Introduction
Lung cancer is currently the most frequent cause of
cancer mortality in the world and non-small-cell lung cancer
(NSCLC) accounts for ∼80% of all lung cancer cases (1).Moreover, the 5-year survival of NSCLC
is only 15% (2), as most patients
are diagnosed at an advanced stage of the disease when surgery
options are limited. Thus, chemotherapy has become the most
important treatment option for treating these patients.
Platinum-based chemotherapy is considered to be the foundation of
treatment for patients with advanced NSCLC, with a median overall
survival (OS) of 8–11 months, and therefore may prolong survival
and improve the quality-of-life for these patients (3). However, current chemotherapeutic
approaches have had limited success and therefore novel agents are
urgently required to improve outcomes. Pemetrexed is a United
States Food and Drug Administration (FDA)-approved therapy for
advanced NSCLC (4) and a large
phase III study has demonstrated that pemetrexed in combination
with cisplatin provides similar efficacy and improved tolerability
compared with gemcitabine in combination with cisplatin for
advanced NSCLC patients (5). The
study also indicated that pemetrexed provides similar improvements
for patients with adenocarcinoma. Pemetrexed is widely used for
first- and second-line and maintenance therapy in NSCLC (4–6).
However, in China Pemetrexed is considered to be an expensive drug
and is therefore not widely used. Consequently, it is critical to
identify patients who may benefit from pemetrexed therapy in order
to reduce the side-effects and provide the most cost-effective
approach. A number of clinical trials have attempted to identify
molecular biomarkers which may be associated with the response and
toxicity of pemetrexed (7,8). The ultimate goal of this type of
research is to create individualized treatment that provides an
improved therapeutic profile by identifying the patients who are
most likely to benefit from personalized therapy.
Pemetrexed is a multi-targeted, folate-based
anti-metabolite that has been shown to have anticancer activity in
other tumor types, including cervical (9), pancreatic (10), colorectal (11), breast (12) and gastric (13) cancers. Pemetrexed inhibits several
enzymes in the thymidine and purine biosynthetic pathways,
including thymidylate synthase (TS), dihydrofolate reductase
(DHFR), glycinamide ribonucleotide formyl transferase
(GARFT) and aminoimidazole carboxamide ribonucleotide-formyl
transferase (AICARFT). Pemetrexed is transported into the
cell by the reduced folate carrier (RFC) system and is then
rapidly and extensively polyglutamated by folylpolyglutamate
synthase (FPGS). The drug is eliminated from the cell by
γ-glutamyl hydrolase (GGH).
TS is a folate-dependent enzyme and the
presence of a single nucleotide polymorphism (SNP) in the TS
promoter has been shown to be an independent factor in metastatic
colorectal cancer patients treated with 5-fluorouracil (5-FU)
(14). Methylenetetrahydrofolate
reductase (MTHFR) is a key enzyme in the folate metabolism
pathway and MTHFR polymorphisms have been reported to
correlate with the outcome of patients treated with methotrexate
(15) and pemetrexed (16,17).
The reduced folate carrier SLC19A1 is responsible for the
transport of pemetrexed and therefore genetic variations in the
gene may affect the transport of the drug (18). The associations between the gene
polymorphisms and drug efficacy remain controversial. At present,
few studies have investigated the correlation between pemetrexed
pathway-associated gene polymophisms and drug efficacy. Therefore,
the present study examined the association between the gene
polymorphisms and therapeutic results of pemetrexed treatment in
NSCLC patients.
Patients and methods
Patient selection
Chemotherapy-naive patients who were ≥18-years-old
with histological or cytological evidence of measurable metastases
or stage IIIB or IV NSCLC were enrolled in the present study. Other
inclusion criteria included an Eastern Cooperative Oncology Group
performance status (ECOG PS) ≤2 and normal organ function,
including adequate hepatic, renal and hematological function.
Patients who had brain metastases were eligible if they had a life
expectancy ≥ three months. Pregnant or lactating women were
excluded from the study. All patients provided written informed
consent prior to the initiation of treatment. Separate informed
consent was obtained for the collection of blood samples for the
study of single-nucleotide polymorphisms (SNPs). This study was
approved by the ethics committee of the Second People’s Hospital of
Lianyungang (Jiangsu, China).
Treatment
Pemetrexed (500 mg/m2) was administered
to patients as a 10 min intravenous (i.v.) infusion on day 1 of a
21 day cycle with cisplatin (75 mg/m2, i.v.)
administered on days 1–3 every three weeks. All patients received
daily oral supplements of folic acid (350–1000 μg) 5–7 days prior
to the first day of administration and the supplementation was
continued for three weeks after the treatment ended. Vitamin
B12 (1000 μg) was administered by intramuscular (i.m.)
injection every three cycles of treatment. Dexamethasone (4 mg) was
administered orally twice a day on the day before, day of and day
after each dose of pemetrexed therapy to prevent allergic
reactions.
