Introduction
The innate immune system serves as an important
first line defense against invading infections (1). Central mediators of this task are the
Toll-like receptors (TLRs), which function as pattern recognition
proteins that detect microbe- and host-derived molecular patterns
(2). To date, 13 mammalian TLRs
have been recognized, and each responds to a different ligand. For
example, TLRs -4 and -5 recognize bacterial lipopolysaccharide
(LPS) and flagellin, respectively, whereas the TLR9 subfamily
members are nucleic acid receptors. More specifically, TLRs -7, -8
and -13 are RNA receptors, while DNA that enters the cell is
recognized by TLR9 (2,3). The various receptors are expressed in
different parts of a cell; TLRs -1, -2 and -4 are expressed and
bind their ligands on the cell surface, while the TLR9 subfamily
resides in intracellular vesicles. Binding of the cognitive ligands
to the various TLRs activates transcription factors, one of the
most significant being nuclear factor-κB (NF-κB). Eventually, TLR
activation results in an immune response, and also in the
activation of the adaptive immune system (1,2).
It is well established that in addition to being
expressed in the immune system, TLR9 is widely expressed in various
cancer cell lines and in clinical cancer specimens, including
breast, brain, gastric, lung, esophageal, prostate and renal cancer
(4–11). Previous studies have demonstrated
that treatment of TLR9-expressing cancer cells with synthetic TLR9
ligands, which mimic the structure of bacterial DNA, stimulates
their invasion in vitro through matrix metalloproteinase-13
(MMP-13) activation (6,12,13).
In prostate cancer cells, native bacterial DNA had similar in
vitro effects (12). Data
concerning the regulation of TLR9 expression are starting to
accumulate, although thus far, not much is known. In breast cancer
cells, TLR9 expression is upregulated by sex steroids and
bicalutamide (14). Testosterone
has been shown to potentiate the TLR9 ligand-induced invasion of
breast cancer cells, without affecting NF-κB signaling in
vitro. However, expression of the estrogen receptor-α (ERα)
inhibited these male sex hormone effects (14). Estradiol also upregulated TLR9
expression in prostate cancer cells in vitro(12). In malignant breast tumors, high TLR9
expression is associated with an ER−, and in breast
cancer cells, overexpression of ERα suppresses TLR9 expression
in vitro(14,15). Notably, steroid hormone receptors
have also been shown to regulate the innate immune response in
Drosophila melanogaster, which is suggestive of
evolutionarily well-conserved regulatory pathways in this immune
system (16). Hypoxia is another
important regulator of TLR9 expression in cancer (17). Finally, various viral infections
have been shown to downregulate TLR9 expression in normal tissues
(18,19), although this has not yet been
demonstrated in cancer cells.
We have previously demonstrated that the level of
TLR9 expression is higher in prostate cancer than in benign
hyperplasia (9). We further showed
that high TLR9 expression is significantly associated with a high
Gleason score (9). Previous
clinical studies similarly suggest that TLR9 may contribute to the
pathogenesis of various types of cancer, where high expression of
TLR9 in tumors has been shown to predict decreased survival in
patients with glioblastoma multiforme and esophageal cancer
(7,8). A high level of TLR9 expression in
prostate cancer tumor cells was also shown to be significantly
associated with a higher probability of biochemical recurrence
(20). On the contrary, we recently
demonstrated that absent or low TLR9 expression in tumors is
associated with poor prognosis in patients with renal cell
carcinoma or triple-negative breast cancer, however not in
ER+ breast cancer patients (10,17).
Therefore, the impact of TLR9 expression in tumors on the prognosis
of a patient appears to be dependent on the type of cancer. The aim
of this study was to investigate whether expression levels of TLR9
in tumors has prognostic value in prostate cancer.
