Introduction
Prostate cancer (PCa) is the most frequently
diagnosed malignancy in males and a common cause of cancer-related
mortality. Hormone deprivation is an option for rapid androgen
ablation in PCa patients. However, cancer cells eventually recur
and progress to the androgen-independent stage. The treatments
available for patients during this stage are limited.
Chloroquine (CQ) is a traditional anti-malarial
medication that has been identified as a potential adjuvant in the
treatment regimen of glioblastoma multiforme (GBM) (1). The results of clinical trials
(2,3) indicate that CQ is a potential adjuvant
therapy for glioblastoma. Moreover, mefloquine (MQ) has been shown
to be more potent than CQ in killing cancer cells in vitro
and is potentially more efficacious than CQ as a chemotherapeutic
agent for GBM patients (1). MQ is a
valuable anti-malarial drug for prophylaxis and treatment for the
majority of patients (4). MQ plasma
concentrations reached 1,692 ng/ml (4.48 μM) for
chemosuppression in Plasmodium falciparum infections
(5,6). Krudsood et al(7) reported that an MQ plasma concentration
of 5,796 ng/ml (15.35 μM) was attained in a clinical study
on P. falciparum-infected adults. Children tolerate MQ
better than adults, and males tolerate it better than females
(8).
This study examined two PCa cell lines (DU145 and
PC3). DU145 and PC3 are the most commonly used PCa cell lines and
their characteristics differ. PC3 was isolated from a bone
metastasis, whereas DU145 cells were isolated from the brain
metastases of prostate carcinoma. However, p53-mutant DU145
(9,10) and p53-null PC3 (9,11)
cells are androgen-independent and proliferate normally in
androgen-deprived media.
This study had the following two objectives: i) to
determine whether MQ possesses anticancer effects at potential
therapeutic concentrations in vitro; and ii) to detail
general MQ features in the context of disrupted cancer cell
proliferation. This study shows that MQ exposure causes immediate
hyperpolarization of mitochondrial membrane potential (MMP) and
increases reactive oxygen species (ROS) generation in PCa cells.
For the first time, the study also shows that MQ-mediated ROS
inhibits Akt phosphorylation and activates c-Jun N-terminal kinase
(JNK), extracellular signal-regulated kinase (ERK) and adenosine
monophosphate-activated protein kinase (AMPK) signaling.
Materials and methods
Cell culture
Human foreskin fibroblast Hs68 cells and the
androgen-independent PCa cell lines, PC3 and DU145, were maintained
in Dulbecco’s modified Eagle’s medium and supplemented with 10%
fetal bovine serum. The PCa cells were continuously cultured in a
regular cell culture medium with 2 mM L-glutamine, 100 μg/ml
streptomycin and 100 U/ml penicillin in a humidified 5%
CO2 atmosphere. This study was approved by the Taipei
Medical University Wan Fang Hospital.
Cell viability assay
Cells were seeded onto 96-well plates at a density
of 5,000 cells per well and incubated for 1 day. Cell viability was
assayed using sulforhodamine B (SRB) staining, as described
previously (12). Absorbance at 570
nm was measured using an ELISA reader. Cell viability is expressed
as the percentage of absorbance of the treated cells relative to
that of the untreated (control) cells.
Colony formation assay
One hundred cells were seeded in a 10-cm culture
dish for 24 h and then incubated with MQ (or without for control).
Colonies >0.5 mm were counted after 2 weeks. Colonies were
washed with phosphate-buffered saline (PBS) then air-dried, stained
with 0.4% crystal violet for 1 min, rinsed in water, air-dried and
then photographed.
MMP assessment
MMP was measured using 40 μM cationic
lipophilic fluorochrome DiOC6. Cells were treated with MQ
(5×105 cells/well) in a 6-well plate for 40 min, then
DiOC6 was added and the cells were cultured continuously without
light for 20 min at 37°C in the presence of MQ. Cells were then
obtained and washed with 1 ml ice-cold PBS. Finally, the cells were
suspended in PBS and analyzed immediately using flow cytometry
(FC500, Beckman Coulter, Miami, FL, USA). DiOC6 was recorded by
fluorescence. The mean value of the MMP in the treated cells was
calculated for comparison with that of the control cells.
