Introduction
Radiotherapy forms a part of the front-line
treatment regimen for nasopharyngeal carcinoma (NPC) due to the
high sensitivity of NPC to radiation treatment (RT).
Intensity-modulated radiation therapy (IMRT) has recently prevailed
in clinical radiotherapy due to its lower radiation doses compared
with other external radiation techniques. The mechanisms of
radiation-induced effects in cancer mainly involve double-strand
breaks (DSBs) which are important in maintaining the stability of
genes. Distant metastases are common in NPC and are the major cause
of treatment failure (1). Methods
to predict distant metastases of NPC have yet to be developed.
Therefore, the identification of prognostic factors that are useful
for the prediction of distant metastases, particularly biological
parameters, may allow the development of individualized strategies
and, thus, improved treatment results.
DSBs are believed to be one of the most lethal forms
of damage induced by DNA damaging agents (2). DNA DSBs are repaired by 2 distinct and
complementary mechanisms, homologous recombination (HR) and
non-homologous end-joining (NHEJ) (3). In the NHEJ pathway, one of the first
enzymes to be attracted to DSBs is the Ku70/80 heterodimer. Upon
binding to DNA, the DNA-Ku70/80 scaffold recruits a large 460-kDa
serine/threonine kinase called the DNA-dependent protein kinase
catalytic subunit (DNA-PKcs) (4,5).
Followed by the recruitment of DNA-PKcs and the associated
end-joining proteins, including Artemis, XRCC4 and DNA ligase IV
(6), DNA-PK activity is stimulated
via phosphorylation which enables the NHEJ reaction to proceed
(7). Unrepaired DNA ends may
contribute to the development of chromosomal translocations by
acting as transposable elements (8). Although it remains to be demonstrated,
DNA-PKcs has a great potential as a tumor-suppressor gene (9). In addtion, DNA-PKcs is a member of the
phosphatidylinositol 3-kinase (PI3K) family. The PI3K/AKT pathway
is involved in many cellular processes, including proliferation,
survival, apoptosis, migration, invasion and cytoskeletal
rearrangements.
The breast cancer 1 (BRCA1) gene was the first
breast cancer susceptibility gene to be identified in 1990
(10). Classically, BRCA1 has been
considered to be implicated as a modulator of response to DNA
damage induced by chemotherapy and radiation therapy through HR
(11,12). The N-terminal RING and C-terminal
domains confer ubiquitinligase activity and specific phosphoprotein
binding to BRCA1, respectively. Until recently, it was believed
that mutations in the BRCA1 C-terminal (BRCT) domain led to reduced
HR, resulting in genomic instability and, ultimately, the
development of cancer (13).
However, the mechanisms by which BRCA1 contributes to HR have thus
far been little studied. A single study claimed that BRCA1 promoted
single-stranded DNA (ssDNA) formation in response to ionizing
radiation, with BRCA1 subsequently accumulating at the resulting
ssDNA sites, possibly contributing to a later step in
homology-dependent repair (14).
Due to distant metastasis occuring mainly in the 2–3
years after treatment in NPC, as well as it being the major cause
of treatment failure, in the present study, we aimed to assess
whether the expression of DNA-PKcs and BRCA1 may be used as
prognostic markers of distant metastasis-free survival in patients
with NPC who underwent IMRT. To the best of our knowledge, no
previous studies have analyzed these two components together in
correlation with therapy outcome and survival.
Methods and materials
Patients
Between May 2007 and February 2012, 87 untreated
patients with NPC, who were due to receive IMRT at the People’s
Hospital of Guangxi Autonomous Region (Nanning, China) were
enrolled in this study. Patients with a history of other cancers or
with distant metastasis were excluded. Of these, 87 cases had
adequate source tissue available for immunohistochemical staining.
The patients’ clinical characteristics are described in Table I. Patients with American Joint
Committee on Cancer (AJCC) stage I–II were treated with IMRT alone
and those with stage III–IVB with concurrent chemoradiotherapy
(CRT). The tumors were staged according to the TNM classification
as presented in the AJCC Cancer Staging manual (6th edition)
(15). At 1 month after completion
of IMRT, the IMRT response was evaluated by clinical examination,
CT or MRI scan when required. After CRT treatment, if the patients
still exhibited residual tumor activity, neck lymph node dissection
surgery was necessary followed by adjuvant chemotherapy. The
patients were followed for a period between 5 and 61 months
(median, 26 months). The study was approved by the Appropriate
Committees for Human Rights in Research in our hospital, and
written informed consent was obtained from each patient.
 | Table IClinical findings of 87 patients with
nasopharyngeal carcinoma. |
Table I
Clinical findings of 87 patients with
nasopharyngeal carcinoma.
