Introduction
Decades of studies have indicated that the tumor
microvasculature has an essential role in malignant tumor growth
and progression (1–3). Thus, multiple anti-angiogenic
compounds have been designed to increase the therapeutic efficacy
of tumor treatments. However, this anti-angiogenic strategy has not
significantly improved the prognosis of patients suffering from
malignant glioma, according to clinical results (4) which indicated that damaged vascular
endothelial cells (ECs) may have an unidentified role in tumor
maintenance or even proliferation.
Researchers have made a concerted effort to
understand how the angiogenic process is regulated by tumor cells
under various conditions (5,6).
However, a limited number of studies have focused on the potential
effect of dying ECs on tumor cell biology. One study reported that
the presence of damaged ECs regulated tumor response to
radiotherapy by facilitating in vivo tumor growth and
contributing to therapy resistance (7). However, the mechanistic details remain
unclear. Another group observed a proliferative effect of apoptotic
cells on wound healing processes, and the caspase 3-mediated
‘phoenix rising’ pathway was found to be involved in this
compensatory proliferation (8).
We hypothesized that ECs exposed to lethal factors
had an enhanced ability to support the proliferation of glioma
cells. Furthermore, the caspase family, which is highly involved in
cell apoptosis, may be the key regulator of this process. To
investigate our hypothesis, the effects of ECs on the proliferation
of glioma cells were evaluated under various conditions. Based on
the present data, it was demonstrated that dying ECs were able to
accelerate glioma cell growth via a caspase 3-mediated pathway
which presented a novel insight into the interaction between
damaged vascular ECs and surrounding tumor cells.
Materials and methods
Cell culture
Three glioma cell lines (U87MG, U251MG and C6MG) and
human umbilical vascular ECs (HUVECs) were used in the present
study. All these cells were purchased from the American Type
Culture Collection (ATCC; Manassas, VA, USA). Cells were cultured
in Dulbecco’s modified Eagle’s medium (DMEM; Gibco-Invitrogen,
Carlsbad, CA, USA) supplemented with 10% fetal bovine serum (FBS;
Gibco-Invitrogen) under normoxic conditions. In coculture
conditions, hypoxia-exposed or untreated HUVECs were seeded on
24-well inserts (0.4 μm; Millipore, Billerica, MA, USA) and
glioma cells were planted on a 24-well receiver plates (Millipore).
For hypoxia exposure, HUVECs were cultured under several hypoxic
conditions, including 6, 2 and 1% O2 using a hypoxia
incubator (Heracell 150; Thermo Scientific, Waltham, MA, USA). The
conditional medium was harvested from HUVECs exposed to 2%
O2 for 48 h and the glioma cell lines were then cultured
in the conditional medium for 8 days before the fluorescence
intensity was measured. This study was approved by the ethics
committee of Xi’an Jiaotong University Medical School, China.
Cell proliferation assay
AlamarBlue assays were performed to evaluate the
proliferation of glioma cells according to the manufacturer’s
instructions (Invitrogen, Carlsbad, CA, USA). Glioma cells were
seeded on 24-transwell receiver plates at a density of
1.0×103 cells/well. For the coculture system,
∼1.0×105 pretreated HUVECs were seeded on each companion
insert. The fluorescence intensity of the glioma cells was measured
with a microplate reader (FLUOstar OPTIMA, BMG Labtech, Offenburg,
Germany) after an 8-h incubation with 10% alamarBlue reagent added
into each well.
Cell viability assay
Trypan blue exclusion tests were performed to assess
the cell viability following exposure to hypoxic conditions. HUVECs
were cultured on 24-well plates under various hypoxic conditions
(6, 2 and 1% O2) at a density of 1.0×105
cells/well with the addition of 10% trypan blue reagent
(Invitrogen). The HUVEC viability was then quantified by counting
the viable and dead cells using a hemocytometer at various time
points (12, 24, 36 and 48 h).
Gene transduction
Lentivirus vectors encoding shRNA against caspase 3,
caspase 6 and calcium-independent phospholipase A2
(iPLA2) were used for gene knockdown according to the
manufacturer’s instructions (Sigma, St. Louis, MO, USA). The
following sequences were used in this study: shCASP3 #1,
CCGGGTGGAATTGATGCGTGATGTTCTCG AGAACATCACGCATCAATTCCACTTTTT; shCASP3
#2, CCGGGCGAATCAATGGACTCTGGAACTCGAGTTCCA GAGTCCATTGATTCGCTTTTT;
shCASP6 #1, CCGGGT TAGGGTGAAGCATTATGGTCCGAGACCATAATGCTTC
ACCCTAACTTTTT; shCASP6 #2, CCGGGCTTTGTG
TGTGTCTTCCTGACTCGAGTCAGGAAGACACACACA AAGCTTTTT; shPLA2G6,
CCGGCCTACTTACTTC CGACCCAATCTCGAGATTGGGTCGGAAGTAAGTAGG TTTTTG.
