Introduction
Lactoferrin (LF) is a single-chain non-haeme
iron-binding glycoprotein with a molecular weight of ∼80 kDa,
consisting of ∼700 amino acids with a high degree of homology
between species (1). The
concentration of LF is high in milk, mainly in colostrum, although
it has been found in other body fluids and secretions such as blood
plasma, tears, saliva, urine, bile, semen and amniotic fluid
(2–4).
LF has a wide range of biological activities,
including antimicrobial properties, improvement of iron status,
anti-inflammation, development of immune function and promotion of
cell proliferation during carcinogenesis (5,6).
Using immunohistochemistry, the distribution of LF
has been investigated in normal human fetal and adult tissues
including the stomach, kidney, lung, pancreas, liver, bone marrow
and skin (7,8). More recently, the immunohistochemical
distribution of LF has been analysed in human embryonic, fetal and
adult bone and cartilaginous tissues (9), in order to investigate whether LF may
be involved in the growth and differentiation of the human
skeleton, similar to that suggested in murine models as well as in
cell culture lines (10–12). In addition, our research group has
also evaluated the immuno histochemical presence of LF in
pathological neoplastic bone and cartilage samples (13,14).
LF immunoreactivity was revealed in chondroblastomas, chondromyxoid
fibromas, giant cell tumours, osteoid osteomas, myelomas and
adamantinomas; while no LF immunoexpression was detected in
enchondromas, osteochondromas, ossifying fibromas, chondrosarcomas
or osteosarcomas (13–15).
As no data regarding the distribution of LF in bone
metastases of cancers that have originated from different organs
are available at present, we set out to analyse the
immunohistochemical pattern of LF in a cohort of these samples as
well as in the corresponding primary neoplasms using a monoclonal
antibody against LF.
Materials and methods
Specimens
LF immunoexpression was investigated in 25 specimens
of human bone metastatic lesions obtained through curettage or
surgery from an equal number of patients (16 females, 9 males; mean
ages, 64 and 92 years, respectively; age range, 28–85 years). Data
concerning the site of occurrence of the metastases as well as
surgical samples of the primary corresponding carcinomas were
obtained from the files at the Department of Human Pathology,
University of Messina, Messina, Italy. The primary carcinoma sites
included breast (8 cases), prostate (4 cases), kidney (4 cases),
lung (3 cases), colon-rectum (2 cases) and uterus (4 cases).
Preparation of specimens
All samples were fixed in 10% neutral formalin for
24–36 h at room temperature (RT), and then embedded in paraffin at
56°C. The bone metastatic specimens were subjected to a
decalcification procedure performed using formic acid (5%) or
ethylenediamine-tetraacetic acid (EDTA; 5%, pH 7.4) for ≤12–24 h,
depending on the size of mineralised samples. From each tissue
block, 4-μm sections were stained with haematoxylin and
eosin (H&E) for microscopic evaluation. Parallel sections were
cut and mounted on silane-coated glass, then dewaxed in xylene and
rehydrated in graded ethanols. Antigen retrieval was performed
prior to the addition of the primary antibody, by heating slides
placed in 0.01 M citrate buffer at pH 6.0 in a microwave oven (750
W) for three 5-min cycles.
Immunohistochemistry
For the immunohistochemical study, sections were
treated in a moist chamber with: i) 0.1% H2O2
in methanol for 30 min at RT, to block the intrinsic peroxidase
activity; ii) normal sheep serum to prevent non-specific adherence
of serum proteins; iii) mouse monoclonal primary antibody
(anti-human) against LF [clone 1A1; working dilution (wd), 1:75;
Biodesign International, Inc., Saco, ME, USA] for 60 min at RT; iv)
sheep anti-mouse immunoglobulin antiserum (wd, 1:25; Behring
Institute, Marburg, Germany) for 30 min at RT; v) mouse
anti-horseradish peroxidase-antiperoxidase complexes (wd, 1:25;
DakoCytomation, Copenhagen, Denmark) for 30 min at RT. To reveal
peroxidase activity, the sections were incubated in the dark for 10
min with 100 mg 3,3′-diaminobenzidine tetrahydrochloride (Sigma,
St. Louis, MO, USA) in 200 ml 0.03% hydrogen peroxide in
phosphate-buffered saline (PBS) solution. The nuclear
counterstaining was performed using Mayer’s haemalum solution.
