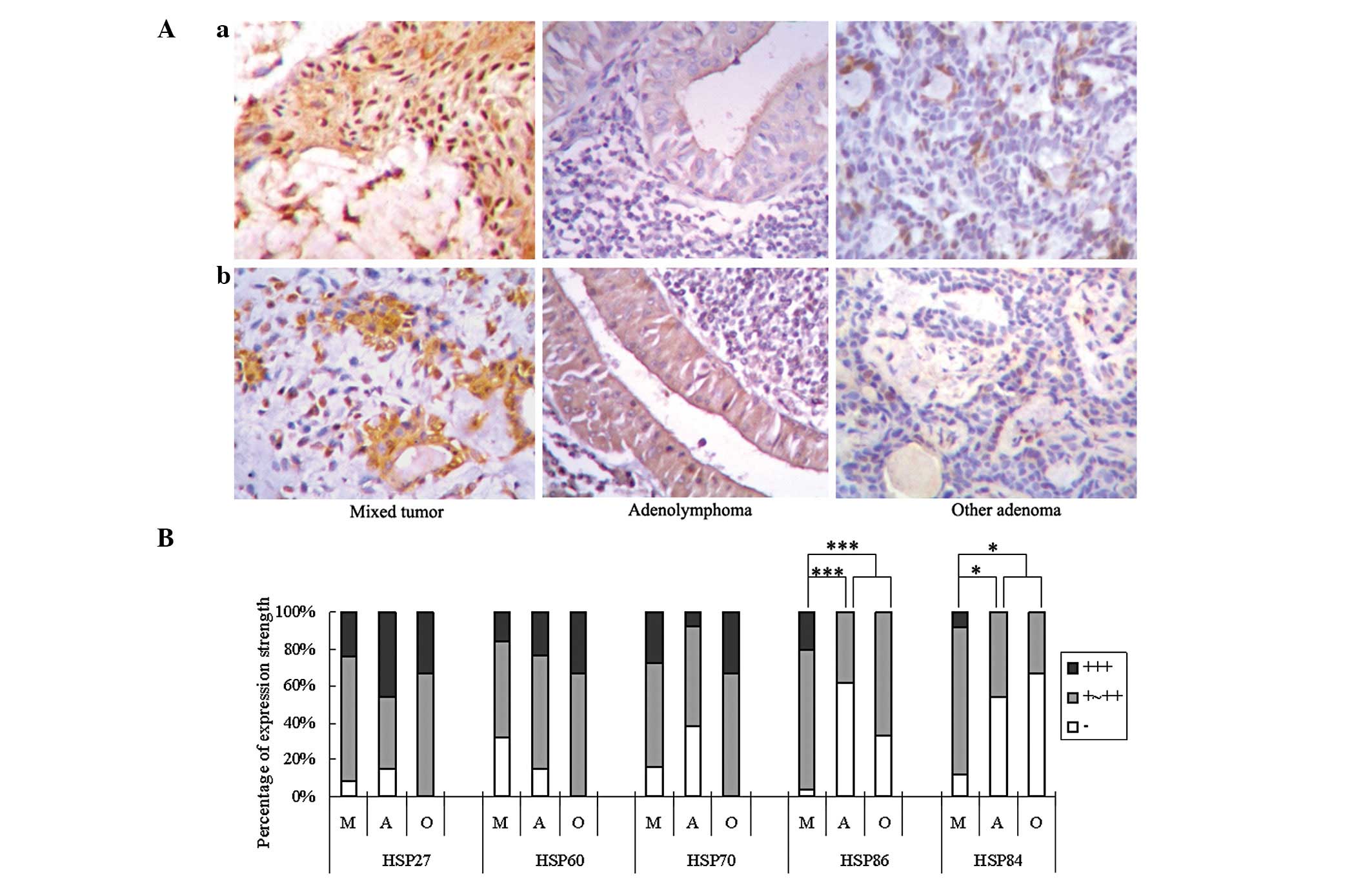
In the three groups of benign tumors, the expression
of HSP86 and HSP84 was greater in the mixed tumors, but less in the
adenolymphoma group and other benign tumor group (Fig. 2A and B). For HSP86, the three
positive grades accounted for 1, 19 and 5 cases, respectively,
among the 25 mixed tumor cases; 8, 5 and 0, respectively, in the 13
adenolymphoma cases; and 1, 2 and 0 in the three other benign tumor
cases (P<0.001). For HSP84, these figures were 3, 20 and 2,
respectively, in the 25 mixed tumor cases; 7, 6 and 0,
respectively, in the 13 adenolymphoma cases; and 2, 1 and 0 in the
three other benign tumor cases (P<0.05). However, the expression
of the 5 HSPs was not significantly different in the malignant
types (each P>0.05).