Intracellular ROS assays
A 5-μM non-fluorescent
2′,7′-dichlorofluorescein-diacetate (DCFH-DA) intracellular probe
was used to detect ROS formation. Cells were treated with MQ
(5×105 cells/well) in a 6-well plate for 40 min, then
DCFH-DA was added and the cells were cultured continuously without
light for 20 min at 37°C. Finally, cells were collected by
centrifuging and suspended in 1 ml ice-cold PBS. The oxidation of
DCFH by ROS was determined by measuring the mean fluorescent
intensity of DCFH by flow cytometry (FC500).
Western blot analysis
PC3 cells were pre-treated with 10 mM NAC for 20 min
and then treated with 10 μM MQ for 1 h. These were compared
to the control cells which were treated with 10 μM MQ for 1
h only. At harvest, total protein extracts were prepared and the
protein concentration was determined using the Bradford method.
Aliquots containing 20 μg total protein each were subjected
to western blot analysis. Antibodies against
glyceraldehyde-3-phosphate dehydrogenase were purchased from Santa
Cruz Biotechnology (Santa Cruz, CA, USA). Antibodies against
phosphorylated Akt, JNK, AMPK and ERK were purchased from Cell
Signaling Technology (Danvers, MA, USA).
Statistical analysis
Error bars represent the standard error of means
(SEM) from independent triplicates (n=3). All data are expressed as
mean ± SEM. We employed Sigma Plot 2001 software for statistical
analysis. P<0.05 was considered to indicate a statistically
significant result.
Results
Effects of MQ on the proliferation of PCa
and Hs68 cells
Two established PCa cell lines (DU145 and PC3) were
examined for their sensitivity to MQ in vitro. We examined
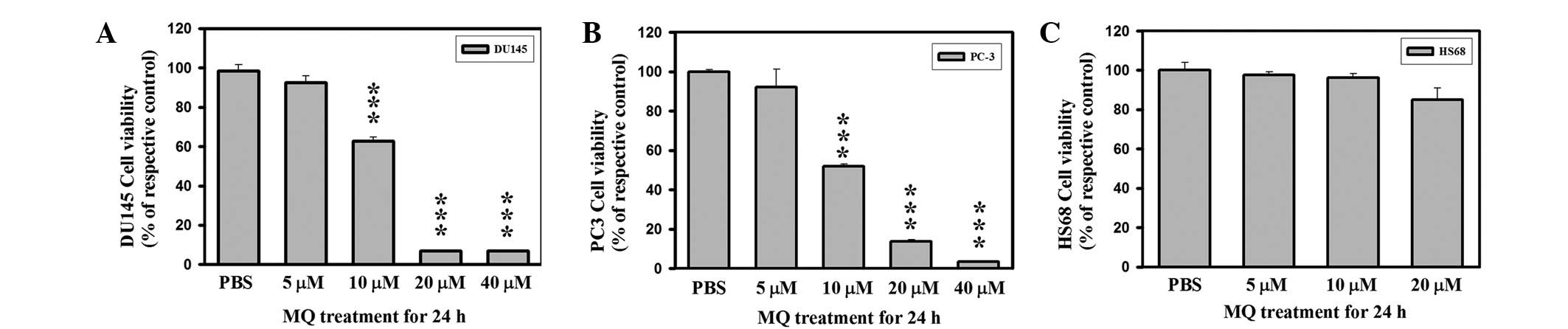
the growth inhibitory effects of MQ on PCa and Hs68 cells using SRB
staining (Fig. 1). The PCa cell
population was reduced after 24 h exposure to MQ (IC50
∼10 μM MQ, Fig. 1A and B).