Treatment evaluation
All patients received complete clinical and
laboratory evaluations prior to each cycle of treatment, including
patient histories, physical examination, complete blood count,
serum chemistry profile, electrocardiography (ECG) and radiological
evaluation. Computed tomography (CT) and/or magnetic resonance
imaging (MRI) were performed to evaluate the tumor response after
every two cycles of therapy. The treatment effect was assessed
according to the Response Evaluation Criteria in Solid Tumors
(RECIST) (19) and categorized as
complete response (CR), partial response (PR), stable disease (SD)
or progressive disease (PD).
DNA extraction
A 5 ml aliquot of blood was collected from each
patient into an EDTA-coated tube before they underwent the first
drug cycle. Genomic DNA was extracted from peripheral blood cells
using the Blood DNA kit (Omega Bio-Tek, Norcross, GA, USA)
according to the manufacturer’s instructions.
TS genotyping
DNA was amplified by polymerase chain reaction (PCR)
using the following primers: 5′-AACTGTGCTGCT GGCTTAGA-3′ (forward)
and 5′-GTCTGTAAGGCGAG GAGGAC-3′ (reverse). The TS gene
promoter repeat and SNP (two or three repeats; G>C within the
second repeat of the 3R allele) polymorphisms were directly
detected by DNA sequencing using an ABI Prism 3730 DNA analyzer
(Applied Biosystems, Carlsbad, CA, USA). The TS genotype was
divided into low expression genotypes (2R/2R, 2R/3C and 3C/3C) and
high expression genotypes (2R/3G, C/3G and 3G/3G) according to the
SNP and TS genotype.
MTHFR and SLC19A1 SNP
MTHFR and SLC19A1 SNPs were detected
using a Taqman minor groove binder (MGB) probe-based assay. The
PCRs for the MTHFR and SLC19A1 genotypes were
performed with a total volume of 5 μl, including 2.5 μl Taqman
Master Mix, 0.25 μl Taqman MGB primer probe, 1 μl DNA and 1.25 μl
DEPC H2O. The PCR cycling parameters were as follows: 45
cycles of 95°C for 10 min, followed by 92°C for 15 sec and 60°C for
90 sec. Genotyping was performed using the ABI 7900 HT Sequence
Detector (Applied Biosystems) and analyzed by the Allelic
Discrimination program (Applied Biosystems).
Statistical analysis
SPSS version 13.0 (SPSS, Inc., Chicago, IL, USA) was
used for the data analyses. The primary end point was
progression-free survival (PFS) and the secondary end points were
objective response rate (RR) by RECIST and OS. PFS was measured
from the time of randomization to disease progression or mortality
from any cause, whichever occurred earlier. OS was measured from
the time of randomization to the time of mortality from any cause.
The Hardy-Weinberg equilibrium for gene polymorphisms was used to
compare the observed distributions of genotype frequencies.
Fisher’s exact test was used to compare the treatment RRs between
the genotypes. Kaplan-Meier curves and log-rank tests were also
used to compare the OS and PFS distributions between the different
SNP subgroups. P≤0.05 was considered to indicate a statistically
significant difference and all values were two-sided.
Results
Characteristics
Between August 2009 and December 2011, a total of 47
patients were enrolled in the trial conducted at the Lianyungang
hospital, which is affiliated with Bengbu Medical College. Two
patients refused additional treatment after the first cycle
administration due to grade 4 neutropenia. There were no
significant differences in the baseline characteristics of the 45
patients and all the patients had adenocarcinoma. The median age
was 63 years (range, 39–81 years). The characteristics of all the
patients are shown in Table I.
 | Table IClinical characteristics. |
Table I
Clinical characteristics.
| Characteristic | Data |
|---|
| Gender, n (5) | |
| Male | 23 (51.1) |
| Female | 22 (48.9) |
| Age (years) | |
| Median age | 63 |
| Range | 39–81 |
| ECOG, n (%) | |
| 0 | 12 (26.7) |
| 1 | 24 (53.3) |
| 2 | 9 (20.0) |
| Stage of disease, n
(%) | |
| IIIB | 21 (46.7) |
| IV | 24 (53.3) |
| Smoking status, n
(%) | |
| Former/current
smoker | 31 (68.9) |
| Never-smoker | 14 (31.1) |
| Number of cycles,
n | |
| Total | 199 |
| Median | 4 |
| Range | 1–6 |
Genotypes
The gene information was obtained from the HapMap
database. Table II shows the
genotype distributions of TS, MTHFR and
SLC19A1. The distributions of the genetic variants followed
the Hardy-Weinberg equilibrium (P>0.05).