Additionally, the pLEX lentiviral vector system (Open Biosystems,
Waltham, MA, USA) was used to deliver activated (ac)
iPLA2 into ECs according to manufacturer’s instructions.
A truncated version of mouse iPLA2 was amplified with
RT-PCR using the following primers: forward,
GACTAGTGCCACCATGCAGCACCAAGGACCTCTTC GACTG; reverse,
ATAAGAATGCGGCCGCGTCCACGA CCATCTTGCCCAG. Pfx polymerase (Invitrogen)
was used for the PCR amplification. The amplified fragment encoded
aa453–679 of murine iPLA2 (equivalent to aa514–733 of
human iPLA2) which has been demonstrated to be a
constitutively active caspase cleavage product (9,10). In
all cases, 293T cells were used to produce viable and recombinant
lentiviral vectors according to manufacturer’s instructions.
Western blot analysis
Immunoblotting was performed to analyze sample
lysates containing protease inhibitor cocktail (Sigma). The BCA
Protein Assay kit (Pierce-Thermo Scientific, Rockford, IL, USA) was
used to measure the protein concentrations and equal amounts of
proteins were loaded onto SDS-PAGE gels (NuPAGE-Invitrogen) for the
electrophoresis and transfer procedures. PVDF membranes
(Invitrogen) were then incubated overnight in a cold room with
multiple primary antibodies, including caspase 3 (Rabbit, 1:1,000;
Abcam, Cambridge, UK), caspase 6 (Rabbit, 1:1,000; Abcam),
iPLA2 (Rabbit, 1:500; Abcam) and β-actin (Rabbit,
1:1,000; Abcam). Signals were amplified using anti-rabbit secondary
antibody (Goat, horseradish peroxidase-conjugated, 1:2,000–1:4,000;
Abcam) followed by detection of enhanced chemiluminiscence.
ELISA
The prostaglandin E2 (PGE2)
production of HUVECs under various conditions was evaluated using a
PGE2 ELISA kit (Abcam). Pretreated or untreated HUVECs
