Introduction
Endometrial carcinoma is the most common malignancy
of the female genital tract. Endometrial endometrioid
adenocarcinoma (EEC) accounts for ∼80% of endometrial tumors
(1). To date, the etiology of
endometrial carcinoma is not fully understood, although there is
evidence that endocrine and genetic factors contribute to its
initiation and progression (2,3).
The claudin family of proteins, the main
transmembrane proteins of tight junctions, has crucial roles in the
control of paracellular transport and maintenance of cell polarity
(4). The recently described
claudins have shown that claudins’ gene expression are frequently
altered in various cancers, including gynecological cancers.
Abnormal expression of claudin molecules, such as claudin-4, by
neoplastic cells is possibly an important determinant of local
invasion and dissemination and claudin represents a promising
target for cancer detection, diagnosis and therapy (5–7).
Recently, gene expression studies of primary uterine serous
papillary cancer (USPC) have demonstrated that claudin-4 is one of
the most highly upregulated genes in USPC when compared to normal
endometrial cells (8). To determine
whether claudin-4 expression has a crucial role in tumor
progression, the expression of claudin-4 in EEC was investigated.
To explore whether claudin-4 could be a potentially useful agent in
the treatment of endometrial cancer, human endometrial cancer
xenograft models were prepared and the change in claudin-4
expression in Ishikawa xenografts after treatment with cytotoxic
drugs was evaluated.
Materials and methods
Tissue samples
Cancerous endometrium was obtained from 62 females
with EEC. The tumors had been graded and staged following the
current recommendations of the International Federation of
Gynecology and Obstetrics (FIGO). The average age was 56.2 years.
Fifty of the 62 women had low-grade EEC (Grades 1 and 2) and 12
were high-grade (Grade 3). Fifty-two patients presented with
early-stage tumors (Stage IA to II) and 10 presented with
advanced-stage tumors (IIIA to IVB). Sixty control normal
endometrial tissues from females without EEC (mean age 53.1 years)
were obtained. Thirty-four were in the proliferative phase and 26
were in the secretory phase. All the formalin-fixed,
paraffin-embedded sections were obtained from the files of the
Department of Pathology of China-Japan Friendship Hospital and
Beijing Chao Yang Hospital of Capital Medical University. The study
was approved by the ethics committee of China-Japan Friendship
Hospital, Beijing, China. No initial hormonal therapy or
radiotherapy was performed prior to endometrium excision.
Hematoxylin-eosin (H&E) stained sections from each case were
reviewed and representative sections from each tumor were
selected.
Immunohistochemical analysis of claudin-4
expression in endometrial carcinoma
Immunohistochemical stains were performed on
5-μm-thick sections of formalin-fixed, paraffin-embedded
tissues. After antigen retrieval, the sections were incubated with
monoclonal mouse anti-claudin-4 (Zymed, San Francisco, CA, USA).
Antigen-bound primary antibody was detected using standard
avidin-biotin immunoperoxidase complex (DAKO Corp., Carpinteria,
CA, USA). Negative controls, in which the primary antibodies were
not added, were processed in parallel. In each case, two
independent observers recorded the distribution of staining,
intensity and localization. Cases were classified as follows
regarding the intensity of protein expression: −, no immunostaining
present; +, weak staining; ++, medium staining and +++, intense
staining.
Real-time PCR analysis of claudin-4
expression in endometrial carcinoma
RNA isolation was performed using TRIzol reagent
(Sangon, Shanghai, China) according to the manufacturer’s
instructions. Total RNA (5 μg) from each sample was reverse
transcribed using M-MLV reverse transcriptase (Promega Corp.,
Madison, WI, USA). The SYBR-Green I assay was used for detecting
real-time PCR products of claudin-4. The primers used were as
follows: claudin-4 (forward, 5′-GTGCCTTGCTCACCGAAAC; reverse,
5′-CCACCACTGCCCAAACCT) and glyceraldehyde phosphate dehydrogenase
(GAPDH) (forward, 5′-GAAGATGGTGATGGGATTTC; reverse, 5′-GAAGGT
GAAGGTCGGAGT). A four-step experimental protocol was used: i)
denaturation program (10 min at 95°C); ii) amplification and
quantification program repeated 40 times (10 sec at 95°C; 5 sec at
57°C for claudin-4 or 5 sec at 55°C for GAPDH; 10 sec at 72°C for
claudin-4 or 15 sec at 72°C for GAPDH with a single fluorescence
measurement); iii) melting curve program (65–95°C with a heating
rate of 0.1°C/sec and a continuous fluorescence measurement); iv)
cooling program down to 40°C. Specificity of the amplified PCR
product was assessed by performing melting curve analysis. The
relative expression is based on the expression ratio of claudin-4
versus GAPDH.