 | Table IIDistribution of TS,
MTHFR and SLC19A1 genotypes. |
Table II
Distribution of TS,
MTHFR and SLC19A1 genotypes.
| Genotype | db SNP id | N (%) |
|---|
| TS | rs45445694 | |
| 2R/2R | | 0 (0) |
| 2R/2C | | 7 (15.6) |
| 3C/3C | | 8 (17.8) |
| 2R/3G | | 12 (26.7) |
| 3G/3G | | 4 (8.9) |
| 3C/3G | | 14 (31.1) |
| MTHFR
C677T | rs1801133 | |
| CC | | 17 (37.8) |
| CT | | 21 (46.7) |
| TT | | 7 (15.6) |
| SLC19A1 exon
6 (2522) C/T | rs1051298 | |
| CC | | 11 (24.4) |
| CT | | 23 (51.1) |
| TT | | 11 (24.4) |
Association between gene polymorphisms
and the treatment response to pemetrexed
There were 14 patients in the study who achieved a
PR, eight with PD, 22 with SD and one who achieved a CR. The
overall response rate (ORR; CR + PR) was 33.3% (15/45). All 45
patients had evaluable data for analysis. No significant
associations were observed between the response rate and
polymorphisms of MTHFR and SLC19A1. Patients with
2R/2R, 2R/3C or 3C/3C genotypes had a significantly higher RR than
patients with 2R/3G, 3C/3G or 3G/3G genotypes (53.3 vs. 23.3%,
respectively; P=0.044; Table
III).
 | Table IIIResponse rate and gene
polymorphisms. |
Table III
Response rate and gene
polymorphisms.
| Gene
polymorphism | CR + PR | PD + SD | P-value |
|---|
| TS | | | 0.044 |
| 2R/3G, 3C/3G,
3G/3G | 7 | 23 | |
| 2R/2R, 2R/3C,
3C/3C | 8 | 7 | |
| MTHFR | | | 0.306 |
| CC | 8 | 9 | |
| CT | 5 | 16 | |
| TT | 2 | 5 | |
| SLC19A1 | | | 0.701 |
| CC | 3 | 8 | |
| CT | 9 | 14 | |
| TT | 3 | 8 | |
Survival analysis in patients with gene
polymorphisms
According to the Kaplan-Meier survival analysis,
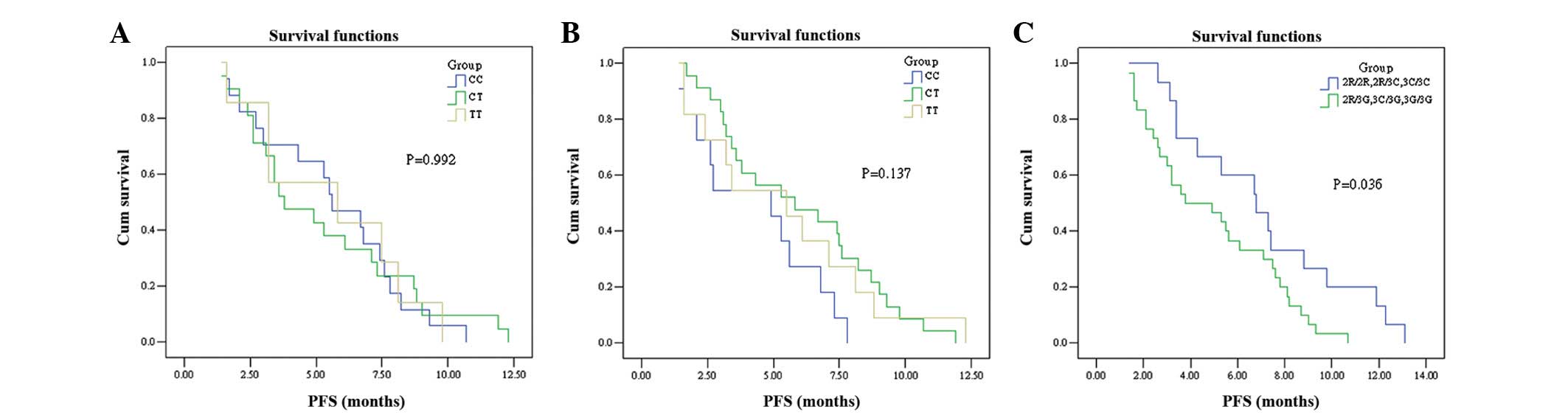
patients with the TS 2R/2R, 2R/3C or 3C/3C genotypes had a
significantly longer median PFS than patients with the 2R/3G, 3C/3G
or 3G/3G genotypes (6.8 vs. 3.8 months, respectively; log-rank x2 =
4.417; P=0.036; Fig. 1A). However,
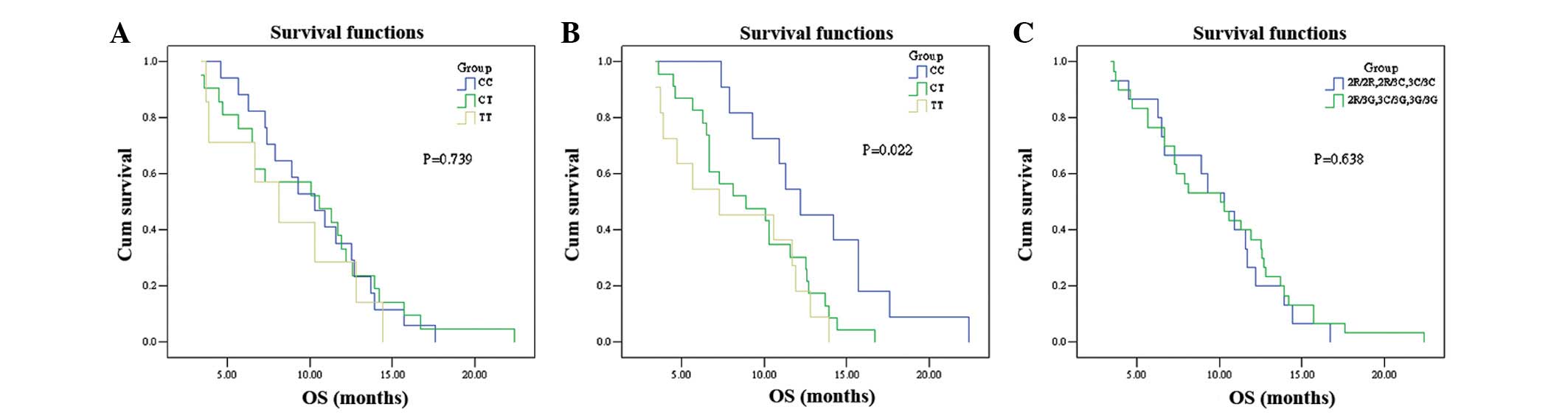