were seeded on 6-well plates at a density of 1.0×105
cells/well. Cells were cultured with DMEM supplemented with 10%
FBS. After a 24-h incubation, the PGE2 concentrations in
the supernatants were measured according to the manufacturer’s
instructions.
Statistical analysis
Student’s t-tests and one-way ANOVA were performed
to analyze the data using SPSS 17.0 software (IBM, Armonk, NY,
USA). P<0.05 was considered to indicate statistically
significant differences.
Results
Growth stimulating effect of dying ECs on
glioma cells
To examine our hypothesis, a series of experiments
were performed to investigate the effects of dying vascular ECs on
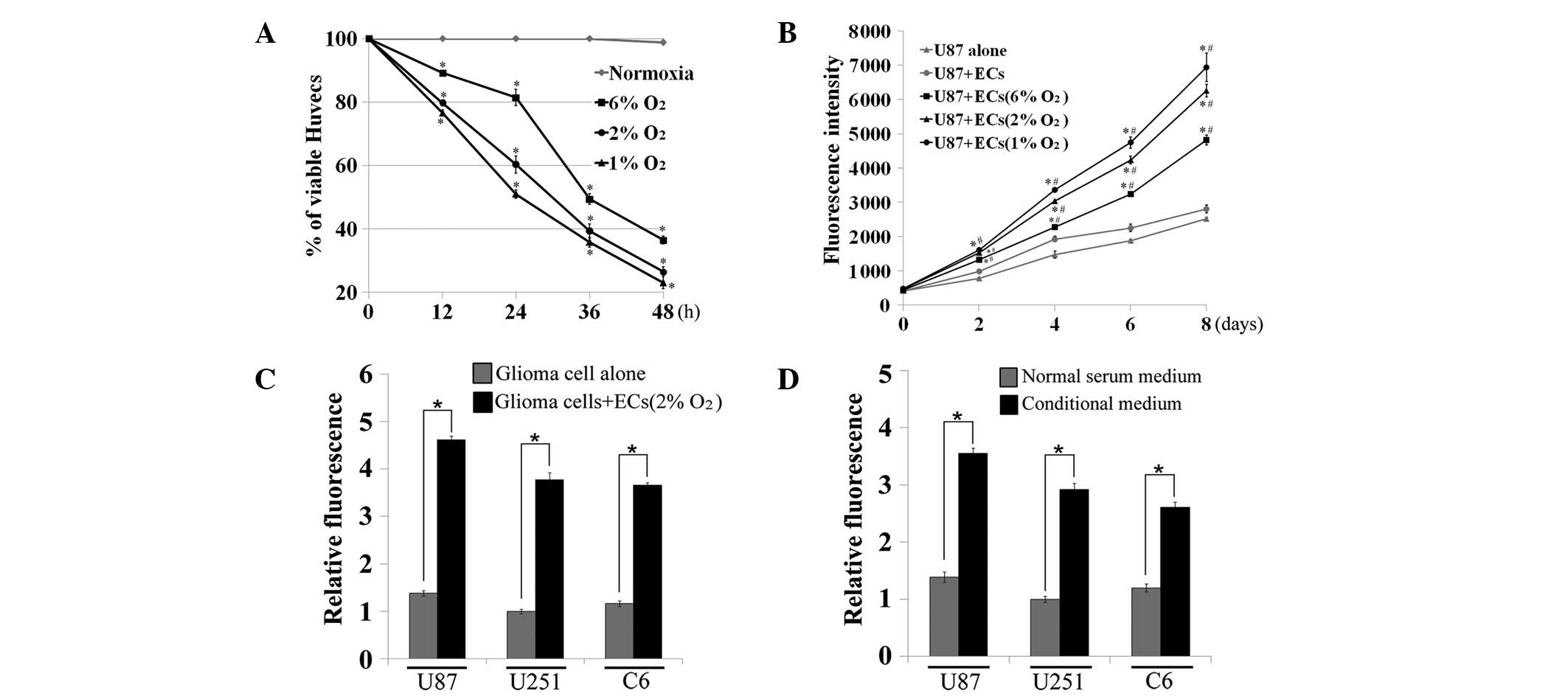
the proliferation of malignant glioma cells. First, there was a
significant decrease in EC viability following exposure to various
severe hypoxic conditions (6, 2 and 1% O2) at various
times, particularly 48 h (P<0.05, one-way ANOVA, Fig. 1A). U87MG cells were cocultured with
pretreated (hypoxia exposure for 48 h) or untreated ECs on
24-transwell plates for up to 8 days, then fluorescence intensity
was measured to evaluate U87MG cell proliferation rates. The
results showed that dying ECs had a more marked ability to
stimulate the growth of cocultured U87MG cells compared with normal
ECs or U87MG cells alone (P<0.05 vs. U87MG alone group;
P<0.05 vs. U87MG and EC coculture group; one-way ANOVA, between
days 2 and 8, Fig. 1B).
Furthermore, this effect occurred in a dose-dependent manner
whereby ECs pretreated with higher levels of hypoxia showed
enhanced growth promoting ability compared with groups with no
treatment or lower levels of hypoxic exposure. To support these