Cell culture
The human endometrial carcinoma cell line Ishikawa
(a high expresser of claudin-4; data not shown) was a generous gift
from Professor Li-Hui Wei (Peking University People’s Hospital,
Beijing, China) and was obtained from American Type Culture
Collection (Manassas, VA, USA). The Ishikawa cell line was
established from a well-differentiated human endometrial carcinoma.
Cells were cultured in RPMI-1640 medium supplemented with 10% fetal
bovine serum (FBS) plus penicillin and streptomycin. They were
subcultured when the density reached 80% using 0.25% trypsin in
Ca2+/Mg2+-free PBS. Cell viability was
determined by trypan blue exclusion. Cells were brought to a
density of 1×107 cells/ml for injection. Harvested cells
with >95% viability by trypan blue exclusion were considered
acceptable for injection.
In vivo antitumor effect of cisplatin and
paclitaxel against Ishikawa endometrial carcinoma cells
Ninety female BALB/c nu/nu mice (5 weeks old) were
obtained from the Zoology Institute of the Chinese Academy of
Sciences (Beijing, China) and housed in a pathogen-free
environment. All experiments were approved by the Institute’s
Animal Care and Use Committee. For subcutaneous xenografts,
1×107 viable Ishikawa cells were injected subcutaneously
into the right flank. After 7 days, when established tumors of ∼6
mm in diameter were detectable, mice were randomized in groups
(n=30) to receive different treatments. One group was treated
intraperitoneally with cisplatin (5 mg/kg/day on days 8, 15 and
22). Paclitaxel was used as reference compound (30 mg/kg/day on
days 8, 15 and 22). Control mice were injected with 0.9% saline
solution. All mice were sacrificed on the 30th day after tumor
implantation. The final weight for each animal was measured just
prior to sacrifice. The tumors were excised from the mice, weighed
for the calculation of mean tumor weight for every group and
prepared for subsequent claudin-4 immunohistochemical staining and
mRNA analysis. The tumor dimensions were measured with calipers and
the tumor volume was calculated using the formula: 0.52 × larger
diameter × (smaller diameter)2.
Immunohistochemical and real-time PCR
analysis of claudin-4 expression in tumor xenografts
Immunohistochemical and real-time PCR analysis of