no significant difference in OS was observed between the two groups
(P=0.638; Fig. 2A). The
Kaplan-Meier survival analysis showed that PFS and OS did not
differ for the three genotypes of MTHFR (PFS: CC, 5.6
months; CT, 3.8 months; TT, 5.8 months; log-rank x2 = 0.016;
P=0.992; OS: 10.3 vs. 10.6 vs. 8.1 months, respectively; P=0.739;
Fig. 1B and 2B). No significant association was
observed between the PFS of patients with the SLC19A1 CC
genotype and patients with the CT and TT genotypes (log-rank x2 =
3.976; P=0.137; Fig. 1C). However,
patients with the SLC19A1 CC genotype had a longer OS
compared with the other two groups (12.2 vs. 8.9 and 7.3 months,
respectively; P=0.022; Fig. 2C).
The PFS and OS summaries based on SNP analysis are shown in
Table IV.
 | Table IVPFS and OS according to SNPs. |
Table IV
PFS and OS according to SNPs.
| Gene genotype | PFS (months)
| OS (months)
|
|---|
| Median | 95% CI | P-value | Median | 95% CI | P-value |
|---|
| TS | | | 0.036 | | | 0.638 |
| 2R/2R, 2R/3C,
3C/3C | 6.8 | 4.3–9.3 | | 10.3 | 6.5–12.7 | |
| 2R/3G, 3C/3G,
3G/3G | 3.8 | 1.0–6.6 | | 10.1 | 7.8–12.8 | |
| MTHFR | | | 0.992 | | | 0.739 |
| CC | 5.6 | 3.7–7.5 | | 10.3 | 7.6–13.0 | |
| CT | 3.8 | 1.6–6.0 | | 10.6 | 4.6–16.6 | |
| TT | 5.8 | 0.0–12.5 | | 8.1 | 4.5–11.7 | |
| SLC19A1 | | | 0.137 | | | 0.022 |
| CC | 4.9 | 2.0–7.8 | | 12.2 | 8.6–15.8 | |
| CT | 5.8 | 2.0–9.6 | | 8.9 | 4.5–13.3 | |
| TT | 5.5 | 2.4–8.6 | | 7.3 | 0.9–13.7 | |
Discussion
The present study was conducted to investigate the
association of polymorphisms in pemetrexed-related genes with the
clinical outcome in advanced NSCLC. The results suggest that the
polymorphisms in the TS 5′-untranslated region (UTR) may
result in differences in the PFS of NSCLC patients treated with
pemetrexed-based chemotherapy. The response rate was higher in
patients with the TS 2R/2R, 2R/3C or 3C/3C genotypes.