Materials and methods
Patient samples
Prostate specimens were obtained from an archive;
these were originally collected from patients who underwent radical
retropubic prostatectomy as a treatment for prostate cancer at Oulu
University Hospital, Oulu, Finland, between 1996 and 2003. During
this period, surgery was performed on 242 males. Following
evaluation of the original diagnostic slides, six cases were
excluded due to the minimal presence of carcinoma tissue. For the
remaining cases, representative areas were located on the slides
and the Gleason score was determined. Gleason scoring was only
carried out for the prostatectomy samples, not for the diagnostic
prostate biopsies. Oulu University Hospital is a tertiary referral
center; patients were referred there to receive surgery after
diagnosis, therefore a number of the diagnostic biopsy slides were
not available. Information concerning the corresponding TNM
classification and prostate-specific antigen (PSA) concentrations
preceding prostatectomy were obtained from patient records. PSA
concentration results were missing for a number of the patients.
Time to biochemical recurrence (PSA progression leading to second
line treatment), time to clinical recurrence (identification of
metastases or histologically confirmed local recurrence),
treatments received and the possible cause of death were also
obtained from patient records. This study was approved by the
Ethics Council of The Northern Ostrobothnia Hospital District.
Immunohistochemistry
Immunohistochemical staining of the specimens was
performed as previously described (9,11,17).
Briefly, 4-μm sections were cut from paraffin-embedded blocks and
mounted onto pre-coated slides. The sections were deparaffinized in
xylene and rehydrated in descending ethanol series. To enhance
immunoreactivity, the sections were incubated in a citrate buffer
(pH 9.0) and boiled. Endogenous peroxidase activity was eliminated
by further incubation in hydrogen peroxide and absolute methanol. A
mouse monoclonal anti-human TLR9 antibody (Img-305A, clone
26C593.2; Imgenex, San Diego, CA, USA; dilution 1:200) was used to
detect specific TLR9 expression. The bound antibodies were
visualized using Envision Detection System (K500711; Dako,
Carpinteria, Denmark A/S). Diaminobenzidine (DAB) was used as a
chromogen (15). All staining was
performed using the LabVision Autostainer™ (LabVision, Fremont, CA,
USA).
Evaluation of immunostaining
TLR9 immunostaining was classified as negative (0),
weakly positive (+1) or strongly positive (+2). Using these
criteria, the immunostained sections were evaluated by two
observers (M-R.V. and M.V.) to reach a consensus.
Statistical analysis
Statistical analysis was carried out using SPSS for
Windows 15 (Chicago, IL, USA). Associations between
clinicopathological variables and TLR9 immunostaining patterns were
assessed using the χ2 test or, in the case of low
expected frequencies, by the Fisher’s exact test. Progression-free
survival rates were calculated using the Kaplan-Meier method, and
the statistical significance between groups was analyzed using the
log-rank test. Hazard ratios (HRs) were assessed using Cox
univariate analysis. Progression-free survival rates in
prostate cancer were calculated from the date of radical
prostatectomy to either biochemical relapse of prostate cancer (as
indicated by increase in serum PSA values) leading to second line
treatments (radiation therapy or hormonal therapies), clinical
progression or the last day of follow-up. Multivariate survival
analysis was carried out using the Cox proportional hazards model.
Two sided P-values were used. P<0.05 was considered to indicate
a statistically significant result.
Results
TLR9 protein expression in prostate
cancer
Baseline patient characteristics are provided in
Table I. There were 186 prostate
cancer samples available for the evaluation of TLR9
immunoreactivity. Evaluation revealed that 124 (66.7%) of the
tumors were strongly positive, 59 (31.7%) were weakly positive and
3 (1.6%) were negative for cytoplasmic TLR9 immunostaining in
cancer cells. For further analysis, the negative and weakly
positive cases were combined and reclassified as TLR9-negative
samples (n=62, 33.3%). Stromal immunoreactivity was also recorded
in these specimens. The majority of the samples (n=114, 61.3%)
exhibited stromal immunoreactivity to TLR9; these were classified
as strongly positive (n=3, 1.6%) or weakly positive (n=111, 59.7%).