claudin-4 expression in tumor xenografts was performed using the
method described above.
Statistical analysis
The statistical significance of the data was
evaluated using one-way ANOVA and Chi-square analysis. P<0.05
was considered to indicate a statistically significant result. All
statistical analysis was calculated using SPSS 11.0 (SPSS, Inc,
Chicago, IL, USA).
Results
Immunohistochemical expression of
claudin-4 in EEC
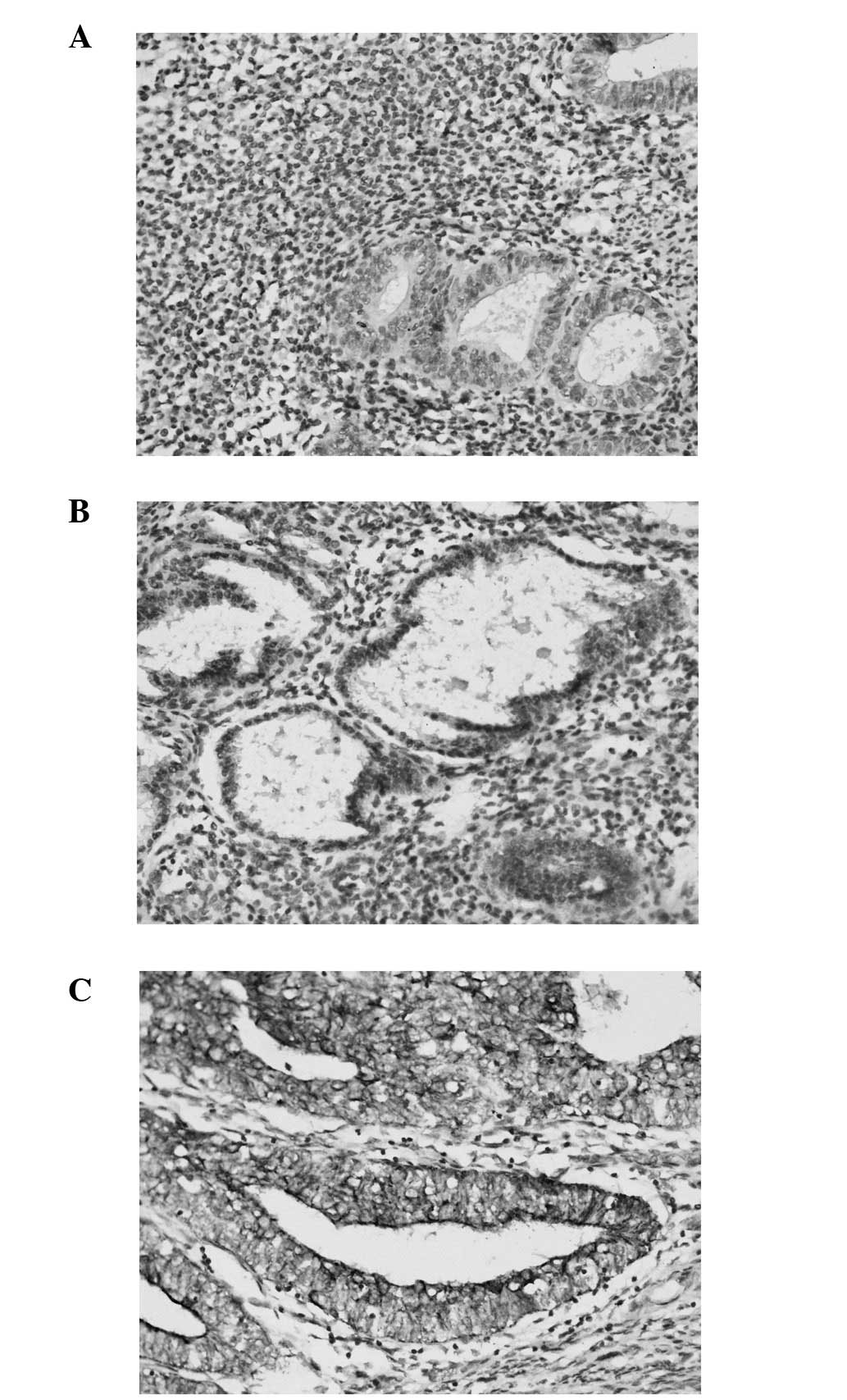
The immunohistochemical analysis of claudin-4 showed
a specific brownish immunostaining localized to the glandular
epithelial cell membrane. There was no signal detected in the
stromal cell. All EEC samples demonstrated some degree of claudin-4
expression. Glandular epithelial cells in EEC exhibited a
circumferential membranous pattern of staining for claudin-4. Among
the EEC samples, 21/62 (33.9%) showed medium staining for claudin-4
and 41/62 (66.1%) showed intense staining for claudin-4. Of the
normal endometrial tissue, 28/60 (46.7%) showed weak staining and
32/60 (53.3%) showed no staining for claudin-4. There was a
statistically significant difference in claudin-4 expression
between EEC and normal endometrial tissue. Claudin-4 immunostaining
was stronger and more diffuse in EEC than in normal cyclic
endometrium (Fig. 1).
Expression of claudin-4 mRNA
According to real-time PCR, the relative quantity of
claudin-4 was 169.7±11.8 in the EEC group and 17.9±3.2 in normal
endometrium. Claudin-4 was found to be highly upregulated in EEC
(P<0.01), consistent with the result obtained using
immunohistochemistry.
Claudin-4 expression in Ishikawa
xenografts after treatment with cytotoxic drugs
A total of 88 out of 90 animals survived treatment
with cisplatin. Statistically significant body weight change was
not found with paclitaxel administration and no significant change
in tumor volume was demonstrated in the paclitaxel group compared
with controls. A significant reduction in tumor growth and weight
loss occurred with cisplatin compared with the group treated with
paclitaxel (Table I).
 | Table IClaudin-4 expression in Ishikawa
xenografts after treatment with cytotoxic drugs. |
Table I
Claudin-4 expression in Ishikawa
xenografts after treatment with cytotoxic drugs.