Renal tubular structures within normal kidney
samples and portions of the parotid gland were utilised as
LF-positive controls. In addition, the LF immunoreactivity
demonstrated in granules of polymorphonuclear neutrophils inside
the neoplastic lesions was utilised as additional positive control.
Finally, to test the inter-run variability of LF immunostaining,
the same LF-positive parotid sample was utilised in every run. To
test the of LF immunoreaction in order to omit the possibility of
non-specific reaction, serial sections of each affected specimen
were tested by replacing the specific antiserum with either PBS,
normal rabbit serum, or by absorption with an excess of purified
human LF from human liver and spleen (Sigma) as well as with
pre-absorbed primary antibody; the results obtained were
negative.
Microscopy
The analysis of immunostained sections was estimated
by light micro scopy using ×20 and ×40 objective lenses and a ×10
eyepiece. Two pathologists used a double-headed microscope to
perform the assessment of LF immunostained sections on a consensus
basis. The percentage of stained neoplastic cells (area of staining
positivity, ASP) was graded as follows: 0, no staining; 1,
>0–5%; 2, >5–50% and 3, >50%. The intensity of staining
(IS; weak, 1; moderate, 2; strong, 3) was also assessed. Then an LF
intensity distribution (ID) score was calculated for each case by
multiplying the values of the ASP and the IS, according to that
described by Tuccari et al(16).
Statistical analysis
The correlations between LF immuno-expression and
the clinical data (age and gender of patients and site of the
lesion) were investigated using either the χ2 or the
Fisher’s exact test, as appropriate. Moreover, the correlation
between the LF immunoreactivity pattern in primary carcinomas and
the corresponding metastatic bone samples was analysed by a
Spearman’s rank correlation test. P<0.05 was considered to
indicate a statistically significant difference. Data were analysed
using the Statistical Package for the Social Sciences (SPSS)
software, version 6.1.3 (SPSS, Inc., Chicago, IL, USA).
Results
Routinely stained H&E sections exhibited a good
morphology, confirming the histopathological diagnosis of all
cases, either in the primary neoplastic lesions or in the bone
neoplastic deposits. Clinicopathological and immunohistochemical
data for LF relative to the 25 analysed bone metastatic samples as
well as the corresponding primary carcinomas are listed in Table I.
 | Table IClinicopathological and LF
immunohistochemical data concerning bone metastases. |
Table I
Clinicopathological and LF
immunohistochemical data concerning bone metastases.