PCa cell proliferation was completely inhibited at MQ
concentrations >20 μM. Assessment of Hs68 cells following
MQ treatment showed no reduction in cell viability at 10 μM
(Fig. 1C). Treatment of the Hs68
cells with MQ resulted in an effect of <IC10 at ∼20
μM MQ at 24 h. Hs68 cells exhibited greater resistance to MQ
exposure compared with the PCa cells. The results show that the
IC50 value of MQ for PCa cells was ∼10 μM. MQ is
a highly cytotoxic drug for PCa cell lines and causes ∼50% cell
death in DU145 and PC3 at clinically achievable concentrations.
Despite its anticancer potency, MQ is relatively nontoxic towards
normal human cells.
Effects of MQ on colony formation in PCa
cells
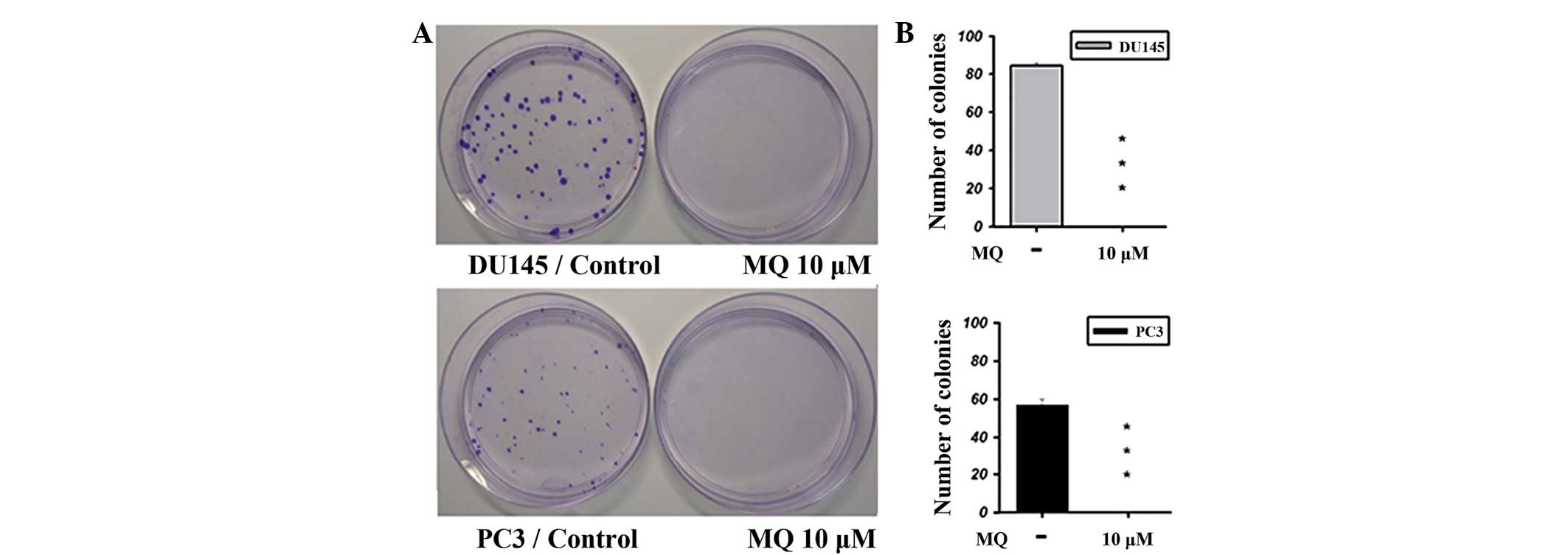
A colony formation assay was performed to further
show the antitumor effect of MQ (Fig.
2). Images of colonies grown in the presence or absence of MQ
are shown in Fig. 2A (top, DU145;
bottom, PC3). A significant antitumor effect was observed for MQ
against the DU145 and PC3 cells (Fig.
2B).