| Characteristics | Value | % |
|---|
| Gender | | |
| Male | 54 | 62 |
| Female | 33 | 38 |
| Age (years) | | |
| Median | 46 | |
| Range | 17–76 | |
| WHO pathology
classification | | |
| I
(keratinizing) | 0 | 0 |
| II
(nonkeratinizig) | 7 | 8 |
| III
(undifferentiatd) | 80 | 92 |
| AJCC group (6th
ed.) | | |
| Stage I | 0 | 0 |
| Stage II | 14 | 16 |
| Stage III | 35 | 40 |
| Stage IVA or
IVB | 38 | 44 |
| T stage | | |
| T1 | 8 | 9 |
| T2 | 27 | 31 |
| T3 | 20 | 23 |
| T4 | 32 | 37 |
| CRT | 73 | 83 |
| RT alone | 14 | 16 |
Immunohistochemical studies
Tumor biopsy specimens obtained from the 87 patients
with NPC before treatment were analyzed by immunohistochemical
methods. The expression of DNA-PKcs or BRCA1 was detected in
formalin-fixed and paraffin-embedded tissues only after using a
high-temperature antigen retrieval technique. Tissue sections (4
μm) on poly-L-lysine-coated slides were treated for antigen
retrieval by boiling in citrate buffer (pH 6.0) for 10–15 min. The
sections were then incubated overnight at 4°C with monoclonal
antibodies to DNA-PKcs or to BRCA1 (Beijing Biosynthesis
Biotechnology Co., Ltd., Beijing, China). The streptavidinbiotin
complex method with peroxidase conjugate was used for detection and
the peroxidase reaction was developed using diaminobenzidine as the
chromogen. The sections were counterstained with Mayer’s
hematoxylin solution and mounted in a nonaqueous mounting medium.
When ≥50% of the tumor cells in the biopsy specimen were
immunopositive for DNA-PKcs or for BRCA1, the patient was
classified in the DNA-PKcs(+) or BRCA1(+) groups, respectively.
When <50% of tumor cells were immunopositive, including loss of
expression, the patient was classified in the corresponding low
expression group. Positive controls were provided by Beijing
Biosynthesis Biotechnology Co., Ltd. Negative controls were
obtained after omission of the primary antibody.
Study design and statistical
analysis
The primary endpoint of the study was the rate of
distant metastasis-free survival. A drawback of the study was that
the follow-up time was relatively short which subsequently led to
further analysis with regard to the 5-year overall survival rate
being required. Survival analysis was performed using the
Kaplan-Meier method, and the curves were compared using the
log-rank test. Cox regression for multivariate analysis was
performed to identify the prognostic factors that influenced
actuarial survival. Correlations with protein expression were
assessed using the nonparametric Spearman correlation test.
Differences in the distribution of patient characteristics were
analyzed using the Chi-square test. P<0.05 was considered to
indicate a statistically significant result. All statistical
analyses were conducted using the SPSS 17.0 statistical software
program (SPSS, IBM, Chicago, IL, USA).
Results
Clinical outcome
Twenty-five (28.7%) and 60 (69%) of the 87 patients
achieved an initial complete response and partial response to
therapy, respectively. Only 2 (2.3%) of the 87 achieved stable
disease. Of the 87 cases, 2 (2.3%) occurred as locoregional
failures at the 8th and 23rd months after treatment, respectively.
Twenty-six (29.9%) patients had a distant metastasis and these
patients accrued for a period of 4 to 57 months (median, 26
months). Distant metastases occurred in the liver in 12 patients,
in the lung in 9 patients and in the bone in 5 patients.