The remaining 72 (38.7%) cases were negative for TLR9
immunostaining in stromal cells.
 | Table IBaseline patient characteristics. |
Table I
Baseline patient characteristics.
| Characteristic | Value |
|---|
| Age (years)
(n=186) | |
| Minimum | 45 |
| Maximum | 72 |
| Mean | 62.0 |
| PSA (ng/ml)
(n=56) | |
| Minimum | 3 |
| Maximum | 50 |
| Mean | 12.2 |
| Androgen deprivation
therapy prior to radical prostatectomy | |
| Yes, n (%) | 25 (13.4) |
| No, n (%) | 161 (86.6) |
Association of cytoplasmic TLR9
expression with clinicopathological characteristics
Twenty-five patients received neoadjuvant hormonal
therapy with LHRH-analogs. These cases were excluded from the
Gleason pattern analysis. Distributions of pT-class, prostatectomy
sample margin status and Gleason score, and their association with
cytoplasmic TLR9 expression are presented in Table II. The mean preoperative PSA values
for patients with negative cytoplasmic TLR9 expression and positive
cytoplasmic TLR9 expression were 15.1 ng/ml (95% CI, 8.36–21.9) and
11.2 ng/ml (95% CI, 9.21–13.2), respectively (P= 0.12). Although
the results were not determined to be statistically significant,
more TLR9-positive staining was observed among samples with higher
Gleason scores.
 | Table IIAssociations between cytoplasmic TLR9
expression and tumor pT-class, surgical margin status and Gleason
score of the prostatectomy sample. |
Table II
Associations between cytoplasmic TLR9
expression and tumor pT-class, surgical margin status and Gleason