Effects of MQ on MMP and ROS in PCa
cells
MMP represents the performance of the electron
transport chain and may indicate a pathological disorder of that
system. A failure of mitochondrial bioenergetics is closely
associated with the onset of apoptosis and necrosis. Since MQ is
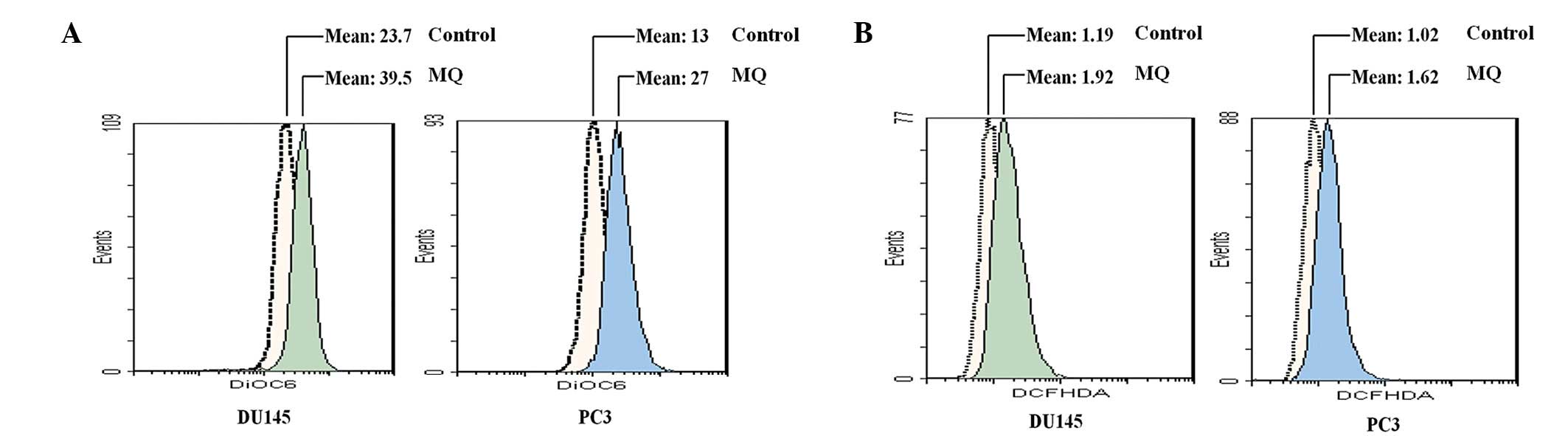
closely related to the alteration of MMP (13), this study investigated the effects
of MQ on MMP using DiOC6. Following 1 h exposure to MQ (10
μM), the fluorescence mean value of the MMP was ∼1.5- to
2-fold higher than that of the control PCa cells (Fig. 3A). Since MQ has a rapid effect on
the hyperpolarization of MMP, the level of ROS produced within 1 h
was further analyzed. The intracellular ROS levels in the
MQ-treated PCa cells were assessed (Fig. 3B). PCa cells have a lower mean DCFH
value in the absence of MQ than in its presence. MQ significantly
stimulated the generation of ROS by PCa cells, as observed by the
alteration of DCFH fluorescence. When treated with 10 μM MQ,
the DCFH fluorescence increased from 1.19 to 1.92 and from 1.02 to
1.62 in DU145 and PC3 cells, respectively.