| | | | Claudin-4 expression,
n (%)
|
|---|
| Treatment | n | Body weight (g) | Tumor volume
(cm3) | − | + | ++ | +++ |
|---|
| Control | 30 | 19.32±1.54 | 1.22±0.13 | 0 | 0 | 10 (33.3) | 20 (66.7) |
| Cisplatina | 28 | 0.71±2.23 | 0.79±0.27 | 0 | 6 (21.4) | 18 (64.3) | 4 (14.3) |
| Paclitaxel | 30 | 18.16±2.89 | 1.14±0.18 | 0 | 2 (6.7) | 10 (33.3) | 18 (60.0) |
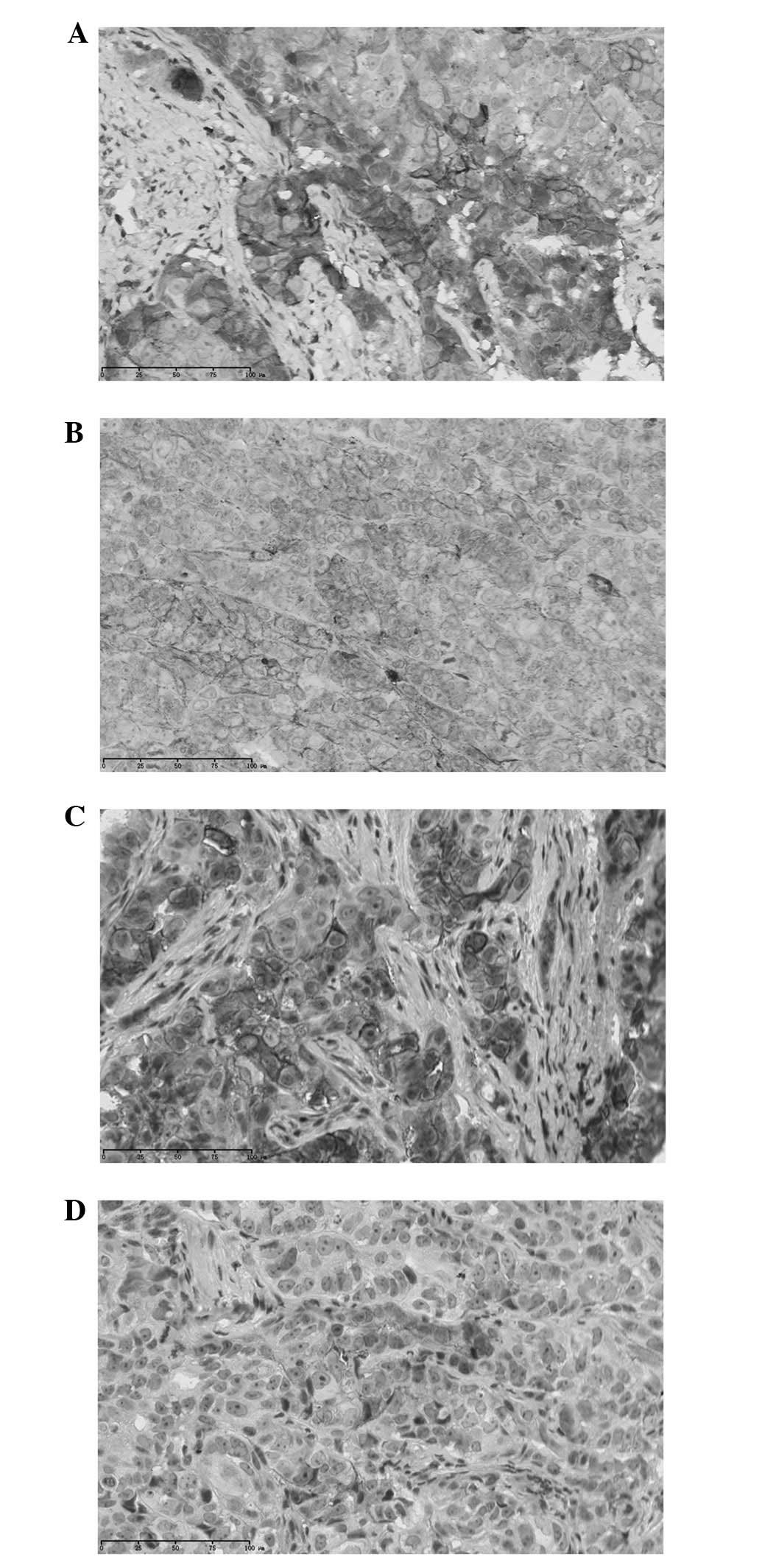
After treatment with cytotoxic drugs, claudin-4
expression in Ishikawa xenografts was detected using
immunohistochemical stain. Claudin-4 was 100% positive in the
control group, and generally had a membranous staining pattern. A
similar result was found in the paclitaxel group, 30 (100%) of 30
cases were positive for claudin-4, with 18 cases showing +++
staining, 10 showing ++ staining and 2 showing + staining. A
significant decrease in claudin-4 expression was observed in the
cisplatin group, with 4 cases showing +++ staining, 18 showing ++
staining and 6 showing + staining (Table I, Fig.