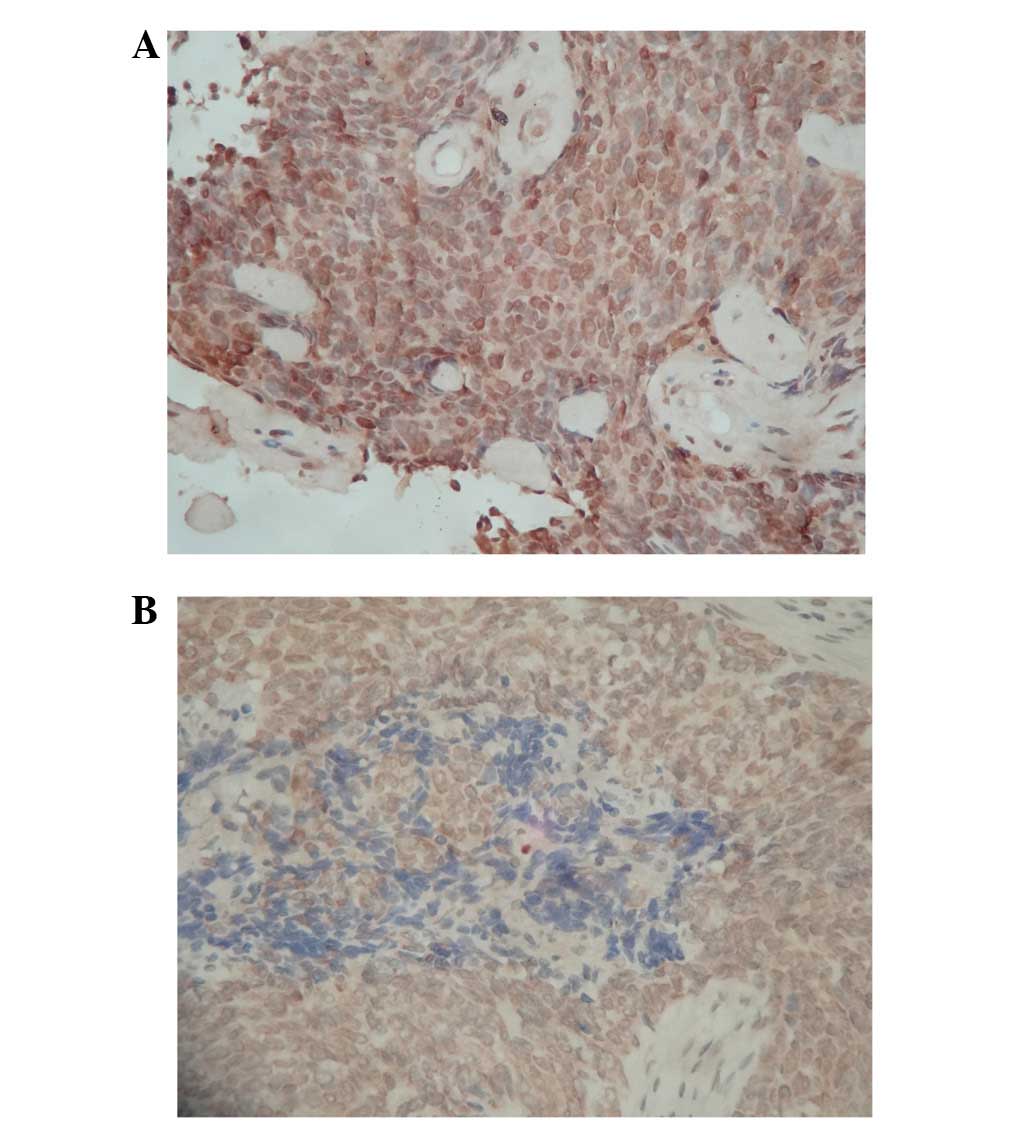
Immunostaining analysis
The patients were divided into groups according to
the expression level of DNA-PKcs and BRCA1 (high and low expression
groups for each candidate marker). High expression levels of
DNA-PKcs (Fig. 1A) and BRCA1
(Fig. 1B) were observed in
paraffin-embedded biopsy specimens from 52 (59.8%) and 37 (42.5%),
respectively, of 87 patients with non-disseminated NPC (Table II). There was no correlation between
the expression level of DNA-PKcs or BRCA1 with age, gender,
pathological subtype, T stage, AJCC stage or treatment.
 | Table IIImmunohistochemical staining results
for Ku70 and DNA-PKcs in nasopharyngeal carcinoma. |
Table II
Immunohistochemical staining results
for Ku70 and DNA-PKcs in nasopharyngeal carcinoma.