| Case no. | Gender | Age | Primary site of
neoplasms | Histotype of
carcinoma | Grading | Site of bone
metastases | LF-ASP | LF-IS | LF-ID score |
|---|
| 1 | F | 58 | Breast | Medullary | - | Femur | 0 | 0 | 0 |
| 2 | M | 59 | Prostate | Cribriform | G2 | Femur | 1 | 2 | 2 |
| 3 | F | 54 | Breast | Ductal
invasive | G3 | Humerus | 0 | 0 | 0 |
| 4 | F | 80 | Breast | Ductal
invasive | G2 | Femur | 2 | 1 | 2 |
| 5 | F | 28 | Uterus | Endometrioid | G2 | Vertebra | 2 | 2 | 4 |
| 6 | F | 80 | Colon-rectum | Adenocarcinoma | G2 | Femur | 1 | 2 | 2 |
| 7 | F | 55 | Breast | Ductal
invasive | G2 | Femur | 1 | 2 | 2 |
| 8 | M | 56 | Lung | Small cell | - | Sternum | 0 | 0 | 0 |
| 9 | F | 70 | Lung | Adenocarcinoma | G3 | Femur | 0 | 0 | 0 |
| 10 | F | 60 | Breast | Lobular
invasive | - | Femur | 0 | 0 | 0 |
| 11 | M | 61 | Lung | Small cell | - | Fibula | 0 | 0 | 0 |
| 12 | M | 74 | Kidney | Clear cell | G2 | Femur | 0 | 0 | 0 |
| 13 | F | 69 | Uterus | Serous | G3 | Humerus | 0 | 0 | 0 |
| 14 | F | 62 | Breast | Ductal
invasive | G1 | Vertebra | 2 | 2 | 4 |
| 15 | F | 69 | Colon-rectum | Adenocarcinoma | G3 | Femur | 0 | 0 | 0 |
| 16 | F | 76 | Uterus | Endometrioid | G2 | Femur | 1 | 1 | 1 |
| 17 | M | 75 | Prostate |