observations, three glioma cells lines were cocultured with dying
ECs (2% O2 exposure for 48 h) and the same growth
promoting effects as previously described were demonstrated
(P<0.05, t-test, Fig. 1C). Next,
the conditional medium was harvested from dying ECs and the growth
stimulating effect of this on three glioma cell lines was
investigated. The addition of the conditional medium increased the
glioma cell growth more than two-fold compared with normal serum
medium (P<0.05, t-test, Fig.
1D).
Caspase 3 regulated growth stimulating
signal in dying ECs
Emerging evidence has shown that caspase 3 is the
key regulator in compensatory proliferation during the wound
healing process (8). Thus we
hypothesized that the caspase family may also be involved in the
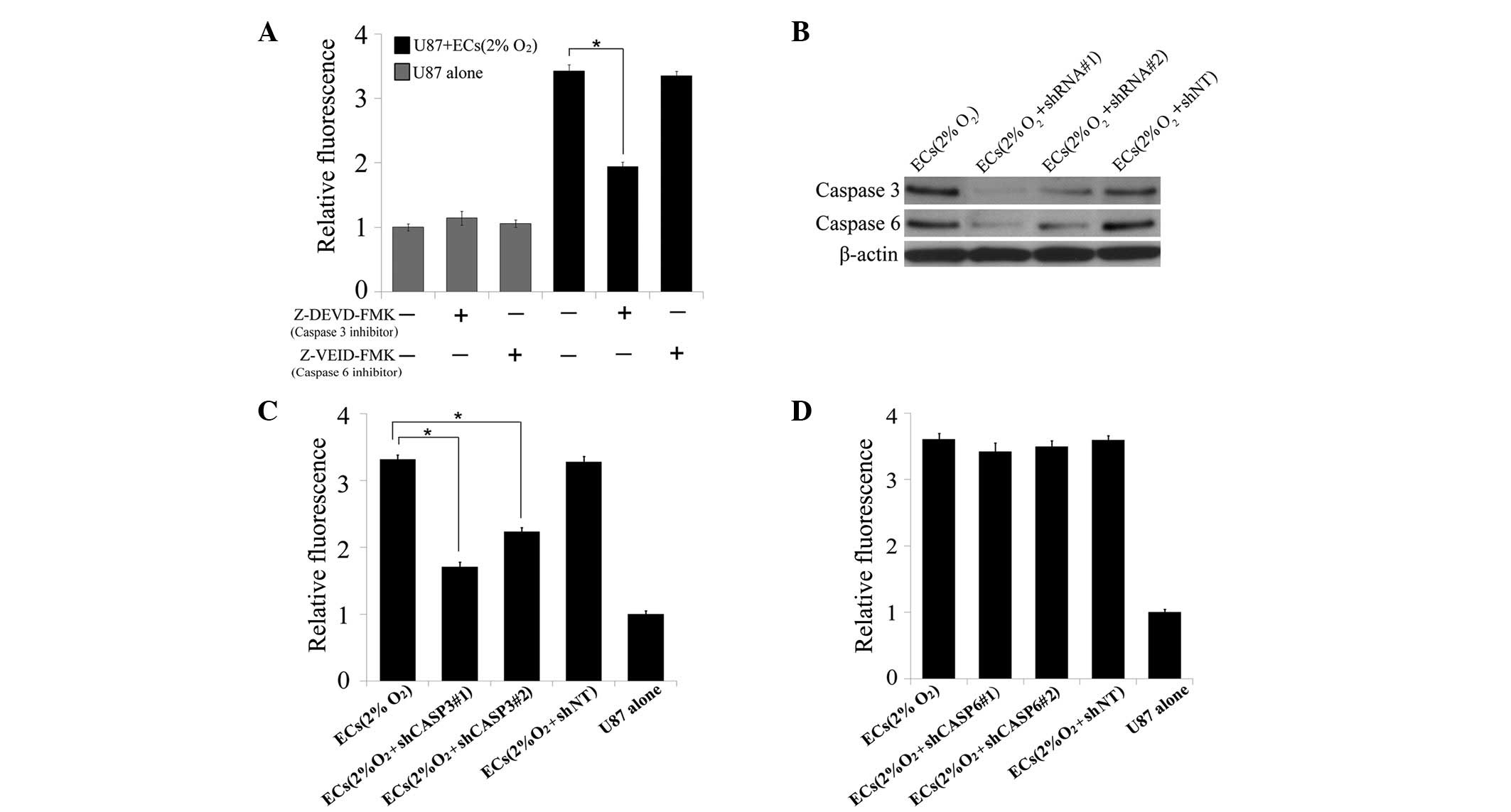
growth stimulating signal released from dying ECs. First, the
blocking effect of two specific caspase inhibitors on the growth
stimulating signals from dying ECs was examined. Notably, caspase 3
inhibitor (Z-DEVD-FMK, 100 μM), but not caspase 6 inhibitor
(Z-VEID-FMK, 100 μM), blocked the growth enhancing effect of
dying ECs on U87MG cells (P<0.05, t-test, Fig. 2A). For further confirmation, shRNA
against caspase 3 and caspase 6 was transduced into ECs which were
then subjected to hypoxic exposure. Western blot analysis was
performed to demonstrate the knockdown effect of caspase 3 and
caspase 6 in the ECs (Fig. 2B).
Knockdown of caspase 3 significantly blocked the growth stimulating
signal in ECs following severe hypoxic pretreatment (P<0.05,
t-test, Fig. 2C), but no effects
were observed in the caspase 6 knockdown group (Fig. 2D). These results indicate that
caspase 3 is a key regulator in the growth stimulating process.