2).
To get highly sensitive measurement of claudin-4 at
the transcript level, a real-time PCR assay was developed. Before
treatment with cytotoxic drugs, the expression levels of claudin-4
in the cisplatin, paclitaxel and control group were 273.1±26.8,
284.7±30.1 and 279.8±28.6, respectively. There was no difference
among the three groups. After treatment with cytotoxic drugs, the
relative quantity of claudin-4 in the three groups was 153.4±34.7,
248.9±28.4 and 262.1±25.9, respectively. Claudin-4 mRNA in the
control group was slightly higher than that in paclitaxel group,
but there was no significant statistical difference. The cisplatin
group showed a significant decrease in mRNA level of claudin-4
compared with the paclitaxel and control group, consistent with the
result obtained using immunohistochemical analysis.
Discussion
Claudins are the major integral membrane proteins
forming the backbone of tight junctions. Increased claudin-4 levels
have been shown in prostate cancer (9), ovarian carcinoma (10) and in several other tumor cell lines
(11). On the basis of these
observations, claudin-4 may represent a useful biomarker for
detection and diagnosis of certain cancers (12). The exact role of claudin-4
overexpression and the functional importance of claudin-4 in the
development of cancer remain unclear.
Recently, Santin et al(13) used gene expression studies to
demonstrate that claudin-4 is among the highest upregulated genes
in USPC when compared with normal endometrial cells. In this study
the expression of claudin-4 was much higher in EEC than in normal
cyclic endometrium. Although EEC and USPC belong to two different
pathogenetic types of endometrial carcinoma, these results have led
to the suggestion that upregulated claudin-4 may be involved in
endometrial carcinogenesis.
It had been shown that cisplatin and paclitaxel had
an anti-tumor effect on human cancer xenografted nude mice
(14,15). To investigate the role of claudin-4
as a marker for the activity of anti-neoplastic agents the same
model was used. In the present study, immunohistochemical and PCR
analysis of tumor xenografts demonstrated that cisplatin treatment
is capable of producing a significant decrease in claudin-4
expression which correlated with the reduction in tumor volume. The
correlation between the expression of claudin-4 and the change in
tumor volume due to chemotherapy demonstrates that therapeutic
inhibition of claudin-4 may reduce claudin-expressing cells and it
further suggests a role of claudin-4 as a marker for the activity
of anti-neoplastic agents.
The antitumor activity of paclitaxel against solid
tumors, such as breast and ovarian cancer, has been well
established (16,17). No significant changes in tumor
volume and claudin-4 expression were shown in the paclitaxel group
in this study, however. The route of administration and dose level
may limit the effect of paclitaxel. Cisplatin seemed to be an
effective anti-neoplastic agent, exhibiting a significantly higher
cytotoxic effect than paclitaxel. Two mice died and a significant
weight loss occurred among the cisplatin-treated animals. The
development of innovative, effective therapy against endometrial
cancer remains a high priority.
Notably, claudin-4 is a receptor for clostridium
perfringens enterotoxin (CPE). Experiments have shown that CPE
has a cytotoxic effect towards cancer cells, provided these cells
express claudin-4 (18–20). Further studies are required to
demonstrate that CPE holds promise for the development of
alternative anticancer agents for endometrial carcinoma.
In conclusion, this study was undertaken to
understand the biological significance of altered claudin-4
expression in endometrial carcinoma. Other mechanisms relevant to
claudin-4 overexpression in endometrial cancer are still under
investigation. The present observations raise the possibility of
exploiting claudin-4 as a potential biomarker for endometrial
carcinoma and may provide an opportunity for therapeutic
intervention.
Acknowledgements
This study was supported by a program
project grant (No. 30901599) from the National Natural Science
Foundation of China (to Xiao-Yu Pan).
References
|
1
|
Di Cristofano A and Ellenson LH:
Endometrial carcinoma. Annu Rev Pathol. 2:57–85. 2007.
|
|
2
|
Macwhinnie N and Monaghan H: The use of
P53, PTEN, and CerbB-2 to differentiate uterine serous papillary
carcinoma from endometrioid endometrial carcinoma. Int J Gynecol
Cancer. 14:938–946. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Wu W, Slomovitz BM, Celestino J, Chung L,
Thornton A and Lu KH: Coordinate expression of Cdc25B and ER-alpha
is frequent in low-grade endometrioid endometrial carcinoma but
uncommon in high-grade endometrioid and nonendometrioid carcinomas.