Undifferentiated | G3 | Pelvis | 0 | 0 | 0 |
| 18 | F | 75 | Breast | Ductal
invasive | G3 | Vertebra | 0 | 0 | 0 |
| 19 | M | 70 | Kidney | Chromophobe | G2 | Femur | 2 | 2 | 4 |
| 20 | M | 85 | Prostate | Adenocarcinoma | G1 | Vertebra | 2 | 2 | 4 |
| 21 | F | 58 | Kidney | Clear cell | G2 | Vertebra | 1 | 1 | 1 |
| 22 | M | 71 | Prostate | Adenocarcinoma | G2 | Femur | 1 | 2 | 2 |
| 23 | F | 52 | Breast | Lobular
invasive | - | Femur | 0 | 0 | 0 |
| 24 | F | 67 | Uterus |
Non-endometrioid | G3 | Pelvis | 0 | 0 | 0 |
| 25 | M | 59 | Kidney | Clear cell | G3 | Fibula | 0 | 0 | 0 |
LF immunostaining with a variable ID score was
encountered in 11/25 (44%) metastatic lesions; LF was identified in
7/16 (43.8%) female and 4/9 (44.4%) male patients. In particular,
LF immunoreactivity was identified with a percentage ranging from
50 to 75% of the cases of bone metastases due to prostatic
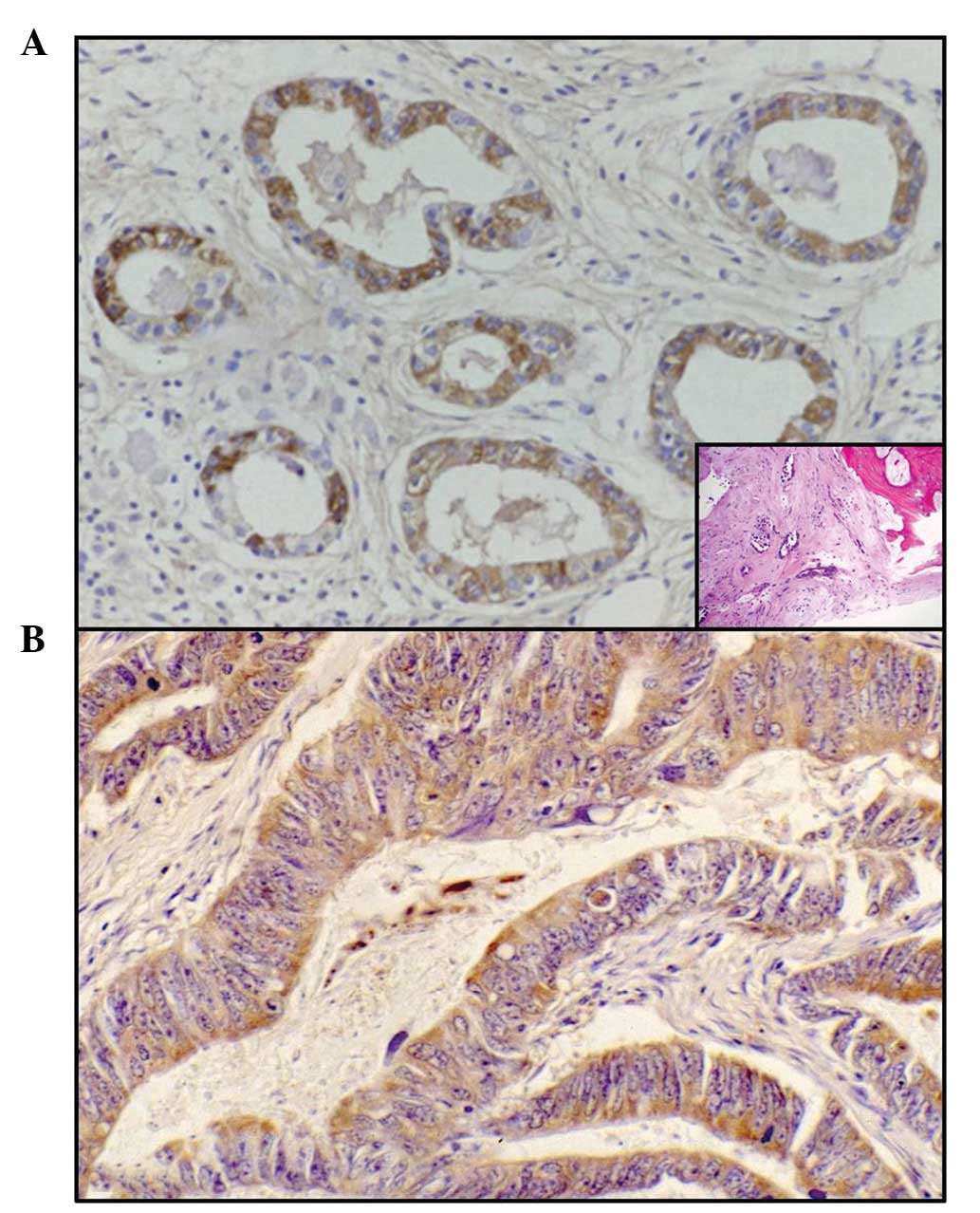
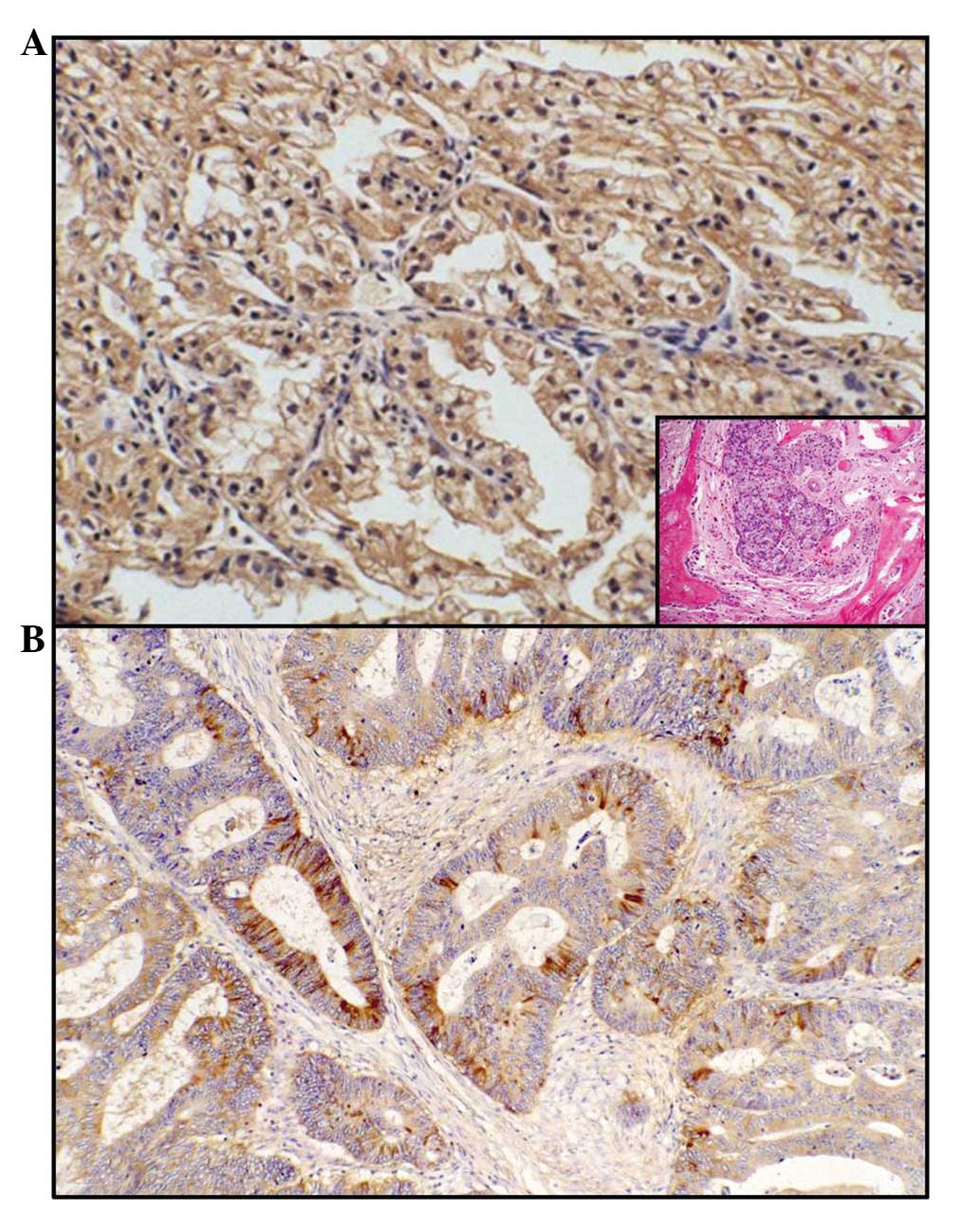
(Fig. 1A, inset), uterine (Fig. 1B), renal (Fig. 2A, inset) and colorectal (Fig. 2B) carcinomas (Table I). Additionally, the positivity was
decreased in breast carcinomas (37.5%) and was completely absent in
lung cancers (Table I). The
immunostaining was mainly localised in the cytoplasm of the
neoplastic elements and occasionally in the nuclei of the same
cells. No differences in LF-ID score were observed between the
primary and metastatic neoplastic localisations with an equivalent
LF immunoreactivity, either regarding the intensity or the
percentage of stained cells.
LF was evident in renal tubular structures, parotid
ductular/acinar portions and in granules of polymorphonuclear
neutrophils utilised as positive controls.
No correlations were observed between LF
immunoexpression and the other parameters investiaged, including
the age and gender of the patients and the localisation of
neoplastic metastatic lesions in bones.
Discussion
A number of studies have demonstrated promising
results in the potential use of LF for the improvement of bone
health (12,17–20).
In particular, LF stimulates the proliferation, differentiation and
survival of osteoblasts (20), as
well as significantly increasing the mineral apposition rate and
bone formation, as demonstrated by the assessment of dynamic
histomorphometric indices (12).
Consequently, it has been suggested that LF may be useful in
pathological states of reduced bone density when used either
systemically or locally.