Involvement of iPLA2 in
caspase 3-mediated growth stimulation of glioma cells
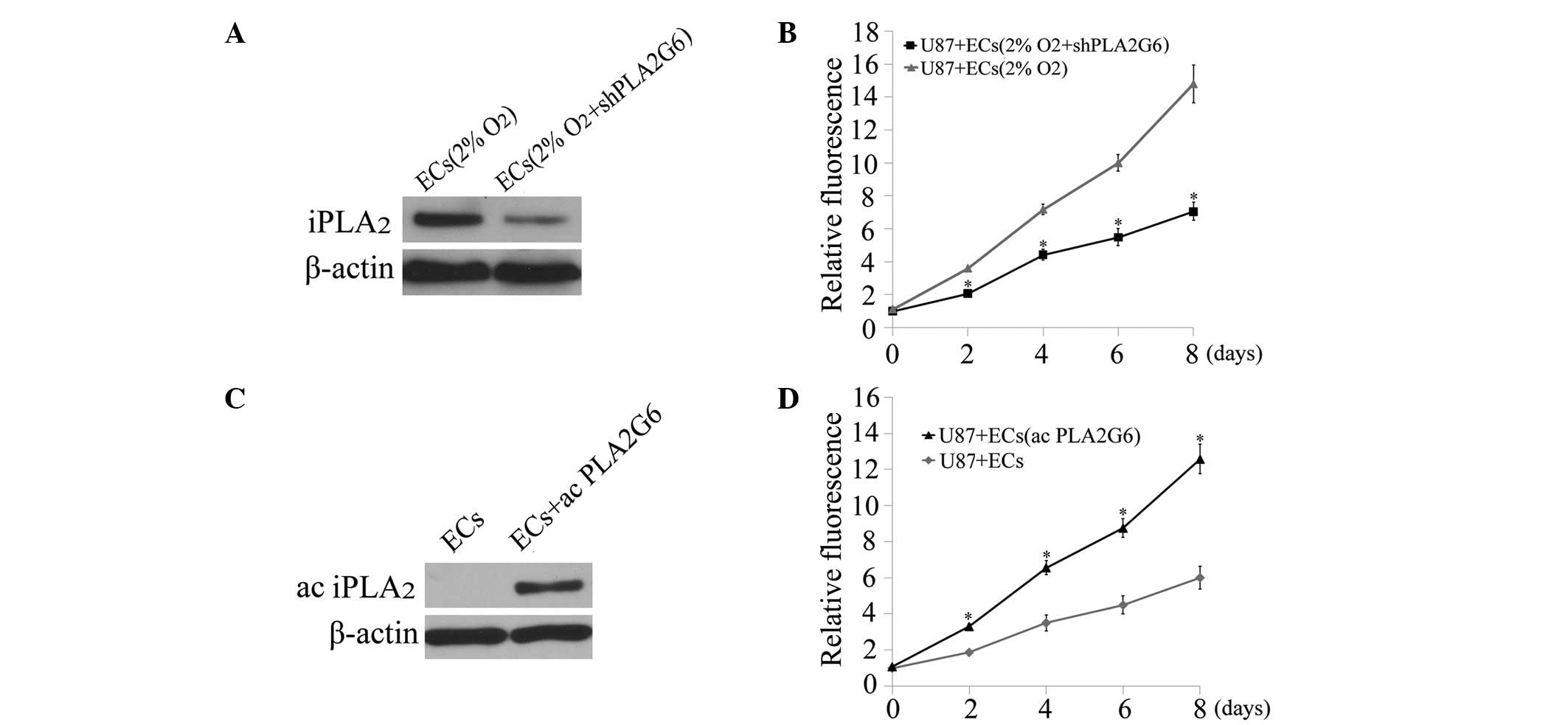
To further understand the mechanism of the growth
stimulating process, group 6 of the iPLA2 family encoded
by PLA2G6, a downstream target of caspase 3 (10), was investigated. First, shRNA
against PLA2G6 was transduced into ECs which were then subjected to
hypoxia exposure. Western blot analysis showed decreased levels of
the iPLA2 signal in dying ECs following PLA2G6 knockdown
(Fig. 3A). The effects of dying ECs
with or without PLA2G6 knockdown on the proliferation of U87MG
cells were then compared. A significant reduction in the growth
stimulating effect was observed in the PLA2G6 knockdown group
(P<0.05, t-test, Fig. 3B). To
further investigate the involvement of iPLA2 in the
growth stimulating process, an expression vector containing the
PLA2G6 gene was transduced into ECs to increase the PLA2G6
expression level (Fig. 3C). ECs
overexpressing PLA2G6 exhibited a more marked ability to promote
the proliferation of U87MG cells under coculture conditions
compared with normal ECs (P<0.05, t-test, between days 2 and 8,
Fig. 3D).