Patients with the SLC19A1 exon 6 (2522) CC genotype had
improved OS when treated with pemetrexed compared with the patients
without the genotype. However, a large-scale randomized prospective
clinical trial is required to confirm these findings.
The TS gene is located on chromosome 18p11.32
and the enzyme encoded by it is essential for cell proliferation as
it catalyzes the methylation of deoxyuridine-5′-monophosphate
(dUMP) to form deoxythymidine-5′-monophophate (dTMP). It is known
that TS is a potential target for fluoropyrimidine and other
drugs, including capecitabine, raltitrexed and pemetrexed. The
promoter enhancer region of the TS gene is prone to
polymorphisms with double or triple repeats of 28-bp tandem repeats
in the 5′-UTR. Mandola et al reported that the 3R alleles
have a G>C SNP at the 12th position of the second repeat
(20). Based on this SNP and the
TS genotype, the 2R/3G, 3C/3G and 3G/3G genotypes were
considered to be high expression types and the 2R/2R, 2R/3C and
3C/3C genotype were considered to be low expression types. However,
no consensus was reached on the correlation between the TS
polymorphisms and efficacy of 5-FU. Several studies have noted that
a C SNP was associated with longer PFS in colorectal cancer
patients treated with 5-FU (21,22).
However, several other studies did not find any significant
difference between TS SNPs and the clinical outcome of
patients treated with 5-FU (23–25).
Few studies have investigated the association
between TS genotypes and the efficacy of pemetrexed. Smit
et al showed that there was no correlation between the high
and low TS expression genotypes and the clinical outcome of
advanced NSCLC patients treated with pemetrexed (16,17).
In the present study, the PFS was significantly longer in patients
with the 2R/2R, 2R/3C or 3C/3C genotypes compared with patients
with the 2R/3G, 3C/3G or 3G/3G genotypes (P=0.036). However, no
significant differences in OS were observed between the two groups
(P=0.638). Further studies in larger populations are required to
confirm these results, however, possible explanations include
differences in the schedules of pemetrexed-based chemotherapy,
types of lung cancer and ethnic populations.
MTHFR is a central regulatory enzyme involved
in folate metabolism and the cellular concentrations of
5,10-methylenetetrahydrofolate are regulated by the activity of
MTHFR(26). Moreover,
optimal TS inhibition occurs when the cellular
concentrations of 5,10-methylenetetrahydrofolate are elevated
(27). The most common gene
polymorphism linked with enzyme activity is MTHFR677 C/T
(28). At present, the association
between MTHFR SNPs and the efficacy of 5-FU is inconclusive
and few studies have investigated the association of SNPs in this
gene with the efficacy of pemetrexed. Jakobsen et al
reported that the RR was significantly higher in patients with the
MTHFR TT genotype compared with two other genotype groups in
metastatic colorectal cancer patients treated with 5-FU (29). However, several large-scale clinical
trials did not identify a clear association (30–32).
Moreover, a large meta-analysis indicated that MTHFR 677C/T
is not a reliable predictor of the efficacy of FU-based therapy
(33). Smit et al reported
that patients with an MTHFR TT homozygous mutation had
increased PFS compared with wild-type or heterozygous NSCLC
patients treated with pemetrexed [7.9 months, (95% CI, 3.9–16
months) vs. 2.9 months, (95% CI, 2.8–3.4 months); P=0.03] in
(16). However, Argiris et
al reported contrasting results (17). In the present study, no correlations
were observed between the MTHFR genotypes and efficacy of
pemetrexed in advanced adenocarcinoma lung cancer. It is possible
that the present results were due to the small sample size of the
study or due to only one SNP in MTHFR being investigated.
This may have prevented the identification of possible
pharmacogenetic associations, since drugs may exert their
anticancer effects through multistep, multigenic cascades (30).