Correlation between expression of HSPs
and patient gender and age, occurrence site of tumor, size of
tumor, malignant degree and occurrence of neural invasion and
metastasis
Table I shows that
the expression rates (χ2=4.1831 and 7.3758,
respectively) and expression strengths (χ2=6.579 and
7.516, respectively) of HSP27 and HSP60 were significantly lower in
the tumor from the patients aged ≥50 years than those from the
patients aged <50 years (each P<0.05). This suggests that the
expression strengths of HSP27 and HSP60 were both negatively
correlated with patient age (r=−0.365 and −0.404, respectively;
each P<0.05).
 | Table ICorrelation between expression of HSPs
and patient gender and age, occurrence site of tumor, size of
tumor, malignant degree and occurrence of neural invasion and
metastasis (n=40). |
Table I
Correlation between expression of HSPs
and patient gender and age, occurrence site of tumor, size of
tumor, malignant degree and occurrence of neural invasion and
metastasis (n=40).
| |
Expression of
HSPs
|
|---|
| | HSP27
| HSP60
| HSP70
| HSP86
| HSP84
|
|---|
| Group | n | − | +/++ | +++ | − | +/++ | +++ | − | +/++ | +++ | − | +/++ | +++ | − | +/++ | +++ |
|---|
| Gender | | | | | | | | | | | | | | | | |
| Male | 20 | 7 | 11 | 2 | 6 | 12 | 9 | 0 | 14 | 9 | 0 | 11 | 9 | 2 | 13 | 5 |
| Female | 20 | 7 | 12 | 1 | 8 | 10 | 2 | 0 | 10 | 10 | 0 | 11 | 9 | 2 | 16 | 2 |
| Age (years) | | r=−0.365
P=0.021a | r=−0.404
P=0.010a | | | |
| <50 | 23 | 5 | 15 | 3 | 4 | 16 | 3 | 0 | 11 | 12 | 0 | 13 | 10 | 2 | 16 | 5 |
| ≥50 | 17 | 9 | 8 | 0 | 10 | 6 | 1 | 0 | 10 | 7 | 0 | 9 | 8 | 2 | 13 | 2 |
| Location | | | | | | | | | r=0.480
P=0.002b | | r=0.482
P=0.002b | | | | | |
| Parotid
gland | 26 | 8 | 15 | 3 | 11 | 13 | 2 | 0 | 18 | 8 | 0 | 19 | 7 | 4 | 19 | 3 |
| Submandibular
gland | 5 | 1 | 4 | 0 | 2 | 2 | 1 | 0 | 2 | 3 | 0 | 1 | 4 | 0 | 4 | 1 |
| Minor salivary
gland | 9 | 5 | 4 | 0 | 1 | 7 | 1 | 0 | 1 | 8 | 0 | 2 | 7 | 0 | 6 | 3 |
| Size (cm) | |
χ2=10.660
P=0.031 a | | | | |
| ≤2 | 12 | 4 | 8 | 0 | 5 | 6 | 1 | 0 | 5 | 7 | 0 | 7 | 5 | 1 | 9 | 2 |
| 2–4 | 20 | 4 | 13 | 3 | 4 | 14 | 2 | 0 | 13 | 7 | 0 | 11 | 9 | 1 | 16 | 3 |
| >4 | 8 | 6 | 2 | 0 | 5 | 2 | 1 | 0 | 3 | 5 | 0 | 4 | 4 | 2 | 4 | 2 |
| Grading | | | | | | | | | | | r=0.487
P=0.001 b | | | | | |
| Low | 9 | 2 | 6 | 1 | 4 | 5 | 0 | 0 | 7 | 2 | 0 | 9 | 0 | 1 | 8 | 0 |
| Middle-high | 31 | 12 | 17 | 2 | 10 | 17 | 4 | 0 | 14 | 17 | 0 | 13 | 18 | 3 | 21 | 7 |
| Neural
invasion | | r=−0.474
P=0.002 b | | r=0.447
P=0.004b | r=0.355
P=0.025 a | |
| − | 31 | 7 | 21 | 3 | 11 | 17 | 3 | 0 | 20 | 11 | 0 | 20 | 11 | 3 | 23 | 5 |