Pre-treatment with N-acetyl cysteine
(NAC) protects DU145 and PC3 cells against MQ-induced anticancer
effects and ROS-mediated signaling
Previous studies have suggested that preincubation
with NAC is required to inhibit the cytotoxicity of generated ROS
(14–16). NAC, which contains a sulfhydryl
group, acts as a ROS scavenger and a precursor of intracellular
reduced glutathione to regulate the redox status in the cells. To
confirm the role of ROS in MQ-induced anticancer effects, cell
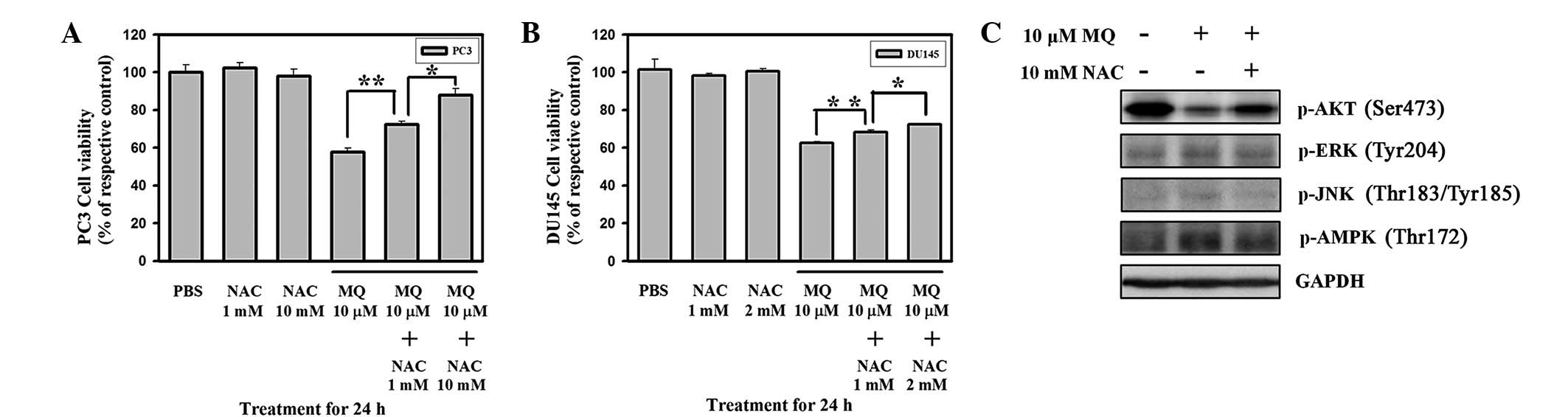
viability was detected in DU145 and PC3 cells pre-treated with NAC
for 20 min and then treated with 10 μM MQ for 24 h. Cell
viability was determined using SRB staining. NAC pre-treatment
significantly inhibited MQ-induced anticancer effects in PC3 and
DU145 cells (Fig. 4A and B). The
NAC control groups (PC3, 1 and 10 mM; DU145, 1 and 2 mM) showed no
significant changes in cell viability compared with the untreated
controls. These results indicate that MQ-mediated cytotoxicity is
quenched by the antioxidant NAC. These data may also indicate that
increased ROS generation is essential in MQ-mediated cell death.
MQ-mediated signal transduction pathways involving ROS-induced
signaling modulation were also investigated (Fig. 4C). We observed that MQ-mediated ROS
simultaneously downregulated Akt Ser473 phosphorylation and
activated ERK, JNK and AMPK signaling in PC3 cells. Moreover, Akt
signaling was rescued and phosphorylation of ERK Tyr204, JNK
Thr183/Tyr185 and AMPK Thr172 was decreased by pre-treatment with
NAC, the ROS scavenger.
Discussion
MQ is a prophylactic anti-malarial drug that has
been shown to possess antineoplastic activity against an
experimental GBM tumor (1). Dow
et al(17) showed that
higher MQ blood levels are achieved under therapeutic regimens (2.1
to 23 μM) than under prophylaxis (3.8 μM). This study
was designed to understand the efficacy underlying MQ-induced
anticancer effects. Human PCa cell lines, including commonly used
androgen-independent DU145 and PC3 (18), were used.
Exposure to 10 μM MQ for 24 h decreased the
populations of DU145 and PC3 cells as determined by SRB staining
(Fig. 1). The clonogenic effect was
most significantly diminished when examined using a colony
formation assay (Fig. 2). However,
a normal human skin fibroblast cell line (Hs68) exhibited a
different response to MQ exposure. Hs68 cells continued to
proliferate normally in the presence of 10–20 μM MQ, whereas
the cancer cells were sensitive to the cytotoxic effects of MQ at
10 μM. This shows that MQ has highly selective anticancer
capabilities.