| DNA-PKcs(−) | DNA-PKcs(+) | Total |
|---|
| BRCA(−) | 28 | 22 | 50 |
| BRCA(+) | 7 | 30 | 37 |
| Total | 35 | 52 | 87 |
Correlation between clinical outcome and
expression of DNA-PKcs and BRCA1
Due to the improvement of radiation therapy
technology, locoregional failure occurred in 2 (2.3%) of the 87
patients, which was too low to compare the differ ences between the
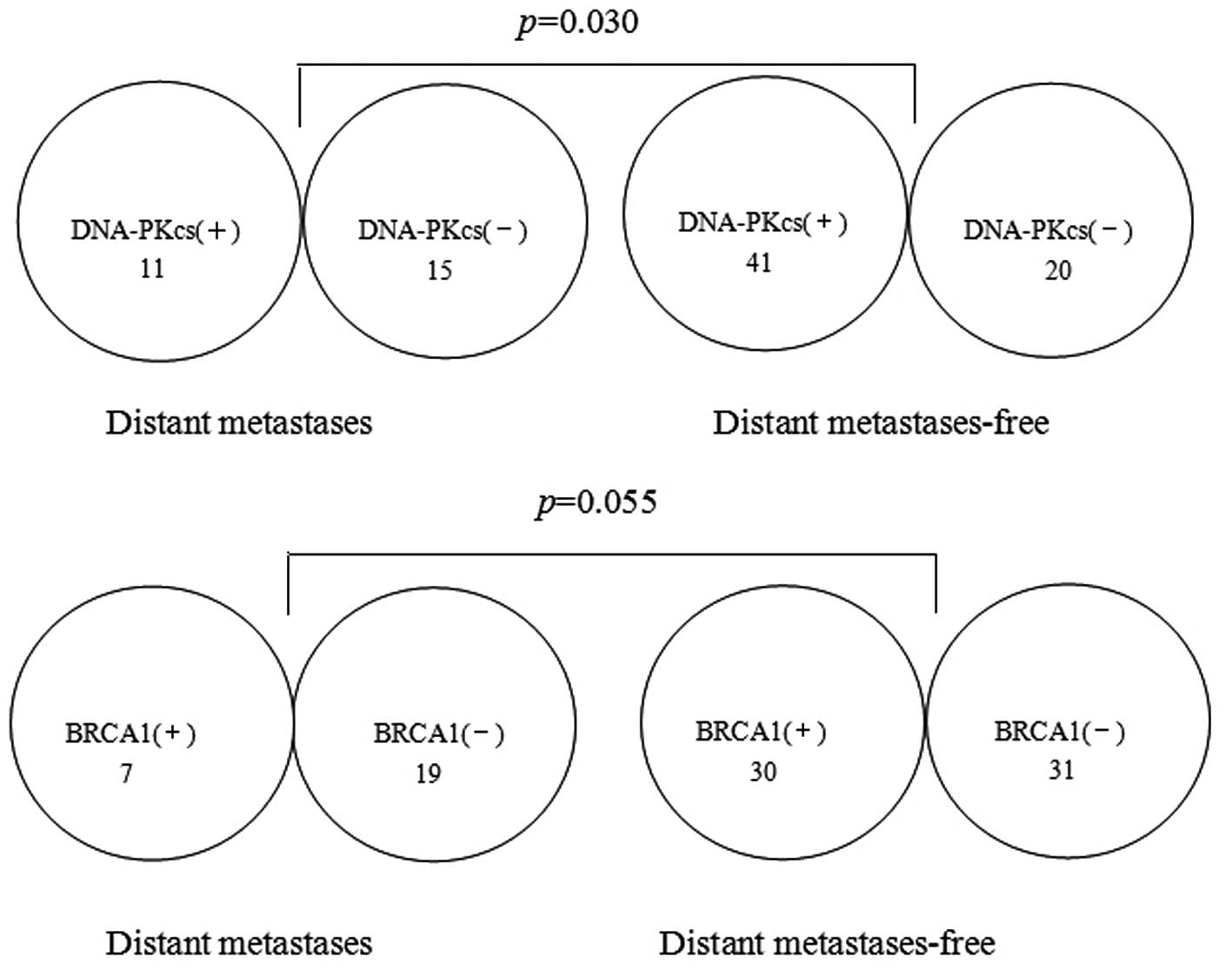
DNA-PKcs and BRCA1 groups. There was a significant positive
correlation between the expression level of DNA-PKcs and BRCA1
(r=0.374; P=0.001; Table II).
Furthermore, a significant correlation was identified between the
expression level of DNA-PKcs and distant metastasis-free rate
(P=0.030; Fig. 2). Distant
metastasis occurred in 15 (42.9%) of the 35 patients in the
DNA-PKcs(−) group and in 11 (21.2%) of 52 patients in the
DNA-PKcs(+) group. However, there was no significant correlation
between the expression level of BRCA1 and distant metastasis-free
rate. Distant metas tasis occurred in 19 (38.0%) of the 50 patients
in the BRCA1(−) group and in 7 (18.9%) of the 37 patients in the
BRCA1(+) group (P=0.055; Fig. 2).