| + | 9 | 7 | 2 | 0 | 3 | 5 | 1 | 0 | 1 | 8 | 0 | 2 | 7 | 1 | 6 | 2 |
| Metastasis | | r=−0.351
P=0.026a |
χ2=6.916
P=0.031a | r=0.376
P=0.017a | r=0.406
P=0.009 b | |
| − | 30 | 7 | 21 | 2 | 8 | 20 | 2 | 0 | 19 | 11 | 0 | 20 | 10 | 3 | 24 | 3 |
| + | 10 | 7 | 2 | 1 | 6 | 2 | 2 | 0 | 2 | 8 | 0 | 2 | 8 | 1 | 5 | 4 |
The positive strengths of HSP70 and HSP86 were
weaker in the parotid group, but stronger in the submandibular and
minor salivary gland groups (χ2= 6.1017, P<0.05 and
χ2=10.2228 and P<0.01, respectively). There was a
significantly positive correlation among the above three groups
(r=0.480, P=0.002 and r=0.4822, P<0.01, respectively). In
particular, both HSP70 and HSP8 were both significantly stronger in
the minor salivary gland group than in the parotid group
(χ2=9.8873 and 7.2865, respectively; each
P<0.01).
The samples were divided into 3 groups based on the
diameter of the tumor tissue; <2 cm group, 2–4 cm group and
>4 cm group. Using these groupings, the expression rate of HSP27
was significantly lower in the >4 cm group
(χ2=7.6190, P=0.022), but no difference existed between
the <2 cm group and the 2–4 cm group. If the samples were
divided into 2 groups based on the diameter of the tumor tissue;
<4 cm group and >4 cm group, then a negative correlation
between the expression rate of HSP27 and tumor size was observed
(χ2= 6.8089, P= 0.009, r=−0.4193, P<0.001). Compared
with the 2–4 cm group, the expression rate and positive strength of
HSP27 were also significantly negatively correlated in the >4 cm
group (r=−0.5185 and −0.3790, respectively; each P<0.05).
Compared with the low grading malignant group, the
expression strength of HSP86 was significant higher in the
middle-high grade malignant group (χ2=12.886, P=000),
and was positively correlated with malignant grading (r=0.487,
P=0.001).
Upon comparing accompanying neural invasion or
metastasis, it was revealed that the expression rates of HSP27
significantly decreased (χ2=9.3411 and 7.1795,
respectively, each P<0.05). The expression strength of HSP27
also decreased with neural invasion or metastasis,
(χ2=9.655 and 8.169, respectively, each P<0.05) and
was negatively correlated with neural invasion and metastasis
(r=−0.474, P<0.01 and r=−0.351, P<0.05, respectively).
By contrast, the expression strength of HSP70
(χ2=8.749 and 5.914, respectively) and HSP86
(χ2=5.192 and 6.852, respectively) increased with neural
invasion and metastasis (each P<0.05) and a positive correlation
was found between the expression strength and neural invasion
(r=0.447, P<0.01; r= 0.355, P<0.05, respectively) or
metastasis (r=0.376, P<0.05 and r=0.406, P<0.01,
respectively).