Mitochondria are required as the primary source of
ROS in cells. Mitochondrial dysfunction, oxidative stress and
impaired cerebral energy metabolism contribute to cell death
(19). The mitochondria are the
primary organelles for production of high-energy phosphate. MMP
hyperpolarization has also been associated with subcellular
necrosis (20). In cell suicide
(apoptosis and necrosis), mitochondrial hyperpolarization is
required for specific steps of cell death (21). Previous studies have indicated that
MQ treatment induces the cytotoxic effect that is dependent on the
increase in oxidative stress in primary rat cortical neurons
(22). MMP hyperpolarization may
also increase ROS production (23).
Exogenous oxidative stress may induce transient hyperpolarization
of MMP and a delayed burst of endogenous ROS (24). MMP hyperpolarization and ROS
generation are early molecular events that precede cell death
(25). In certain types of
necrosis, cell death is mediated by an increase in the
MMP-dependent overproduction of ROS (21). A recent study (26) has shown that massive mitoptosis may
result in cell death. ROS-initiated mitoptosis is presumed to
eliminate the mitochondria which overproduce ROS (21). MMP hyperpolarization-induced ROS
cause oxidative stress-induced necrotic cell death (24).
MMP alteration and oxidative stress in cancer cells
treated with MQ have not been examined previously. The current
study investigated MMP alteration induced by 1 h of exposure to MQ
in PCa cells. In the alteration of MMP, a change in the state of
ROS was also observed. In our study, MQ treatment led to
significant MMP hyperpolarization. This indicates that MMP
hyperpolarization during MQ treatment mediated intracellular ROS
generation which inhibited PCa cells at concentrations >10
μM.
There are several signaling factors, such as Akt,
AMPK and the mitogen-activated protein kinase (MAPK) family, that
regulate the progression of various tumors. Akt is activated by
Ser473 phosphorylation, which affects cell growth, proliferation
and survival. The increased ROS levels may enhance MAPK activities.
The phosphorylation of AMPK Thr172 depends on cellular metabolic
stress. Previous studies have implied that the signaling factors
Akt, AMPK and the MAPK family are potentially associated with
ROS-triggered biological responses. ROS are a significant type of
molecule that mediate numerous signal transduction pathways and
have a critical function in cells. To confirm the function of ROS
generation in MQ treatment, NAC was used to scavenge the ROS.
Pre-treatment of DU145 and PC3 cells with NAC abrogated ROS
induction by MQ and significantly protected these cells against
MQ-induced anticancer effects. Exposure of PC3 cells to MQ for 1 h
caused a decrease in phosphorylation of Akt at Ser473.
Simultaneously, we also observed the activation of AMPK (at Thr172)
and MAPK family members (ERK at Tyr204 and JNK at Thr183/Tyr185) in
response to MQ treatment. Pre-treatment with NAC confirmed the
function of Akt, ERK, JNK and AMPK in MQ treatment. Blocking ROS
generation with NAC had a significantly preventative effect on Akt
activation, and inhibited ERK, JNK and AMPK. This study indicates
an anticancer action of MQ that is dependent on Akt signaling
disruption and JNK, ERK and AMPK signaling upregulation in PCa
cells; this action is dependent on ROS generation. Mitochondrial
function and ROS generation are involved in PI3K-Akt-mTOR and MAPK
(JNK/ERK) signaling for autophagy in cancer (27). AMPK regulates the ROS/redox balance
which indicates that AMPK signaling is critical in intracellular
homoeostasis during autophagy (28). The intricate relationships between
ROS-mediated signaling (involving Akt, JNK, ERK and AMPK) and
autophagy require further study. We corroborate that MQ induces ROS
generation which then affects cancer cell proliferation by
modulating signaling transduction, specifically by ERK, JNK and
AMPK activation and Akt inhibition.
Thus, we assert that MQ has a strong inhibitory
effect on cell viability by producing ROS in DU145 and PC3 cells.