score of the prostatectomy sample.
| Cytoplasmic TLR9
expression
|
|---|
| Characteristic | Negative, n(%) | Positive, n(%) | P-value |
|---|
| pT | | | |
| 2a | 13 (32) | 28 (68) | 0.63 |
| 2b | 4 (22) | 14 (78) | |
| 2c | 36 (38) | 59 (62) | |
| 3a | 2 (40) | 3 (60) | |
| 3b | 7 (26) | 20 (74) | |
| Surgical margin
status | | | |
| Negative | 37 (33) | 76 (67) | 0.87 |
| Positive | 25 (34) | 48 (66) | |
| Gleason score | | | |
| 4 | 4 (67) | 2 (33) | 0.36 |
| 5 | 17 (40) | 25 (60) | |
| 6 | 20 (29) | 49 (71) | |
| 7 | 16 (29) | 40 (71) | |
| 8 | 3 (38) | 5 (62) | |
| 9 | 2 (40) | 3 (60) | |
Prognostic significance of TLR9
expression in prostate cancer
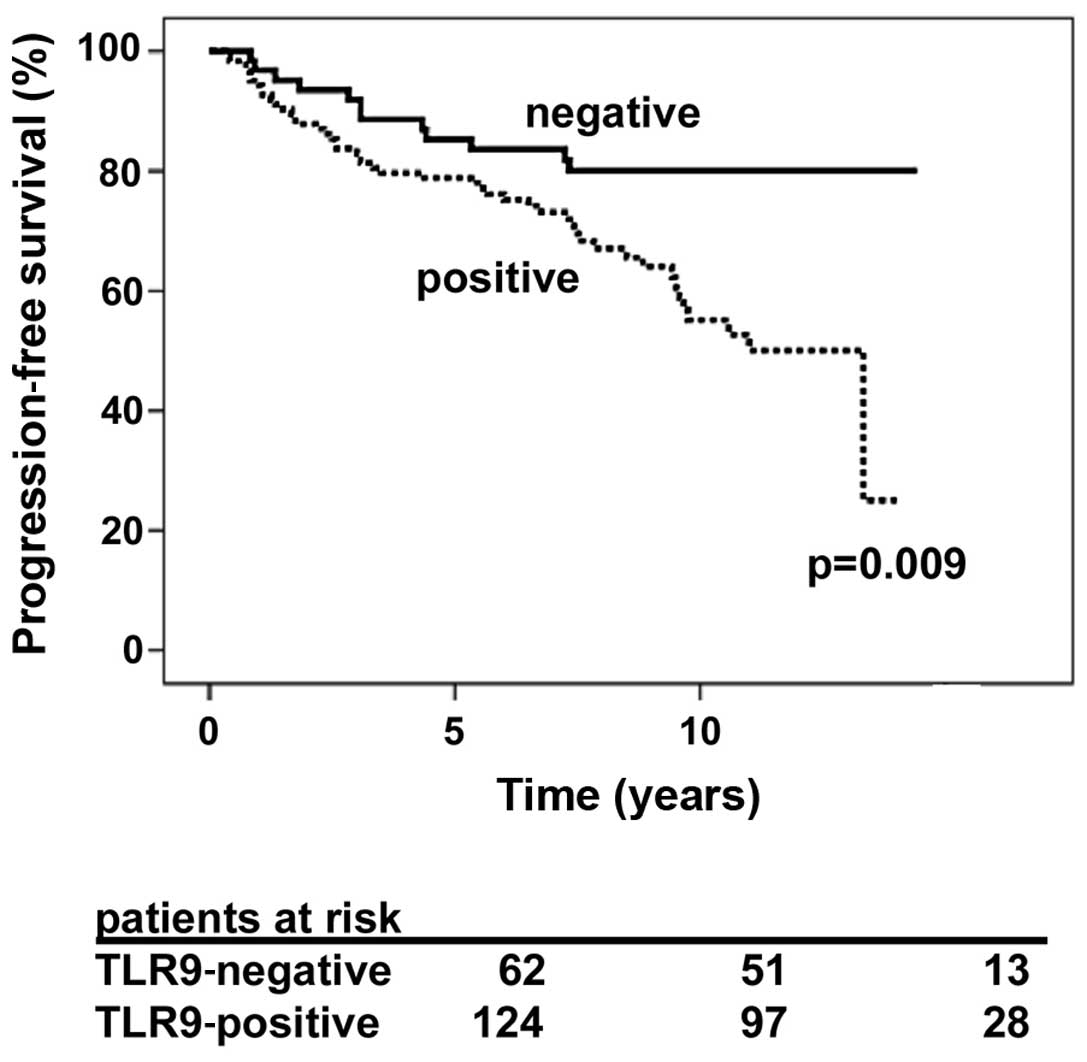
The prostate cancer-specific progression-free
survival rate was significantly longer for patients whose tumors
were graded as negative for cytoplasmic TLR9 expression, as
compared with patients whose tumors were strongly immunopositive
for cytoplasmic TLR9 (P=0.009; Fig.
1). The HR of patients with TLR9-expressing tumors was 2.27
(95% CI, 1.20–4.28, P=0.007). The mean prostate cancer-specific
progression-free survival times for TLR9-negative and TLR9-positive
tumors were 147 (95% CI, 138–161) and 116 (95% CI, 105–128) months,
respectively (P=0.009). The Cox regression analysis results for
age, cytoplasmic TLR9 expression and Gleason score were stratified
as: Gleason score, ≤7 vs. ≥8; pT class, ≤T2c vs. T3a or T3b; and
prostatectomy resection surgical margin status, negative vs.
positive, as shown in Table III.