Correlation analysis of HSPs with PI in
malignant salivary gland tumors
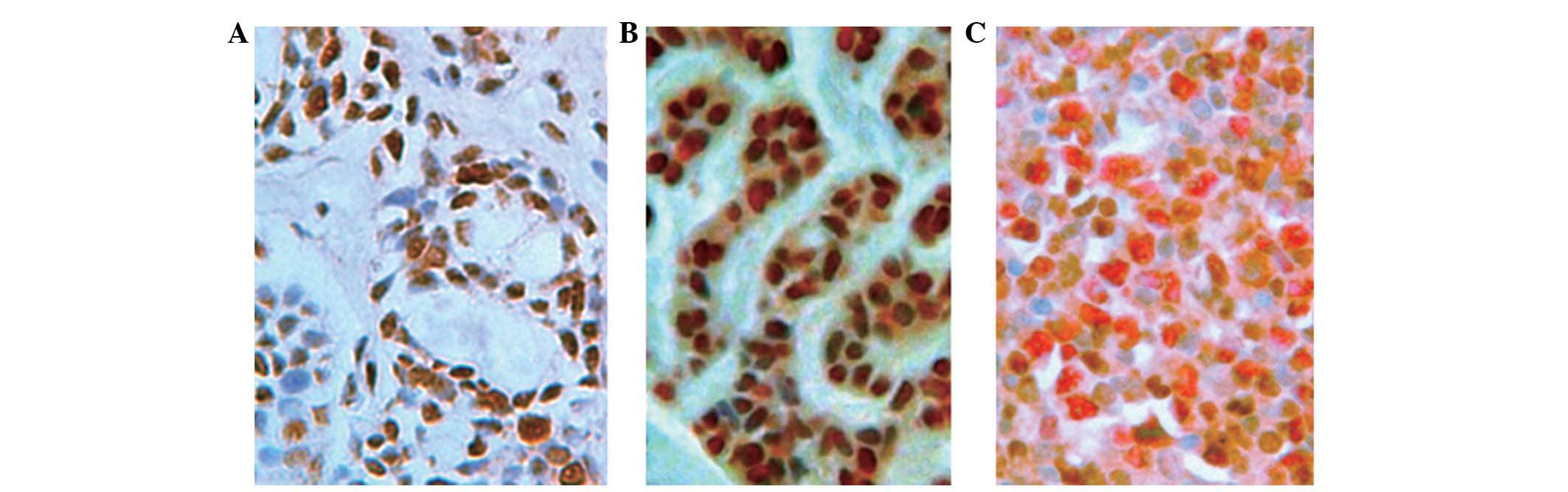
PCNA was mainly located in the nucleus of tumor
epithelial cells (Fig. 3A) and
little was expressed in non-tumor salivary gland tissues. However,
its expression ratio increased in both benign and malignant tumor
tissues. In the benign tumor group, the expression rate was 58.54%
(24/41) with PI reaching 23.46±33.177, while in the malignant tumor
group, it was 100% and 49.95±14.569. Therefore, significant
differences existed between the benign group and the malignant
group (t=−4.6323, P=0.0000).
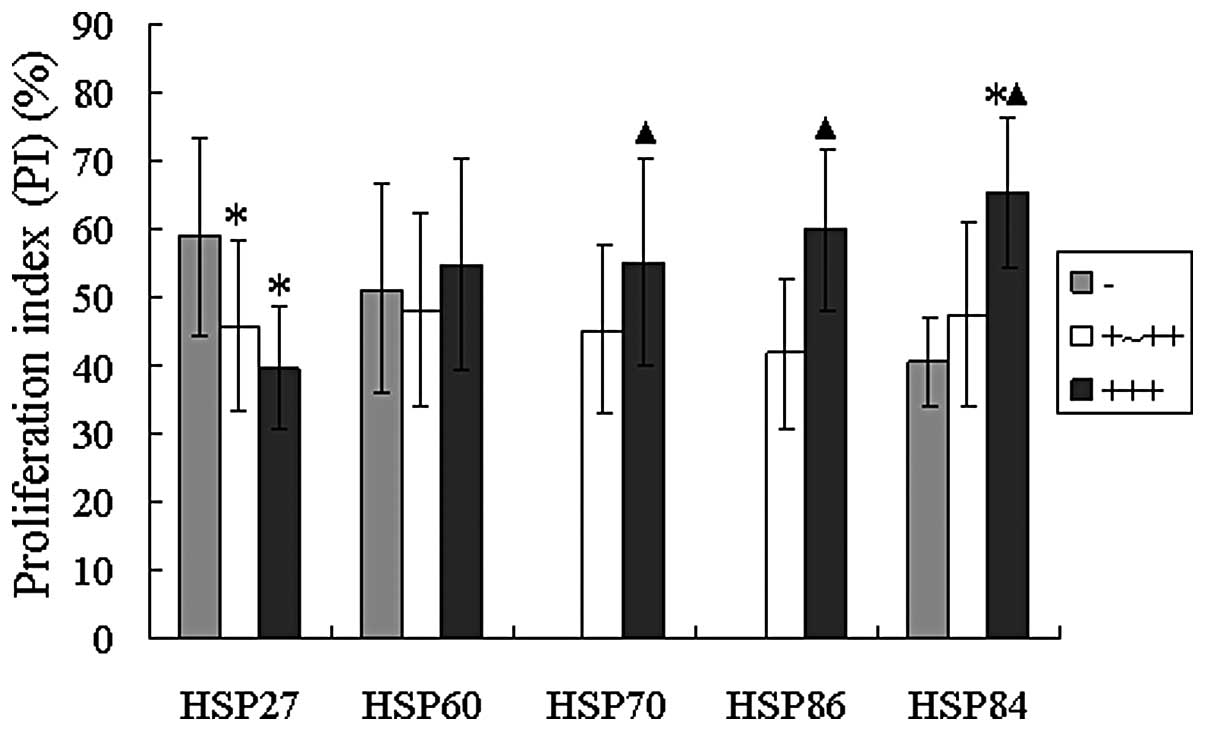
The correlation between the expression of the 5 HSPs
and PI was analyzed in malignant tumors. (Figs. 3B and C and 4) demonstrated that the PCNA-PI was
significantly higher (P<0.05), while the expression strength of
HSP70, HSP86 and HSP84 was enhanced in the malignant tumors
(t=−4.0687, −5.0744 and −3.1779, respectively). Also, PCNA-PI was
highest with the lack of HSP27 expression, but HSP27 positive
(+/++, +++) PCNA-PI was significantly lower (t=2.9185 and 2.1834,
respectively). The above results of duration t-test all had
statistical significance (each P<0.05).