There was a weak correlation between the expression level of BRCA1
and distant metastasis rate, although this correlation was not
statistically significant. If follow-up time was elongated and the
number of patients were increased, the distant metastasis-free
survival difference of the BRCA1 groups would be statistical
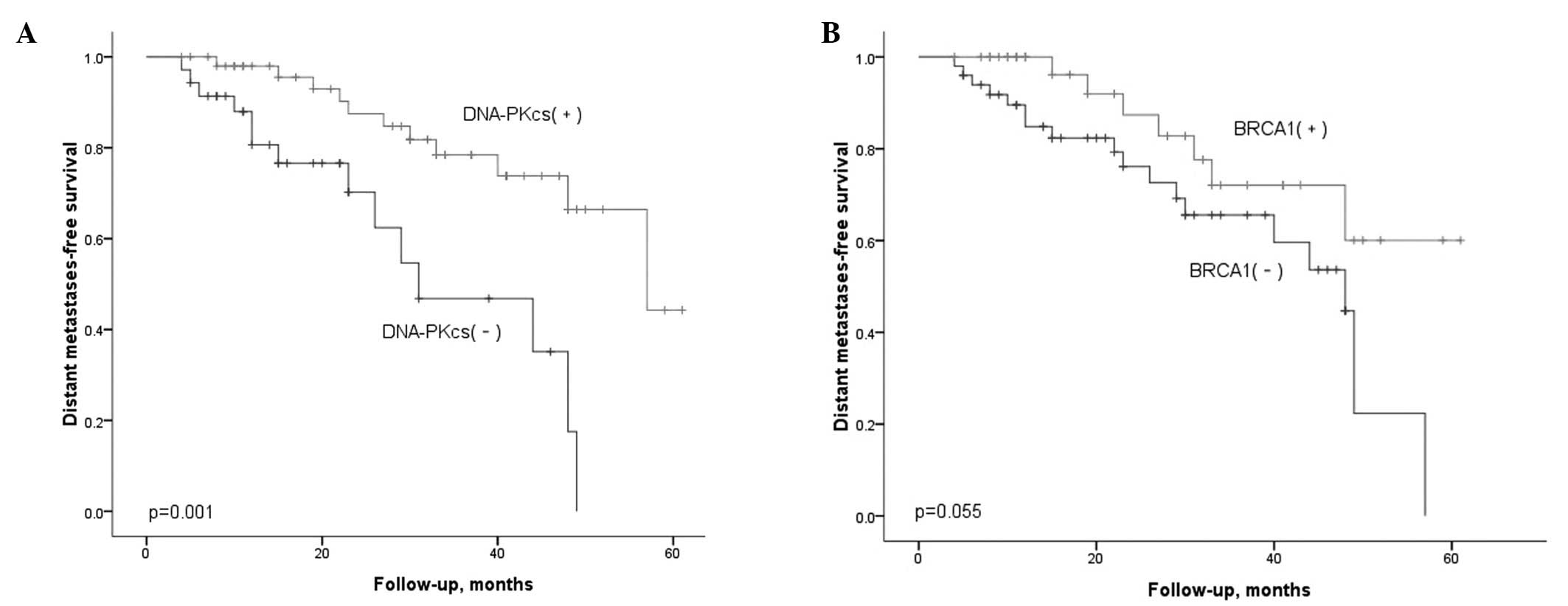
significance. Figs. 3 and 4 illustrate the Kaplan-Meier estimated
survival curves for distant metastasis-free survival in patients
classified according to the levels of DNA-PKcs and BRCA1 expression
in the tumor, respectively (P=0.001, P=0.055, P=0.001). We further
performed a Cox regression for multivariate analysis (Table III). The expression levels of
DNA-PKcs and AJCC staging demonstrated significant correlation with
the distant metastasis-free survival. No significant differences
were found between age, gender, ethnicity, pathological subtype or
treatment in correlation with distant metastasis-free survival.
However, no similar results were identified in the BRCA1
groups.
 | Table IIICox regression prognosis analysis for
distant metastasis-free survival in the DNA-PKcs groups. |
Table III
Cox regression prognosis analysis for
distant metastasis-free survival in the DNA-PKcs groups.
| Variate | B | SE | Wald | P-value | Exp(B) | 95.0% CI for
Exp(B) |
|---|
| Age | −0.003 | 0.023 | 0.014 | 0.906 | 0.997 | 0.953 to 1.044 |
| Pathological
subtype | 0.442 | 0.661 | 0.449 | 0.503 | 1.557 | 0.427 to 5.680 |
| AJCC stage | 1.466 | 0.527 | 7.730 | 0.005 | 4.331 | 1.541 to
12.173 |
| DNA-PKcs(+) | −1.593 | 0.461 | 11.966 | 0.001 | 0.203 | 0.082 to 0.501 |
Discussion
In our study, all of the patients received IMRT
treatment. This is mainly since a larger volume of normal tissue is
capable of being exposed to lower radiation doses with IMRT as
compared with other external radiation techniques (16). NPC may frequently be cured by IMRT
but metastasis and recurrence usually result in NPC treatment
failure. Thus, prognostic tests for IMRT outcome based on
biological markers are of particular interest to radiation
oncologists. Therefore, we have examined the immunoreactivity of 2
molecules involved in our previous study (17) and also in the repair of damaged DNA.