Pemetrexed is transported into cells by the RFC
protein, which is encoded by the SLC19A1 gene. In previous
studies, the SLC19A1 exon 6 (2522) CC genotype was observed
to be associated with longer PFS and three-month progression-free
status (progression-free at three months) in NSCLC patients treated
with a combination of pemetrexed and bevacizumab chemotherapies
(34). Adjei et al also
demonstrated that a polymorphism in SLC19A1 may predict
survival differences in pemetrexed-treated NSCLC patients (18). In the present study, patients with
the SLC19A1 CC genotype had a longer median OS than patients
with the other two genotypes (12.2 vs. 8.9 and 7.3 months,
respectively; log-rank χ2 = 2.957; P=0.022). The present
results are similar to those of the two previously described
trials.
In summary, polymorphisms in the 5′-UTR of the
TS gene and exon 6 (2522C/T) of the SLC19A1 gene
appear to be potential prognostic factors for NSCLC patients
treated with pemetrexed. Notably, germline polymorphisms have been
reported to correlate with response and/or toxicity. The ultimate
aim of this research is to aid the identification of the patients
who are most likely to benefit from pemetrexed-based chemotherapy.
Therefore, SNPs may be a useful tool for creating optimal
individualized treatment regimens.
Acknowledgements
This study was funded by the
Lianyungang Science and Technology Project (SH0805).
References
|
1
|
Jemal A, Siegel R, Ward E, Hao Y, Xu J,
Murray T and Thun MJ: Cancer statistics, 2008. CA Cancer J Clin.
58:71–96. 2008. View Article : Google Scholar
|
|
2
|
Ries LAG, Melbert D, Krapcho M, Stinchcomb
DG, Howlader N, Horner MJ, Mariotto A, Miller BA, Feuer EJ,
Altekruse SF, Lewis DR, Clegg L, Eisner MP, Reichman M and Edwards
BK: SEER Cancer Statistics Review, 1975–2005. National Cancer
Institute; Bethesda, MD: 2008
|
|
3
|
Ramalingam S and Belani C: Systemic
chemotherapy for advanced non-small cell lung cancer: recent
advances and future directions. Oncologist. 13(Suppl 1): 5–13.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hanna N, Shepherd FA, Fossella FV, Pereira
JR, De Marinis F, von Pawel J, Gatzemeier U, Tsao TC, Pless M,
Muller T, Lim HL, Desch C, Szondy K, Gervais R, Shaharyar, Manegold
C, Paul S, Paoletti P, Einhorn L and Bunn PA Jr: Randomized phase
III trial of pemetrexed versus docetaxel in patients with
non-small-cell lung cancer previously treated with chemotherapy. J
Clin Oncol. 22:1589–1597. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Scagliotti GV, Parikh P, von Pawel J,
Biesma B, Vansteenkiste J, Manegold C, Serwatowski P, Gatzemeier U,
Digumarti R, Zukin M, Lee JS, Mellemgaard A, Park K, Patil S,
Rolski J, Goksel T, de Marinis F, Simms L, Sugarman KP and Gandara
D: Phase III study comparing cisplatin plus gemcitabine with
cisplatin plus pemetrexed in chemotherapy-naive patients with
advanced-stage non-small-cell lung cancer. J Clin Oncol.
26:3543–3551. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ciuleanu T, Brodowicz T, Zielinski C, Kim
JH, Krzakowski M, Laack E, Wu YL, Bover I, Begbie S, Tzekova V,
Cucevic B, Pereira JR, Yang SH, Madhavan J, Sugarman KP, Peterson
P, John WJ, Krejcy K and Belani CP: Maintenance pemetrexed plus
best supportive care versus placebo plus best supportive care for
non-small-cell lung cancer: a randomised, double-blind, phase 3
study. Lancet. 374:1432–1440. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Chen JS, Chao Y, Bang YJ, Roca E, Chung
HC, Palazzo F, Kim YH, Myrand SP, Mullaney BP, Shen LJ and Linn C:
A phase I/II and pharmacogenomic study of pemetrexed and cisplatin
in patients with unresectable, advanced gastric carcinoma.
Anticancer Drugs. 21:777–784. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Chen CY, Chang YL, Shih JY, Lin JW, Chen
KY, Yang CH, Yu CJ and Yang PC: Thymidylate synthase and
dihydrofolate reductase expression in non-small cell lung
carcinoma: The association with treatment efficacy of pemetrexed.