Important role of PGE2 in the
growth stimulating process
As previously described, it was observed that the
conditional medium from dying ECs also had the ability to
facilitate the growth of glioma cells, indicating the secretion of
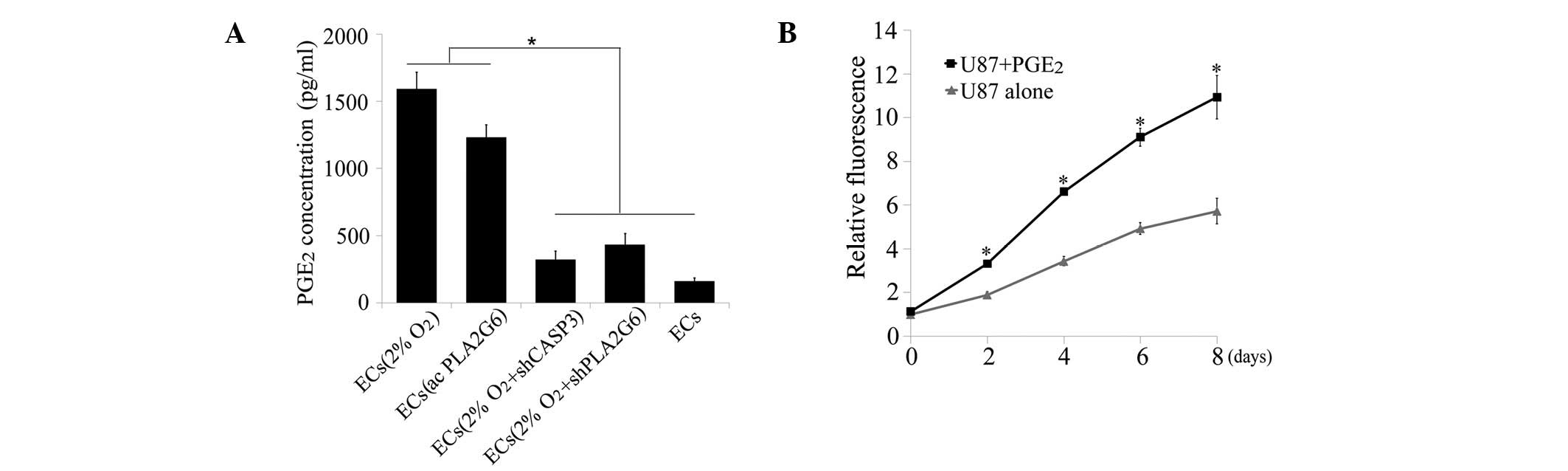
growth factors during this process. Consequently, the concentration
of PGE2, a downstream growth factor of
iPLA2(11), was measured
in the supernatants obtained from the various EC groups. Severe
hypoxic exposure and overexpression of PGE2 in ECs
significantly increased the production of PGE2 compared
with normal ECs (P<0.05, one-way ANOVA, Fig. 4A). By contrast, knockdown of caspase
3 or PLA2G6 blocked the signal that increased PGE2
production in dying ECs. Next, U87MG cells were treated with 1,500
pg/ml PGE2 added to the culture medium which
significantly promoted the growth of U87MG cells compared with no
treatment (P<0.05, t-test, between days 2 and 8, Fig. 4B).
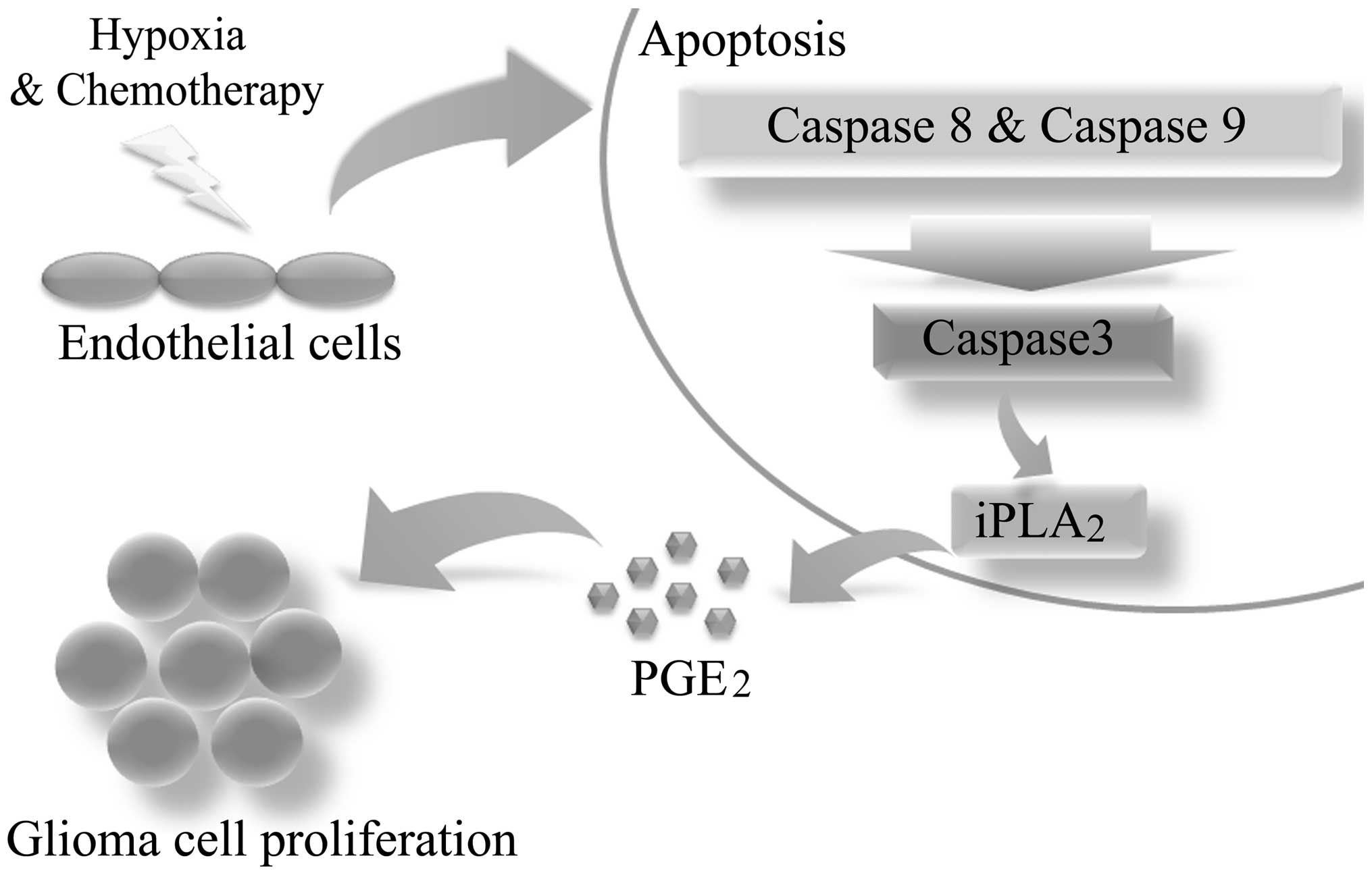
Overall, the present study has described the growth
stimulating ability of dying ECs surrounding malignant glioma
cells. Furthermore, caspase 3 was identified as the key regulator
of this process (Fig. 5).
Discussion
Previously, it was considered that apoptosis was an
isolated process in apoptotic cells which did not affect
neighboring cells (12,13). However, emerging evidence indicates
that dying cells are not inactive and have the ability to
accelerate the growth of surrounding cells, termed ‘compensatory
proliferation’ (14–16). In the present study, the presence of
dying ECs was observed to possess a clear growth promoting effect
on three glioma cell lines. Furthermore, this process was under the
control of caspase 3, an executor during cell apoptosis, but not
caspase 6. The gene iPLA2, a downstream target of
caspase 3, was reported to regulate the release of arachidonic acid
which is further modified into eicosanoids such as
PGE2(17). Additionally,
PGE2 is known to be an important growth factor and
regulator in cell biology (18,19).
Taken together, this evidence reveals the key role of the caspase
3-iPLA2-PGE2 signaling pathway in the growth
stimulation process, as demonstrated by in vitro gene
knockdown and overexpression. Although these results appear