Discussion
In this study, 5 HSPs were found to be expressed in
salivary gland tumors (expression rates, 64.71 to 100%) and
non-tumor salivary ducts, but not in salivary glands. The
expression of the 5 HSPs was closely correlated with the occurrence
of salivary gland tumors originating from salivary ducts, but that
varied with the tumor type.
HSP27 is a member of the small HSP family, and is
associated with tumor formation and metastatic potential (9,10). It
may be a potential target for the periodontal regeneration process
involved in cell migration (11).
Our findings indicated that the significant decrease in expression
rate and strength in malignant tumors was similar to that of low
class gliomas (12). Thus, HSP27
may be correlated with cell differentiation of salivary gland
tumors, and its weak expression may be used as a marker of low
differentiation in malignant tumors. Due to the growth rate, the
ability to invade and metastasize was often stronger in the low
differentiated tumors, so the expression of HSP27 significantly
decreased in both the >4 cm diameter group and groups where
neural invasion or metastasis occurred (each P<0.05). Therefore,
low or no expression of HSP27 may be a useful biomarker to evaluate
the biological behavior of malignant salivary gland tumors.
HSP60 (also known as Cpn60) is a chaperonin protein,
which plays an important role in cell physiology and survival, and
is closely correlated with functions of the innate and adaptive
immune system in mammals (13).
Thus, it is essential in tumor immunity, and is a good biomarker
for assessment of prognosis of patients with lung adenocarcinoma
(14,15). We found that the expression of HSP60
was negatively correlated with the age of patients; this suggests
that as an immune adjuvant, the age of the patients is a negative
control factor. Its decreasing expression may lead to a decline in
antitumor immune function and may be correlated with occurrence of
metastasis. HSP60 may participate in tumor immune action in the
early stage of tumor development. Similarly, age factors may also
be an important factor associated with HSP27 expression. However,
the exact underlying mechanisms for the effect of the patient age
on the expression of HSP60 and HSP27 remain unclear.
By far the most conserved molecular chaperone, HSP70
is an important apoptosis inhibitor (16) and is often increasingly synthesized
in malignant tumors (17,18), including hepatocellular carcinoma
HepG (2) cells. When the HSP70 gene
was silenced, cell death induced by chemotherapy drugs was enhanced
(19).
HSP86 (90α) and HSP84 (90β) are two subtypes of
HSP90, which are functionally important in the structure and
stability of client proteins in tumor cells, which affect
proliferation, survival, differentiation, mobility, angiogenesis
and metastasis of tumor cells (20,21).
Hop (Hsp70/Hsp90 organising protein), also called stress-inducible
protein 1, which interacts with HSP70 and HSP90 was overexpressed
in human colonic carcinoma (22),
invasive pancreatic cancer cell lines and malignant tissues of
pancreatic cancer patients, and knockdown of Hop gene may decrease
the invasiveness of pancreatic cancer cells, possibly by means of
modulation of HSP90 activity (23).
Our results demonstrated that HSP70, HSP86 and HSP84
were all increasingly expressed in salivary gland tumors and were
positively correlated with malignant tumors (r= 0.360, 0.457 and
0.290, respectively); particularly for HSP86 (r= 0.487). The
expression of HSP70 and HSP86 were also positively correlated with
the occurrence of neural invasion (r= 0.447 and 0.355,
respectively) and metastasis (r= 0.376 and 0.406, respectively), as
demonstrated in previous studies (18,21,22).