Cytoplasmic TLR9 expression, Gleason score 8–10 and pT3a-pT3b, were
statistically significant factors in prostate cancer-specific
progression-free survival (Table
III). PSA concentration was excluded from the Cox regression
analysis, due to the numerous cases which were missing a
preoperative PSA value. Based on the results of this study, high
TLR9 expression is an independent marker of poor prognosis in
prostate cancer.
 | Table IIICox multivariate progression-free
survival analysis in 186 patients with prostate cancer treated
using radical prostatectomy. |
Table III
Cox multivariate progression-free
survival analysis in 186 patients with prostate cancer treated
using radical prostatectomy.
| Co-variate | Hazard ratio | 95% CI | P-value |
|---|
| Age | 0.99 | 0.94–1.04 | 0.74 |
| Gleason score
≤7 | 1 (ref) | | |
| Gleason score
≥8 | 2.47 | 1.02–5.99 | 0.044 |
| pT2a-pT2c | 1 (ref) | | |
| pT3a-pT3b | 2.38 | 1.34–4.23 | 0.003 |
| Surgical
margin-negative | 1 (ref) | | |
| Surgical
margin-positive | 1.29 | 0.75–2.24 | 0.36 |
| Positive
cytoplasmic TLR9 expression | 2.27 | 1.18–4.37 | 0.014 |
Discussion
The possible pathophysiological significance of
cellular DNA receptor TLR9 in various types of cancer has attracted
research interest, after studies have established that it is widely
expressed in malignant tumor cells (6,9,11,12).
TLR9 recognizes DNA from bacteria, viruses and the host. DNA
recognition by TLR9 takes place in the endosomal-lysosomal
compartment of cells. The eventual outcome of TLR9 stimulation is
inflammation, characterized by increased expression of
proinflammatory cytokines and other inflammatory mediators.
Stimulation of TLR9 by synthetic DNA ligands or bacterial DNA also
stimulates cancer cell invasion (6,12,21).
Our previous studies with breast cancer cells further suggested
that TLR9 expression may regulate cancer cell invasion, even in the
absence of ligands (17).
Using a cohort of 186 prostate cancer samples and
their associated clinical data, this study showed that high TLR9
expression in tumor cells is an independent marker of poor
prognosis in prostate cancer. TLR9 staining was also detected in
prostate cancer stroma, however there are no published results with
regard to the prognostic role of TLR9 staining in prostate cancer
stroma. Stromal staining for TLR9 appeared to be markedly less than
that detected in epithelial cancer cells. Our results agree with
those of Gonzáles-Reyes et al(20), who demonstrated that high TLR9 mRNA
expression in prostate tumors is significantly associated with
recurrence and higher probability of biochemical recurrence.
Although the results of this study were not statistically
significant, higher TLR9 expression scores were observed in tumors
with higher Gleason scores. This has also been demonstrated in
previous studies (9,20).
If TLR9 is significant in the pathophysiology of
prostate cancer, the mechanism by which it promotes prostate cancer
must be determined. There are several possibilities; firstly, TLR9
may promote the spread of prostate cancer by facilitating prostate
cancer invasion. This hypothesis is supported by our previous
findings, which show that knocking out TLR9 expression from cancer
cells results in decreased invasion, and that E. coli DNA
promotes prostate cancer cell invasion in vitro(12,17,21).
Notably, testosterone promotes TLR9 ligand-induced invasion in
breast cancer cells in vitro(14). Whether the effects are similar in
prostate cancer cells requires further investigation. Secondly,
TLR9 stimulation by DNA results in inflammation, which has been
associated with prostate carcinogenesis (22–25).
Finally, TLR9 expression appears to be upregulated by sex hormones
in breast and prostate cancer cells in vitro(12,14).
Considering the importance of sex hormones in the aggressive
behavior of prostate cancer, this effect on TLR9 may also
contribute to the explanation. These issues require further
characterization in pre-clinical models of prostate cancer.
In conclusion, this study shows that increased
expression of TLR9 is associated with poor prognosis in prostate
cancer. The question that remains, however, is whether TLR9 is a
driver or a passenger in prostate cancer. This should be answered
via research in pre-clinical prostate cancer models, using prostate
cancer cells with manipulated TLR9 expression levels.