contrary to the predominant view of apoptotic cells and caspase 3
function, the same phenomenon has recently been reported in other
types of cancer. For example, Huang et al noted that 4T1 (a
breast cancer cell line) and mouse embryonic fibroblast (MEF) cells
showed a marked growth promoting ability towards the surrounding
breast cancer cells when exposed to lethal doses of radiation
(20). In addition, caspase 3 was
demonstrated to be the key regulator in this tumor repopulation
model by in vitro and in vivo evidence.
Furthermore, the presence of compensatory
proliferation in dying ECs may aid in improving the understanding
of therapy resistance in malignant glioma. One example is that the
effect of anti-angiogenic agents, which mainly target vascular ECs
during tumor therapy, may be compromised by growth stimulating
signals released from dying ECs, according to the present findings.
This may be one of the potential reasons why it is so difficult to
obtain as high anti-angiogenic efficacy in clinical cases as is
expected (21,22). Furthermore, there have also been
several reports that certain tumors became more aggressive
following treatment with angiogenic inhibitors in animal models,
indicating the potential role of dying ECs during tumor progression
(23,24). Thus, more attention should be paid
to the compensatory signals from dying ECs and more in vivo
experiments should be performed in the future to further study this
mechanism.
Finally, the present study described a model of
compensatory proliferation in tumors that was not only performed by
damaged cancer cells or their supporting stromal cells, but also
dying ECs. This method of compensatory proliferation was shown to
be performed by the growth stimulating ability of ECs towards
surrounding tumor cells via a caspase 3-mediated pathway. This
phenomenon demonstrates the importance of blocking compensatory
signals from dying cells during tumor therapy. Additionally,
blocking caspase 3-mediated signaling pathways in combination with
current tumor therapeutic strategy may be a promising approach for
improving the dismal prognosis of malignant tumors.
Acknowledgements
The present study was supported by the
Key Clinical Program of the Ministry of Health Grant in China
(2010–2012). Dr Ping Mao was supported by the China Scholarship
Council.