These findings suggest that HSP70 and HSP90 and the client proteins
synthesized by the lasting induction of HSP70 and HSP90 work
together to enhance the occurrence and development of malignant
tumors. Thus, in increasing order, the expression of HSP70 and
HSP86 was arranged as parotid gland, submandibular and minor
salivary glands (r=0.480 and 0.482, respectively).
In 41 cases of benign tumors, expression of HSP84
and HSP86 was significantly greater in mixed tumors, particularly
when compared with that in the adenolymphoma group. This may be due
to the higher recurrence and malignant transformation rates in
mixed tumor compared to adenoma and adenolymphoma. As previously
reported (22), HSP90 may be a
critical molecular chaperone in tumor recurrence and malignant
transformation.
PCNA is a nucleoprotein that is widely expressed
during S phase of the cell cycle and is associated with cell
proliferation potential (24);
therefore, it is often found at the front of invasive tumors and
correlated with poor prognosis (25).
PCNA-PI was mainly found in the tumor cells and
increased with HSP70, HSP86 and HSP84 expression (each P<0.05).
Moreover, significantly higher levels of PCNA-PI were observed in
malignant than in the benign tumors (t=−4.6323, P=0.0000). This
indicated that the malignant tumor cells have a stronger
proliferative potentiality and this may explain why more HSP70,
HSP86 and HSP84 were synthesized. As important molecular
chaperones, these HSPs maintained the high proliferation
capabilities of the tumor cells, particularly that of the malignant
tumors, through interaction with their client proteins. This may
explain why the double staining results revealed the higher
co-expression correlation between PCNA and HSP70, HSP86 and
HSP84.
Salinthone et al(26) reported that HSP27 may inhibit cell
proliferation. The present study also demonstrated that high
expression of HSP27 prevented the proliferation and immortalization
of tumor cells in benign tumors and although its expression was
weakened, the proliferation and malignant transformation rate of
the tumor cell increased. Therefore, PCNA-PI was higher in the
group where HSP27 expression was negative (t=2.9185 and 2.1834,
respectively; each P<0.05). The reversely proportional
correlation identified between HSP27 expression and PCNA based on
the double staining results may indicate that HSP27 only has an
incomplete inhibition effect on the expression of PCNA in tumor
cells and certain other unknown pathways may be involved. The
decrease in the expression of HSP27 and positive PCNA may work
together to control tumor proliferation and the malignant
transformation process.
Acknowledgements
This study was funded by the Priority
Academic Program Development of Jiangsu Higher Education
Institutions and the Science Foundation of Nantong City, Jiangsu,
China (No. S2006009 and HS2012070).
References
|
1
|
Khalil AA, Kabapy NF, Deraz SF and Smith
C: Heat shock proteins in oncology: diagnostic biomarkers or
therapeutic targets? Biochim Biophys Acta. 1816:89–104.
2011.PubMed/NCBI
|
|
2
|
Scroggins BT, Robzyk K, Wang D, et al: An
acetylation site in the middle domain of Hsp90 regulates chaperone
function. Mol Cell. 25:151–159. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kim LS and Kim JH: Heat shock protein as
molecular targets for breast cancer therapeutics. J Breast Cancer.
14:167–174. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Park SJ, Kostic M and Dyson HJ: Dynamic
interaction of Hsp90 with its client protein p53. J Mol Biol.
411:158–173. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Rérole AL, Gobbo J, De TA, et al: Peptides
and aptamers targeting HSP70: a novel approach for anticancer
chemotherapy. Cancer Res. 71:484–495. 2011.
|
|
6
|
Guo QY, Yuan M, Peng J, Cui XM, Song G,
Sui X and Lu SB: Antitumor activity of mixed heat shock
protein/peptide vaccine and cyclophosphamide plus interleukin-12 in
mice sarcoma. J Exp Clin Cancer Res. 30:242011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Okui T, Shimo T, Hassan NM, et al:
Antitumor effect of novel HSP90 inhibitor NVP-AUY922 against oral
squamous cell carcinoma. Anticancer Res. 31:1197–1204.
2011.PubMed/NCBI
|
|
8
|
Barnes L, Eveson JW, Reichart P and
Sidransky D: World Health Organization Classification of Tumours.