Acknowledgements
Mr. Kari Mononen and Mrs. Anita
Tikkala assisted with data collection. We also thank Pasi Ohtonen
for assistance with the statistical analysis. This study was funded
by a grant from Finnish Urological Association.
References
|
1
|
Jinushi M: The role of innate immune
signals in antitumor immunity. Oncoimmunology. 1:189–194. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Akira S and Hemmi H: Recognition of
pathogen-associated molecular patterns by TLR family. Immunol Lett.
85:85–95. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hidmark A, von Saint Paul A and Dalpke AH:
Cutting edge: TLR13 Is a Receptor for Bacterial RNA. J Immunol.
189:2717–2721. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Chang YJ, Wu MS, Lin JT and Chen CC:
Helicobacter pylori-induced invasion and angiogenesis of
gastric cells is mediated by cyclooxygenase-2 induction through
TLR2/TLR9 and promoter regulation. J Immunol. 175:8242–8252. 2005.
View Article : Google Scholar
|
|
5
|
Droemann D, Albrecht D, Gerdes J, Ulmer
AJ, Branscheid D, Vollmer E, Dalhoff K, Zabel P and Goldmann T:
Human lung cancer cells express functionally active Toll-like
receptor 9. Respir Res. 6:12005. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Merrell MA, Ilvesaro JM, Lehtonen N, Sorsa
T, Gehrs B, Rosenthal E, Chen D, Shackley B, Harris KW and Selander
KS: Toll-like receptor 9 agonists promote cellular invasion by
increasing matrix metalloproteinase activity. Mol Cancer Res.
4:437–447. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Takala H, Kauppila JH, Soini Y, Selander
KS, Vuopala KS, Lehenkari PP, Saarnio J and Karttunen TJ: Toll-like
receptor 9 is a novel biomarker for esophageal squamous cell
dysplasia and squamous cell carcinoma progression. J Innate Immun.
3:631–638. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kauppila JH, Takala H, Selander KS,
Lehenkari PP, Saarnio J and Karttunen TJ: Increased Toll-like
receptor 9 expression indicates adverse prognosis in oesophageal
adenocarcinoma. Histopathology. 59:643–649. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Väisänen MR, Väisänen T, Jukkola-Vuorinen
A, Vuopala KS, Desmond R, Selander KS and Vaarala MH: Expression of
toll-like receptor-9 is increased in poorly differentiated prostate
tumors. Prostate. 70:817–824. 2010.PubMed/NCBI
|
|
10
|
Ronkainen H, Hirvikoski P, Kauppila S,
Vuopala KS, Paavonen TK, Selander KS and Vaarala MH: Absent
Toll-like receptor-9 expression predicts poor prognosis in renal
cell carcinoma. J Exp Clin Cancer Res. 30:842011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Jukkola-Vuorinen A, Rahko E, Vuopala KS,
Desmond R, Lehenkari PP, Harris KW and Selander KS: Toll-like
receptor-9 expression is inversely correlated with estrogen
receptor status in breast cancer. J Innate Immun. 1:59–68. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ilvesaro JM, Merrell MA, Swain TM,
Davidson J, Zayzafoon M, Harris KW and Selander KS: Toll like
receptor-9 agonists stimulate prostate cancer invasion in vitro.
Prostate. 67:774–781. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ren T, Xu L, Jiao S, Wang Y, Cai Y, Liang
Y, Zhou Y, Zhou H and Wen Z: TLR9 signaling promotes tumor
progression of human lung cancer cell in vivo. Pathol Oncol Res.