Lung Cancer. 74:132–138. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lorusso D, Ferrandina G, Pignata S,
Ludovisi M, Viganò R, Scalone S, Scollo P, Breda E, Pietragalla A
and Scambia G: Evaluation of pemetrexed (Alimta, LY231514) as
second-line chemotherapy in persistent or recurrent carcinoma of
the cervix: the CERVIX 1 study of the MITO (Multicentre Italian
Trials in Ovarian Cancer and Gynecologic Malignancies) Group. Ann
Oncol. 21:61–66. 2010. View Article : Google Scholar
|
|
10
|
Mazzer M, Zanon E, Foltran L, De Pauli F,
Cardellino G, Iaiza E, Ermacora P, Aprile G and Fasola G:
Second-line pemetrexed-oxaliplatin combination for advanced
pancreatic adenocarcinoma. J Clin Oncol. 27(Suppl): e155972009.
|
|
11
|
Atkins JN, Jacobs SA, Wieand HS, Smith RE,
John WJ, Colangelo LH, Vogel VG, Kuebler JP, Cescon TP, Miller BJ,
Geyer CE Jr and Wolmark N: Pemetrexed/oxaliplatin for first-line
treatment of patients with advanced colorectal cancer: A phase II
trial of the National Surgical Adjuvant Breast and Bowel Project
Foundation Research Program. Clin Colorectal Cancer. 5:181–187.
2005. View Article : Google Scholar
|
|
12
|
Pippen J, Elias AD, Neubauer M, Stokoe C,
Vaughn LG, Wang Y, Orlando M, Shonukan O, Muscato J, O’Shaughnessy
JA and Gralow J: A phase II trial of pemetrexed and gemcitabine in
patients with metastatic breast cancer who have received prior
taxane therapy. Clin Breast Cancer. 10:148–153. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Celio L, Sternberg CN, Labianca R, La
Torre I, Amoroso V, Barone C, et al: Pemetrexed in combination with
oxaliplatin as a first-line therapy for advanced gastric cancer: a
multi-institutional phase II study. Ann Oncol. 20:1062–1067. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Martinez-Balibrea E, Manzano JL,
Martinez-Cardus A, Moran T, Cirauqui B, Catot S, Taron M and Abad
A: Combined analysis of genetic polymorphisms in thymidylate
synthase, uridine diphosphate glucoronosyltransferase and X-ray
cross complementing factor 1 genes as a prognostic factor in
advanced colorectal cancer patients treated with 5-fluorouracil
plus oxaliplatin or irinotecan. Oncol Rep. 17:637–645. 2007.
|
|
15
|
Urano W, Taniguchi A, Yamanaka H, Tanaka
E, Nakajima H, Matsuda Y, Akama H, Kitamura Y and Kamatani N:
Polymorphisms in the methylenetetrahydrofolate reductase gene were
associated with both the efficacy and the toxicity of methotrexate
used for the treatment of rheumatoid arthritis, as evidenced by
single locus and haplotype analyses. Pharmacogenetics. 12:183–190.
2002. View Article : Google Scholar
|
|
16
|
Smit EF, Burgers SA, Biesma B, Smit HJ,
Eppinga P, Dingemans AM, Joerger M, Schellens JH, Vincent A, van
Zandwijk N and Groen HJ: Randomized phase II and pharmacogenetic
study of pemetrexed compared with pemetrexed plus carboplatin in
pretreated patients with advanced non-small cell lung cancer. J
Clin Oncol. 27:2038–2045. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Argiris A, Karamouzis MV, Gooding WE,
Branstetter BF, Zhong S, Raez LE, Savvides P and Romkes M: Phase II
trial of pemetrexed and bevacizumab in patients with recurrent or
meta-static head and neck cancer. J Clin Oncol. 29:1140–1145. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Adjei AA, Salavaggione OE, Mandrekar SJ,
Dy GK, Ziegler KL, Endo C, Molina JR, Schild SE and Adjei AA:
Correlation between polymorphisms of the reduced folate carrier
gene (SLC19A1) and survival after pemetrexed-based therapy
in non-small cell lung cancer. J Thorac Oncol. 5:1346–1353.
2010.PubMed/NCBI
|
|
19
|
Therasse P, Arbuck SG, Eisenhauer EA,
Wanders J, Kaplan RS, Rubinstein L, Verweij J, Van Glabbeke M, van
Oosterom AT, Christian M and Gwyther SG: New guidelines to evaluate
the response to treatment in solid tumors: European Organization
for Research and Treatment of Cancer, National Cancer Institute of
the United States, National Cancer Institute of Canada. J Natl
Cancer Inst. 92:205–216. 2000. View Article : Google Scholar
|
|
20
|
Mandola MV, Stoehlmacher J, Muller-Weeks
S, Cesarone G, Yu MC and Lenz HJ: 2898–2904. 2003.
|
|
21
|
Kawakami K and Watanabe G: Identification
and functional analysis of single nucleotide polymorphism in the
tandem repeat sequence of thymidylate synthase gene. Cancer Res.