Pathology and Genetics of Head and Neck Tumours. IARC Press; Lyon:
pp. 209–281. 2005
|
|
9
|
Gibert B, Hadchity E, Czekalla A, et al:
Inhibition of heat shock protein 27 (HspB1) tumorigenic functions
by peptide aptamers. Oncogene. 30:3672–3681. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Banerjee S, Lin CF, Skinner KA, et al:
Heat shock protein 27 differentiates tolerogenic macrophages that
may support human breast cancer progression. Cancer Res.
71:318–327. 2011. View Article : Google Scholar
|
|
11
|
Kwon SM, Kim SA, Fujii S, Maeda H, Ahn SG
and Yoon JH: Transforming growth factor β1 promotes migration of
human periodontal ligament cells through heat shock protein 27
phosphorylation. Biol Pharm Bull. 34:486–489. 2011.
|
|
12
|
Shen G, Liang S, Xu Z, et al:
Dowuregulated expression of HSP27 in human low-grade glioma tissues
discovered by a quautitative proteomic analysis. Proteomic Sci.
26:17–28. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Quintana FJ and Cohen IR: The HSP60 immune
system network. Trends Immunol. 32:89–95. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Cappello F, de Macario EC, Zummo G and
Macario AJ: Immunohistochemistry of human Hsp60 in health and
disease: from autoimmunity to cancer. Methods Mol Biol.
787:245–254. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Xu X, Wang W, Shao W, et al: Heat shock
protein-60 expression was significantly correlated with the
prognosis of lung adenocarcinoma. J Surg Oncol. 104:598–603. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kayama M, Nakazawa T, Thanos A, et al:
Heat shock protein 70 (HSP70) is critical for the photoreceptor
stress response after retinal detachment via modulating
anti-apoptotic Akt kinase. Am J Pathol. 178:1080–1091. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhang J, Wang K, Zhang J, Liu SS, Dai L
and Zhang JY: Using proteomic approach to identify tumor-associated
proteins as biomarkers in human esophageal squamous cell carcinoma.
J Proteome Res. 10:2863–2872. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kocsis J, Mészáros T, Madaras B, et al:
High levels of acute phase proteins and soluble 70 kDa heat shock
proteins are independent and additive risk factors for mortality in
colorectal cancer. Cell Stress Chaperones. 16:49–55. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhu Q, Xu YM, Wang LF, et al: Heat shock
protein 70 silencing enhances apoptosis inducing factor-mediated
cell death in hepatocellular carcinoma HepG2 cells. Cancer Biol
Ther. 8:792–798. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Beck R, Dejeans N, Glorieux C, et al:
Molecular chaperone Hsp90 as a target for oxidant-based anticancer
therapies. Curr Med Chem. 18:2816–2825. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Taiyab A and Rao ChM: HSP90 modulates
actin dynamics: inhibition of HSP90 leads to decreased cell
motility and impairs invasion. Biochim Biophys Acta. 1813:213–221.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kubota H, Yamamoto S, Itoh E, et al:
Increased expression of co-chaperone HOP with HSP90 and HSC70 and
complex formation in human colonic carcinoma. Cell Stress
Chaperones. 15:1003–1011. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Walsh N, Larkin A, Swan N, Conlon K,
Dowling P, McDermott R and Clynes M: RNAi knockdown of Hop
(Hsp70/Hsp90 organising protein) decreases invasion via MMP-2 down
regulation. Cancer Lett. 306:180–189. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Abike F, Tapisiz OL, Zergeroglu S, Dunder
I, Temizkan O and Payasli A: PCNA and Ki-67 in endometrial
hyperplasias and evaluation of the potential of malignancy. Eur J
Gynaecol Oncol. 32:77–80. 2011.PubMed/NCBI
|
|
25
|
Kato K, Kawashiri S, Yoshizawa K, et al:
Expression form of p53 and PCNA at the invasive front in oral
squamous cell carcinoma: correlation with clinicopathological
features and prognosis. J Oral Pathol Med. 40:693–698. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Salinthone S, Ba M, Hanson L, Martin JL,
Halayko AJ and Gerthoffer WT: Overexpression of human Hsp27
inhibits serum-induced proliferation in airway smooth muscle
myocytes and confers resistance to hydrogen peroxide cytotoxicity.
Am J Physiol Lung Cell Mol Physiol. 293:1194–1207. 2007. View Article : Google Scholar
|