15:623–630. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Sandholm J, Kauppila JH, Pressey C,
Tuomela J, Jukkola-Vuorinen A, Vaarala M, Johnson MR, Harris KW and
Selander KS: Estrogen receptor-alpha and sex steroid hormones
regulate Toll-like receptor-9 expression and invasive function in
human breast cancer cells. Breast Cancer Res Treat. 132:411–419.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kakonen SM, Selander KS, Chirgwin JM, Yin
JJ, Burns S, Rankin WA, Grubbs BG, Dallas M, Cui Y and Guise TA:
Transforming growth factor-beta stimulates parathyroid
hormone-related protein and osteolytic metastases via Smad and
mitogen-activated protein kinase signaling pathways. J Biol Chem.
277:24571–24578. 2002. View Article : Google Scholar
|
|
16
|
Flatt T, Heyland A, Rus F, Porpiglia E,
Sherlock C, Yamamoto R, Garbuzov A, Palli SR, Tatar M and Silverman
N: Hormonal regulation of the humoral innate immune response in
Drosophila melanogaster. J Exp Biol. 211:2712–2724. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Tuomela J, Sandholm J, Karihtala P,
Ilvesaro J, Vuopala KS, Kauppila JH, Kauppila S, Chen D, Pressey C,
Harkonen P, Harris KW, Graves D, Auvinen PK, Soini Y,
Jukkola-Vuorinen A and Selander KS: Low TLR9 expression defines an
aggressive subtype of triple-negative breast cancer. Breast Cancer
Res Treat. 135:481–493. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Vincent IE, Zannetti C, Lucifora J, Norder
H, Protzer U, Hainaut P, Zoulim F, Tommasino M, Trepo C, Hasan U
and Chemin I: Hepatitis B virus impairs TLR9 expression and
function in plasmacytoid dendritic cells. PLoS One. 6:e263152011.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Yu SL, Chan PK, Wong CK, Szeto CC, Ho SC,
So K, Yu MM, Yim SF, Cheung TH, Wong MC, Cheung JL, Yeung AC, Li EK
and Tam LS: Antagonist-mediated down-regulation of toll-like
receptors increases the prevalence of human papillomavirus
infection in systemic lupus erythematosus. Arthritis Res Ther.
14:R802012. View
Article : Google Scholar : PubMed/NCBI
|
|
20
|
González-Reyes S, Fernandez JM, González
LO, Aguirre A, Suarez A, González JM, Escaff S and Vizoso FJ: Study
of TLR3, TLR4, and TLR9 in prostate carcinomas and their
association with biochemical recurrence. Cancer Immunol Immunother.
60:217–226. 2011.PubMed/NCBI
|
|
21
|
Ilvesaro JM, Merrell MA, Li L, Wakchoure
S, Graves D, Brooks S, Rahko E, Jukkola-Vuorinen A, Vuopala KS,
Harris KW and Selander KS: Toll-like receptor 9 mediates CpG
oligonucleotide-induced cellular invasion. Mol Cancer Res.
6:1534–1543. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Nakai Y and Nonomura N: Inflammation and
prostate carcinogenesis. Int J Urol. Jul 31–2012.(Epub ahead of
print). View Article : Google Scholar
|
|
23
|
Kundu SD, Lee C, Billips BK, Habermacher
GM, Zhang Q, Liu V, Wong LY, Klumpp DJ and Thumbikat P: The
toll-like receptor pathway: a novel mechanism of infection-induced
carcinogenesis of prostate epithelial cells. Prostate. 68:223–229.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Di JM, Pang J, Sun QP, Zhang Y, Fang YQ,
Liu XP, Zhou JH, Ruan XX and Gao X: Toll-like receptor 9 agonists
up-regulates the expression of cyclooxygenase-2 via activation of
NF-kappaB in prostate cancer cells. Mol Biol Rep. 37:1849–1855.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Di JM, Pang J, Pu XY, Zhang Y, Liu XP,
Fang YQ, Ruan XX and Gao X: Toll-like receptor 9 agonists promote
IL-8 and TGF-beta1 production via activation of nuclear factor
kappaB in PC-3 cells. Cancer Genet Cytogenet. 192:60–67. 2009.
View Article : Google Scholar : PubMed/NCBI
|