Cancer Res. 63:6195–6199. 2003.PubMed/NCBI
|
|
4
|
Matter K and Balda MS: Functional analysis
of tight junctions. Methods. 30:228–234. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Swisshelm K, Macek R and Kubbies M: Role
of claudins in tumorigenesis. Adv Drug Deliv Rev. 57:919–928. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Morin PJ: Claudin proteins in human
cancer: promising new targets for diagnosis and therapy. Cancer
Res. 65:9603–9606. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yoshida H, Sumi T, Zhi X, Yasui T, Honda K
and Ishiko O: Claudin-4: a potential therapeutic target in
chemotherapy-resistant ovarian cancer. Anticancer Res.
31:1271–1277. 2011.PubMed/NCBI
|
|
8
|
Hayes MP and Ellenson LH: Molecular
alterations in uterine serous carcinoma. Gynecol Oncol.
116:286–289. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Long H, Crean CD, Lee WH, Cummings OW and
Gabig TG: Expression of Clostridium perfringens enterotoxin
receptors claudin-3 and claudin-4 in prostate cancer epithelium.
Cancer Res. 61:7878–7881. 2001.
|
|
10
|
Zhu Y, Brännström M, Janson PO and
Sundfeldt K: Differences in expression patterns of the tight
junction proteins, claudin 1, 3, 4 and 5, in human ovarian surface
epithelium as compared to epithelia in inclusion cysts and
epithelial ovarian tumours. Int J Cancer. 118:1884–1891. 2006.
View Article : Google Scholar
|
|
11
|
Nichols LS, Ashfaq R and Iacobuzio-Donahue
CA: Claudin 4 protein expression in primary and metastatic
pancreatic cancer: support for use as a therapeutic target. Am J
Clin Pathol. 121:226–230. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Shang X, Lin X, Alvarez E, Manorek G and
Howell SB: Tight junction proteins claudin-3 and claudin-4 control
tumor growth and metastases. Neoplasia. 14:974–985. 2012.PubMed/NCBI
|
|
13
|
Santin AD, Bellone S, Marizzoni M, et al:
Overexpression of claudin-3 and claudin-4 receptors in uterine
serous papillary carcinoma: novel targets for a type-specific
therapy using Clostridium perfringens enterotoxin (CPE).
Cancer. 109:1312–1322. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Villena-Heinsen C, Friedrich M, Ertan AK,
Farnhammer C and Schmidt W: Human ovarian cancer xenografts in nude
mice: chemotherapy trials with paclitaxel, cisplatin, vinorelbine
and titanocene dichloride. Anticancer Drugs. 9:557–563. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Armstrong DK, Bundy B, Wenzel L, et al:
Gynecologic Oncology Group. Intraperitoneal cisplatin and
paclitaxel in ovarian cancer. N Engl J Med. 354:34–43. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Liu Y, Chen L, He X, et al: Enhancement of
therapeutic effectiveness by combining liposomal honokiol with
cisplatin in ovarian carcinoma. Int J Gynecol Cancer. 18:652–659.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Rosanò L, Cianfrocca R, Spinella F, Di
Castro V, Natali PG and Bagnato A: Combination therapy of
zibotentan with cisplatinum and paclitaxel is an effective regimen
for epithelial ovarian cancer. Can J Physiol Pharmacol. 88:676–681.
2010.PubMed/NCBI
|
|
18
|
Kominsky SL, Vali M, Korz D, et al:
Clostridium perfringens enterotoxin elicits rapid and
specific cytolysis of breast carcinoma cells mediated through tight
junction proteins claudin 3 and 4. Am J Pathol. 164:1627–1633.
2004. View Article : Google Scholar
|
|
19
|
Litkouhi B, Kwong J, Lo CM, et al:
Claudin-4 overexpression in epithelial ovarian cancer is associated
with hypomethylation and is a potential target for modulation of
tight junction barrier function using a C-terminal fragment of
Clostridium perfringens enterotoxin. Neoplasia. 9:304–314.
2007. View Article : Google Scholar
|
|
20
|
Gao Z, Xu X, McClane B, et al: C terminus
of Clostridium perfringens enterotoxin downregulates CLDN4
and sensitizes ovarian cancer cells to Taxol and Carboplatin. Clin
Cancer Res. 17:1065–1074. 2011.
|