63:6004–6007. 2003.PubMed/NCBI
|
|
22
|
Fernández - Contreras ME, Sánchez -
Prudencio S, Sánchez-Hernández JJ, García de Paredes ML, Gisbert
JP, Roda-Navarro P and Gamallo C: Thymidylate synthase expression
pattern, expression level and single nucleotide polymorphism are
predictors for disease-free survival in patients of colorectal
cancer treated with 5-fluorouracil. Int J Oncol. 28:1303–1310.
2006.
|
|
23
|
Fariña-Sarasqueta A, Gosens MJ, Moerland
E, et al: TS gene polymorphisms are not good markers of
response to 5-FU therapy in stage III colon cancer patients. Cell
Oncol (Dordr). 34:327–335. 2011.
|
|
24
|
Vignoli M, Nobili S, Napoli C, et al:
Thymidylate synthase expression and genotype have no major impact
on the clinical outcome of colorectal cancer patients treated with
5-fluorouracil. Pharmacol Res. 64:242–248. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ruzzo A, Graziano F, Loupakis F, Rulli E,
Canestrari E, Santini D, et al: Pharmacogenetic profiling in
patients with advanced colorectal cancer treated with first-line
FOLFOX-4 chemotherapy. J Clin Oncol. 25:1247–1254. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Marcuello E, Altés A, Menoyo A, Rio ED and
Baiget M: Methylenetetrahydrofolate reductase gene polymorphisms:
genomic predictors of clinical response to fluoropyrimidine-based
chemotherapy? Cancer Chemother Pharmacol. 57:835–840. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Longley DB, Harkin DP and Johnston PG:
5-fluorouracil: mechanisms of action and clinical strategies. Nat
Rev Cancer. 3:330–338. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
28
|
Frosst P, Blom HJ, Milos R, Goyette P,
Sheppard CA, Matthews RG, Boers GJH, den Heijer M, Kluijtmans LA,
van den Heuvel LP, et al: A candidate genetic risk factor for
vascular disease: a common mutation in methylenetetrahydrofolate
reductase. Nat Genet. 10:111–113. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Jakobsen A, Nielsen JN, Gyldenkerne N and
Lindeberg J: Thymidylate synthase and methylenetetrahydrofolate
reductase gene polymorphism in normal tissue as predictors of
fluorouracil sensitivity. J Clin Oncol. 23:1365–1369. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Ruzzo A, Graziano F, Kawakami K, Watanabe
G, Santini D, Catalano V, Bisonni R, Canestrari E, Ficarelli R,
Menichetti ET, Mari D, Testa E, Silva R, Vincenzi B, Giordani P,
Cascinu S, Giustini L, Tonini G and Magnani M: Pharmacogenetic
profiling and clinical outcome of patients with advanced gastric
cancer treated with palliative chemotherapy. J Clin Oncol.
24:1883–1891. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Boige V, Mendiboure J, Pignon JP, Loriot
MA, Castaing M, Barrois M, Malka D, Trégouët DA, Bouché O, Le Corre
D, Miran I, Mulot C, Ducreux M, Beaune P and Laurent-Puig P:
Pharmacogenetic assessment of toxicity and outcome in patients with
metastatic colorectal cancer treated with LV5FU2, FOLFOX, and
FOLFIRI: FFCD 2000-05. J Clin Oncol. 28:2556–2564. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Goekkurt E, Al-Batran SE, Hartmann JT,
Mogck U, Schuch G, Kramer M, Jaeger E, Bokemeyer C, Ehninger G and
Stoehlmacher J: Pharmacogenetic analyses of a phase III trial in
metastatic gastroesophageal adenocarcinoma with fluorouracil and
leucovorin plus either oxaliplatin or cisplatin: a study of the
arbeitsgemeinschaft internistische onkologie. J Clin Oncol.
27:2863–2873. 2009. View Article : Google Scholar
|
|
33
|
Zintzaras E, Ziogas DC, Kitsios GD,
Papathanasiou AA, Lau J and Raman G: MTHFR gene
polymorphisms and response to chemotherapy in colorectal cancer: a
meta analysis. Pharmacogenomics. 10:1285–1294. 2009. View Article : Google Scholar
|
|
34
|
Adjei AA, Mandrekar SJ, Dy GK, Molina JR,
Adjei AA, Gandara DR, Ziegler KL, Stella PJ, Rowland KM Jr, Schild
SE and Zinner RG: A phase II trial of pemetrexed plus bevacizumab
for second-line therapy of patients with advanced non-small cell
lung cancer (NSCLC): an NCCTG and SWOG Study N0426. J Clin Oncol.
28:614–619. 2010. View Article : Google Scholar : PubMed/NCBI
|