To the best of our knowledge, no studies have reported the
predictive value of combining DNA-PKcs and BRCA1 protein expression
in biopsy specimens for IMRT responsiveness in cases of NPC. Even
single detection of the expression levels of BRCA1 in NPC has yet
to be reported. We selected the immuno histochemical method using
paraffin-embedded tumor tissues. This method is practical and
efficient for clinical practice, since most cases of NPC are
diagnosed only on the basis of the examination of small
punch-biopsy specimens. If the immunoreactivity of these proteins
are able to be used as in vivo indicators, specific
treatment strategies may be designed for individual cases.
In the present study, numerous patients had stage
III–IVB disease which led to low complete response rates, however,
if the patients possessed residual tumor activity, neck lymph node
dissection surgery was necessary followed by adjuvant chemotherapy.
We first described the positive correlation between the expression
levels of DNA-PKcs and BRCA1. This may be due to interaction
between DNA-PKcs and BRCA1 which are capable of interacting to
maintain genetic stability (3).
Univariate analyses demonstrated that patients with a lower
proportion of DNA-PKcs(−) tended to have a higher rate of distant
metastasis than those with higher DNA-PKcs(+) in NPC (Fig. 3a). The improved distant
metastasis-free survival in the patient group with high expression
of DNA-PKcs was noteworthy, as we expected high-expressing tumors
with an improved ability to carry out DNA DSB repair to be
radioresistant. However, if the level of DNA-PKcs is low or even
absent and p53 is mutated, cells may behave differently. Friesland
et al(18) observed that
p53− (p53-negatove) and high DNA-PKcs levels were
detected among patients with improved outcome, and the lowest
survival rate was observed in patients with tumors that were
p53+ (p53-positive) and had low DNA-PKcs expression
which supported our hypothesis. We suggest that if both DNA-PKcs
and wild-type p53 are present, the DNA damage is detected by
DNA-PKcs and p53 activates an apoptotic response. If both the
DNA-PKcs and p53 functions are defective, DNA damage is neither
detected nor is p53-dependent apoptosis induced. If the level of
DNA-PKcs is low or even absent and p53 is mutated, cells may behave
differently. However, the exact mechanism of this phenomenon is
unknown.
The close correlation between DNA-PKcs expression
levels and metastasis may be due to it being a member of the PI3K
family, including ataxia telangiectasia-mutated (ATM) and ATM- and
Rad3-related (ATR) in response to DNA damage. The PI3K/AKT cell
signaling pathway is implicated in cell migration and invasion
(19). Our study also confirmed
earlier studies by Lee et al(20,21),
who reported that negative expression of DNA-PKcs in surgical
specimens was significantly associated with tumor progression and
poor patient survival rate in gastric cancer. In addition, DNA-PK
activity may be determined by the transcription of the DNA-PKcs
gene (22). Someya et al
substantiate that the lower DNA-PK activity is correlated with
chromosomal instability. Reduced DNA-PK activity profoundly affects
the ability to repair DNA DSBs, resulting in the perpetuation of
chromosome damage. Genetic instability correlated with low DNA-PK
activity may cause a higher frequency of distant metastasis
(23). Based on these observations,
DNA-PKcs expression was thought to be a good candidate for a
prognostic marker for NPC. To date, several studies support a
central role for DNA-PKcs in radiation sensitivity (24,25).
However, it is unclear whether radiation sensitivity was a
predictor of the clinical outcome of an individual (26). Due to the application of IMRT,
patients with radiation resistance were rarely observed in our
study.
In the present study, no significant difference
between the expression level of BRCA1 and clinical outcome was
observed. BRCA1 is involved in a multitude of cellular functions,
including HR and perhaps some forms of NHEJ. Until recently, it was
believed that mutations in the BRCA1 C-terminal domain resulted in
reduced HR, resulting in genomic instability and, ultimately, the
development of cancer (13). As
mentioned above, genetic instability may cause a higher frequency
of distant metastasis. In the present study, there was a weak
correlation between the expression level of BRCA1 and distant
metastasis rate, although this correlation was not statistically
significant. This may be due to different mutations in BRCA1
exerting different effects on the recognition and processing of DNA
damage. In addition, the BRCA1 protein is implicated in numerous
complex cellular processes that are correlated with chromosome
sensitivity in mutagens. There are no other descriptions of BRCA1
immunoreactivity in NPC, so further studies will be necessary to
compare with our results.