These results indicate that MQ may be a potential candidate for
clinical trials of cancer applications in the future.
Abbreviations:
|
MQ
|
mefloquine;
|
|
PCa
|
prostate cancer;
|
|
ROS
|
reactive oxygen species;
|
|
MMP
|
mitochondrial membrane potential;
|
|
NAC
|
N-acetyl cysteine
|
Acknowledgements
This study was supported by the
Research Fund of the Center of Excellence for Cancer Research,
Taipei Medical University, Taipei, Taiwan (Project Number
DOH100-TD-C-111-008) and the National Science Council (Grant NSC
100-2314-B-038-039).
References
|
1
|
Geng Y, Kohli L, Klocke BJ and Roth KA:
Chloroquine-induced autophagic vacuole accumulation and cell death
in glioma cells is p53 independent. Neuro Oncol. 12:473–481.
2010.PubMed/NCBI
|
|
2
|
Sotelo J, Briceno E and López-González MA:
Adding chloroquine to conventional treatment for glioblastoma
multiforme: a randomized, double-blind, placebo-controlled trial.
Ann Intern Med. 144:337–343. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Briceño E, Reyes S and Sotelo J: Therapy
of glioblastoma multiforme improved by the antimutagenic
chloroquine. Neurosurg Focus. 14:e32003.PubMed/NCBI
|
|
4
|
Taylor WR and White NJ: Antimalarial drug
toxicity: a review. Drug Saf. 27:25–61. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Simpson JA, Price R, ter Kuile F,
Teja-Isavatharm P, Nosten F, Chongsuphajaisiddhi T, Looareesuwan S,
Aarons L and White NJ: Population pharmacokinetics of mefloquine in
patients with acute falciparum malaria. Clin Pharmacol Ther.
66:472–484. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kollaritsch H, Karbwang J, Wiedermann G,
Mikolasek A, Na-Bangchang K and Wernsdorfer WH: Mefloquine
concentration profiles during prophylactic dose regimens. Wien Klin
Wochenschr. 112:441–447. 2000.PubMed/NCBI
|
|
7
|
Krudsood S, Looareesuwan S, Wilairatama P,
Leowattana W, Tangpukdee N, Chalermrut K, Ramanathan S, Navaratnam
V, Olliaro P, Vaillant M, Kiechel JR and Taylor WR: Effect of
artesunate and mefloquine in combination on the Fridericia
corrected QT intervals in Plasmodium falciparum infected
adults from Thailand. Trop Med Int Health. 16:458–465. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
ter Kuile FO, Nosten F, Thieren M,
Luxemburger C, Edstein MD, Chongsuphajaisiddhi T, Phaipun L,
Webster HK and White NJ: High-dose mefloquine in the treatment of
multidrug-resistant falciparum malaria. J Infect Dis.
166:1393–1400. 1992.
|
|
9
|
Isaacs WB, Carter BS and Ewing CM:
Wild-type p53 suppresses growth of human prostate cancer cells
containing mutant p53 alleles. Cancer Res. 51:4716–4720.
1991.PubMed/NCBI
|
|
10
|
Gurova KV, Rokhlin OW, Budanov AV,
Burdelya LG, Chumakov PM, Cohen MB and Gudkov AV: Cooperation of
two mutant p53 alleles contributes to Fas resistance of prostate
carcinoma cells. Cancer Res. 63:2905–2912. 2003.PubMed/NCBI
|
|
11
|
Bajgelman MC and Strauss BE: The DU145
human prostate carcinoma cell line harbors a temperature-sensitive
allele of p53. Prostate. 66:1455–1462. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Keepers YP, Pizao PE, Peters GJ, van
Ark-Otte J, Winograd B and Pinedo HM: Comparison of the
sulforhodamine B protein and tetrazolium (MTT) assays for in vitro
chemosensitivity testing. Eur J Cancer. 27:897–900. 1991.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
McArdle JJ, Sellin LC, Coakley KM, Potian
JG and Hognason K: Mefloquine selectively increases asynchronous
acetylcholine release from motor nerve terminals.