References
|
1
|
Franses JW and Edelman ER: The evolution
of endothelial regulatory paradigms in cancer biology and vascular
repair. Cancer Res. 71:7339–7344. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Calabrese C, Poppleton H, Kocak M, et al:
A perivascular niche for brain tumor stem cells. Cancer Cell.
11:69–82. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Carmeliet P and Jain RK: Angiogenesis in
cancer and other diseases. Nature. 407:249–257. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Carmeliet P and Jain RK: Molecular
mechanisms and clinical applications of angiogenesis. Nature.
473:298–307. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Sakurai T and Kudo M: Signaling pathways
governing tumor angiogenesis. Oncology. 81(Suppl 1): 24–29. 2011.
View Article : Google Scholar
|
|
6
|
Ribatti D: Cancer stem cells and tumor
angiogenesis. Cancer Lett. 321:13–17. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Garcia-Barros M, Paris F, Cordon-Cardo C,
et al: Tumor response to radiotherapy regulated by endothelial cell
apoptosis. Science. 300:1155–1159. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Li F, Huang Q, Chen J, et al: Apoptotic
cells activate the ‘phoenix rising’ pathway to promote wound
healing and tissue regeneration. Sci Signal. 3:ra132010.
|
|
9
|
Zhao X, Wang D, Zhao Z, et al:
Caspase-3-dependent activation of calcium-independent phospholipase
A2 enhances cell migration in non-apoptotic ovarian
cancer cells. J Biol Chem. 281:29357–29368. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lauber K, Bohn E, Kröber SM, et al:
Apoptotic cells induce migration of phagocytes via
caspase-3-mediated release of a lipid attraction signal. Cell.
113:717–730. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Cohen D, Papillon J, Aoudjit L, Li H,
Cybulsky AV and Takano T: Role of calcium-independent phospholipase
A2 in complement-mediated glomerular epithelial cell
injury. Am J Physiol Renal Physiol. 294:F469–F479. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Böhm I and Schild H: Apoptosis: the
complex scenario for a silent cell death. Mol Imaging Biol. 5:2–14.
2003.
|
|
13
|
Bär PR: Apoptosis - the cell’s silent
exit. Life Sci. 59:369–378. 1996.
|
|
14
|
Chera S, Ghila L, Dobretz K, et al:
Apoptotic cells provide an unexpected source of Wnt3 signaling to
drive hydra head regeneration. Dev Cell. 17:279–289.
2009.PubMed/NCBI
|
|
15
|
Fan Y and Bergmann A: Distinct mechanisms
of apoptosis-induced compensatory proliferation in proliferating
and differentiating tissues in the Drosophila eye. Dev Cell.
14:399–410. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Hwang JS, Kobayashi C, Agata K, Ikeo K and
Gojobori T: Detection of apoptosis during planarian regeneration by
the expression of apoptosis-related genes and TUNEL assay. Gene.
333:15–25. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Balsinde J, Pérez R and Balboa MA:
Calcium-independent phospholipase A2 and apoptosis.
Biochim Biophys Acta. 1761:1344–1350. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lawlor G, Doran PP, MacMathuna P and
Murray DW: MYEOV (myeloma overexpressed gene) drives colon cancer
cell migration and is regulated by PGE2. J Exp Clin
Cancer Res. 29:812010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Rasmuson A, Kock A, Fuskevåg OM, et al:
Autocrine pros-taglandin E2 signaling promotes tumor
cell survival and proliferation in childhood neuroblastoma. PLoS
One. 7:e293312012.
|
|
20
|
Huang Q, Li F, Liu X, et al: Caspase
3-mediated stimulation of tumor cell repopulation during cancer
radiotherapy. Nat Med. 17:860–866. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Bergers G and Hanahan D: Modes of
resistance to anti-angiogenic therapy. Nat Rev Cancer. 8:592–603.
2008. View
Article : Google Scholar : PubMed/NCBI
|
|
22
|
Rini BI and Atkins MB: Resistance to
targeted therapy in renal-cell carcinoma. Lancet Oncol.
10:992–1000. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Pàez-Ribes M, Allen E, Hudock J, et al:
Antiangiogenic therapy elicits malignant progression of tumors to
increased local invasion and distant metastasis. Cancer Cell.
15:220–231. 2009.PubMed/NCBI
|
|
24
|
Ebos JM, Lee CR, Cruz-Munoz W, Bjarnason
GA, Christensen JG and Kerbel RS: Accelerated metastasis after
short-term treatment with a potent inhibitor of tumor angiogenesis.
Cancer Cell. 15:232–239. 2009. View Article : Google Scholar : PubMed/NCBI
|