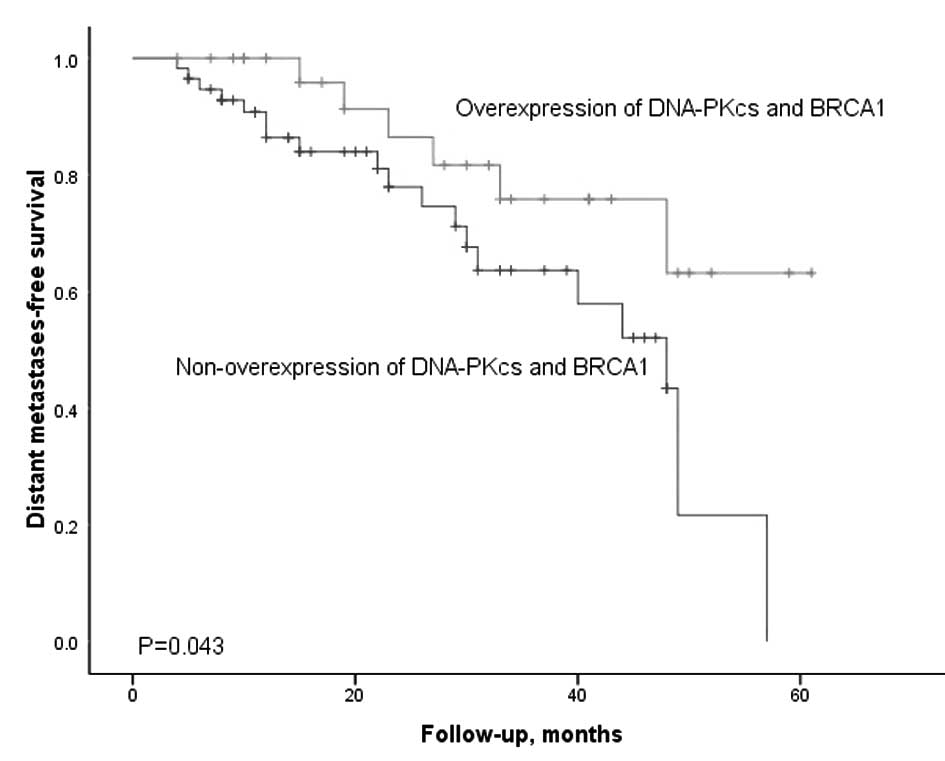
Univariate analyses demonstrated a statistically
significant difference between the overexpression and
non-overexpression groups (P=0.001, Fig. 4). Patients expressing a high level
of DNA-PKcs and BRCA1 had an improved distant metastasis-free
survival than those who were not. DNA-PKcs and BRCA1 are capable of
interacting to maintain genetic stability. They are two key players
in the NHEJ and HR pathway of DNA DSB repair, respectively. In our
study, we confirmed that there was a significant positive
correlation between the expression level of DNA-PKcs and BRCA1
(r=0.374, P=0.001). These factors supported our results that
co-overexpression patients have a better distant metastasis-free
survival despite their internal mechanism remaining unclear. To the
best of our knowledge, this is the first report of the expression
of BRCA1 and DNA-PKcs in paraffin-embedded NPC tumor tissues.
Finally, we identified that a high proportion of
DNA-PKcs(+) cells and the AJCC stage were significant, independent
predictors of patient distant metastasis-free after IMRT. In this
study, age, gender, ethnicity, treatment, complete response rate, T
stage and pathological type were not significantly associated with
the probability of distant metastasis-free survival. Generally,
AJCC stage is the most important prognostic factor for survival
(1). The results of this study also
indicated that the high proportion of DNA-PKcs-positive cells
provide a strong molecular marker of improved distant
metastasis-free survival in patients with NPC who are treated with
IMRT.
In summary, cancer patients with lower DNA-PKcs
tended to have the higher distant metastasis and the poorer
prognosis. DNA-PKcs may thus be used as a marker to possibly
predict the distant metastasis and poorer prognosis for patients
with NPC. BRCA1 may have a synergistic effect with DNA-PKcs in
predicting the distant metastasis and poorer prognosis. In the
present study, we described the immunoreactivity of DNA-PKcs and
BRCA1 in NPC for the first time. More studies will be necessary to
confirm our results.
Acknowledgements
The authors would like to thank
Professor Yu BB and the Center Laboratory of People’s Hospital of
Guangxi Autonomous Region (Nanning, China) for their technical
assistance. This study was supported by GuangXi Natural Science
Fund (No. 2010037) and the National Natural Science Fund (No.
81260348).
References
|
1
|
Lee AW, Poon YF, Foo W, et al:
Retrospective analysis of 5037 patients with nasopharyngeal
carcinoma treated during 1976–1985: overall survival and patterns
of failure. Int J Radiat Oncol Biol Phys. 23:261–270.
1992.PubMed/NCBI
|
|
2
|
Collis SJ, DeWeese TL, Jeggo PA and Parker
AR: The life and death of DNA-PK. Oncogene. 24:949–961. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Shrivastav M, De Haro LP and Nickoloff JA:
Regulation of DNA double-strand break repair pathway choice. Cell
Res. 18:134–147. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Weterings E and Chen DJ: The endless tale
of non-homologous end-joining. Cell Res. 18:114–124. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Peterson SR, Kurimasa A, Oshimura M, et
al: Loss of the catalytic subunit of the DNA-dependent protein
kinase in DNA double-strand-break-repair mutant mammalian cells.