Neuropharmacology. 50:345–353. 2006. View Article : Google Scholar
|
|
14
|
Rogalska A, Koceva-Chyla A and Jóźwiak Z:
Aclarubicin-induced ROS generation and collapse of mitochondrial
membrane potential in human cancer cell lines. Chem Biol Interact.
176:58–70. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Liu H, Jiang C, Xiong C and Ruan J: DEDC,
a new flavonoid induces apoptosis via a ROS-dependent mechanism in
human neuroblastoma SH-SY5Y cells. Toxicol In Vitro. 26:16–23.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lan A, Liao X, Mo L, Yang C, Yang Z, Wang
X, Hu F, Chen P, Feng J, Zheng D and Xiao L: Hydrogen sulfide
protects against chemical hypoxia-induced injury by inhibiting
ROS-activated ERK1/2 and p38MAPK signaling pathways in PC12 cells.
PLoS One. 6:e259212011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Dow GS, Caridha D, Goldberg M, Wolf L,
Koenig ML, Yourick DL and Wang Z: Transcriptional profiling of
mefloquine-induced disruption of calcium homeostasis in neurons in
vitro. Genomics. 86:539–550. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Chlenski A, Nakashiro K, Ketels KV,
Korovaitseva GI and Oyasu R: Androgen receptor expression in
androgen-independent prostate cancer cell lines. Prostate.
47:66–75. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Robertson CL, Scafidi S, McKenna MC and
Fiskum G: Mitochondrial mechanisms of cell death and
neuroprotection in pediatric ischemic and traumatic brain injury.
Exp Neurol. 218:371–380. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Berghe TV, Vanlangenakker N, Parthoens E,
Deckers W, Devos M, Festjens N, Guerin CJ, Brunk UT, Declercq W and
Vandenabeele P: Necroptosis, necrosis and secondary necrosis
converge on similar cellular disintegration features. Cell Death
Differ. 17:922–930. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Skulachev VP: Bioenergetic aspects of
apoptosis, necrosis and mitoptosis. Apoptosis. 11:473–485. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Hood JE, Jenkins JW, Milatovic D, Rongzhu
L and Aschner M: Mefloquine induces oxidative stress and
neurodegeneration in primary rat cortical neurons. Neurotoxicology.
31:518–523. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zheng Z, Chen H, Zhao H, Liu K, Luo D,
Chen Y, Yang X, Gu Q and Xu X: Inhibition of JAK2/STAT3-mediated
VEGF upregulation under high glucose conditions by PEDF through a
mitochondrial ROS pathway in vitro. Invest Ophthalmol Vis Sci.
51:64–71. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Choi K, Kim J, Kim GW and Choi C:
Oxidative stress-induced necrotic cell death via
mitochondria-dependent burst of reactive oxygen species. Curr
Neurovasc Res. 6:213–222. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Di Stefano A, Frosali S, Leonini A,
Ettorre A, Priora R, Di Simplicio FC and Di Simplicio P: GSH
depletion, protein S-glutathionylation and mitochondrial
transmembrane potential hyperpolarization are early events in
initiation of cell death induced by a mixture of isothiazolinones
in HL60 cells. Biochim Biophys Acta. 1763:214–225. 2006.
|
|
26
|
Venditti P, Di Stefano L and Di Meo S:
Mitochondrial metabolism of reactive oxygen species. Mitochondrion.
Jan 29–2013.(Epub ahead of print).
|
|
27
|
Li ZY, Yang Y, Ming M and Liu B:
Mitochondrial ROS generation for regulation of autophagic pathways
in cancer. Biochem Biophys Res Commun. 414:5–8. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Wang S, Song P and Zou MH: AMP-activated
protein kinase, stress responses and cardiovascular diseases. Clin
Sci (Lond). 122:555–573. 2012. View Article : Google Scholar : PubMed/NCBI
|