Proc Natl Acad Sci USA. 92:3171–3174. 1995. View Article : Google Scholar
|
|
6
|
Hsu HL, Yannone SM and Chen DJ: Defining
interactions between DNA-PK and ligase IV/XRCC4. DNA Repair (Amst).
1:225–235. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Lee JW, Yannone SM, Chen DJ, et al:
Requirement for XRCC4 and DNA ligase IV in alignment-based gap
filling for nonhomologous DNA end joining in vitro. Cancer Res.
63:22–24. 2003.PubMed/NCBI
|
|
8
|
Obe G and Durante M: DNA double strand
breaks and chromosomal aberrations. Cytogenet Genome Res. 128:8–16.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kurimasa A, Ouyang H, Dong LJ, et al:
Catalytic subunit of DNA-dependent protein kinase: Impact on
lymphocyte development and tumorigenesis. Proc Natl Acad Sci USA.
96:1403–1408. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Hall JM, Lee MK, Newman B, et al: Linkage
of early-onset familial breast cancer to chromosome 17q21. Science.
250:1684–1689. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Quinn JE, Kennedy RD, Mullan PB, et al:
BRCA1 functions as a differential modulator of chemotherapy-induced
apoptosis. Cancer Res. 63:6221–6228. 2003.PubMed/NCBI
|
|
12
|
Kennedy RD, Quinn JE, Johnston PG and
Harkin DP: BRCA1: mechanisms of inactivaion and implications for
management of patients. Lancet. 360:1007–1014. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Laan R, Baarends WM, Wassenaar E, et al:
Expression and possible functions of DNA lesion bypass proteins in
spermatogenesis. Int J Androl. 28:1–15. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Harris JL and Khanna KK: BRCA1 A-complex
fine tunes repair functions of BRCA1. Aging (Albany NY). 3:461–463.
2011.PubMed/NCBI
|
|
15
|
Greene FL, Page DL, Fleming ID, et al:
AJCC Cancer Staging Manual. 6th edition. Springer-Verlag; New York,
NY: pp. 35–45. 2005
|
|
16
|
Hall EJ: Intensity-modulated radiation
therapy, protons, and the risk of second cancer. Int J Radiat Oncol
Biol Phys. 65:1–7. 2006. View Article : Google Scholar
|
|
17
|
Chen JX, Guo Y, Li Y, et al: Predicting
Radiation Sensitivity of Nasopharyngeal Carcinoma Using Genetic
Fingerprinting. Chinese Journal of Clinical Oncology. 34:1141–1145.
2007.
|
|
18
|
Friesland S, Kanter-Lewensohn L, Tell R,
et al: Expression of Ku86 confers favorable outcome of tonsillar
carcinoma treated with radiotherapy. Head Neck. 25:313–321. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ohta S, Lai EW, Pang AL, et al:
Downregulation of metastasis suppressor genes in malignant
pheochromocytoma. Int J Cancer. 114:139–143. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Lee HS, Yang HK, Kim WH and Choe G: Loss
of DNA-dependent protein kinase catalytic subunit (DNA-PKcs)
expression in gastric cancers. Cancer Res Treat. 37:98–102. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lee HS, Choe G, Park KU, et al: Altered
expression of DNA-dependent protein kinase catalytic subunit
(DNA-PKcs) during gastric carcinogenesis and its clinical
implications on gastric cancer. Int J Oncol. 31:859–866. 2007.
|
|
22
|
Someya M, Sakata K, Monobe M, et al: The
association of DNA-dependent protein kinase activity with
chromosomal instability and risk of cancer. Carcinogenesis.
27:117–122. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Someya M, Sakata KI, Matsumoto Y, et al:
The association of DNA-dependent protein kinase activity of
peripheral blood lymphocytes with prognosis of cancer. Br J Cancer.
104:1724–1729. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Azad A, Jackson S, Cullinane C, et al:
Inhibition of DNA-dependent protein kinase induces accelerated
senescence in irradiated human cancer cells. Mol Cancer Res.
9:1696–1707. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zhuang W, Li B, Long L, et al: Knockdown
of the DNA-dependent protein kinase catalytic subunit
radiosensitizes glioma-initiating cells by inducing autophagy.
Brain Res. 1371:7–15. 2011. View Article : Google Scholar
|
|
26
|
Taghian A, Ramsay J, Allaunis-Turner J, et
al: Intrinsic radiation sensitivity may not be the determinant of
the poor clinical outcome of glioblastoma multiforme. Int J Radiat
Oncol Biol Phys. 25:243–249. 1993. View Article : Google Scholar : PubMed/NCBI
|