As part of a series of studies concerning the
immunohistochemical distribution pattern of LF in human neoplasms
(21), we have previously
investigated this distribution in pathological primary neoplastic
bone and cartilage samples, as well as in the corresponding human
normal embryo-fetal bone and cartilage tissues (9,13). The
observed heterogeneous distribution of LF in tumours, as well as
its independence from benign and malignant characteristics, appear
to contrast with the elsewhere hypothesised role of LF as oncofetal
marker (15). The most aggressive
bone tumours, such as osteosarcomas and chondrosarcomas, were
consistently observed to be unreactive for LF; while the pattern of
LF expression was mainly evident in the early phases of bone
growth, suggesting an important role for LF as a bone growth
regulator in the early phases of skeletal development, particularly
in endochondral ossification (12,15).
Metastatic deposits in bones from carcinomas arising
from breast, colon, endometrium, kidney, lung and prostate are
considered to be a key stage in the natural history of these
neoplasms, although to date no data regarding the
immunohistochemical distribution of LF have been available in the
literature. In the current study, we immunohistochemically detected
a variable ID score for LF in the cytoplasm of 11/25 (44%)
metastatic neoplastic bone lesions as well as in the corresponding
primary carcinomas. Occasionally, the site of LF immuno staining
was appreciable both in the nucleus and cytoplasm, and this
co-localisation was expected, as LF has also been revealed in the
nucleoli and LF is speculated to be involved in ribosomal
biogenesis (21,22). With regard to the site of the
primary carcinomas, LF immunoreactivity was found with a percentage
ranging from 50 to 75% of bone metastases due to colorectal,
uterine, prostatic and renal carcinomas. In addition, the
positivity was decreased in breast carcinomas (37.5%) and was
completely absent in lung cancers. In these primary neoplastic
conditions, previous studies by our research group have
demonstrated a similar variable percentage of immuno expression of
LF (23–26). In detail, a progressive increase of
LF immunostaining was encountered when moving from endometrial
adenocarcinomas (61%) (25) and
renal cell carcinomas (62.5%) (26)
to well-differentiated prostatic adenocarcinomas (66%) (23), and finally to adenocarcinomas and
colloid colorectal carcinomas (80%) (24). The most likely explanation for the
negative LF immunoreactivity observed in a number of cases of the
above mentioned cohorts of tumours has been correlated with
undifferentiated or less differentiated variants of carcinomas
(23–26). Occasional and slight LF staining has
been found in isolated cells of undifferentiated prostatic
carcinomas (23), while only well-
and moderately differentiated colonic carcinomas exhibited a strong
LF reaction. Furthermore, a significantly higher LF-ID score was
evident in the endometrioid type in comparison to the
non-endometrioid type carcinomas of the uterus (25). In addition, significant differences
in the LF-ID score were found among clear cell renal carcinomas
(CCC) and other non-CCC variants, the former exhibited a lower
score (26). By contrast, the
positive rate of LF in breast carcinoma has been identified with a
large variability, ranging from 7.5 to 42% of cases (27,28).
However, LF was more often observed in low-grade ductal carcinomas
with positive estrogen/progesterone receptors, confirming a
decrease in LF immunostaining in less differentiated and more
aggressive breast carcinomas (27–29).
Therefore, LF may be a potential marker for glandular or acinar
differentiation, similar to that previously observed in other
malignancies (28,30,31).
No data concerning LF immunodistribution in primary and metastatic
lung cancer are currently available in literature.
The origin of LF in human malignant primary and
metastatic tumours has not yet been fully elucidated. It is well
known that LF has a high affinity for iron, which is considered to
be an essential nutrient for cells that are dividing rapidly, such
as tumour cells, taking part in various metabolic processes
(including oxydative phosphorylation and RNA/DNA synthesis)
(32,33). Therefore, neoplastic elements may
produce LF in order to provide a greater amount of iron available
for their turnover, as we have previously suggested (21,24,26,34,35).
Alternatively, the localisation of LF in malignant cells may not
reflect an intracellular synthesis, but rather the degree of
transmembranous iron transfer as the consequence of defective or
functionally impaired LF-receptors already documented on the
surface of target cells as well as in human neoplastic cell lines
(36,37). It has been suggested that LF is
involved in the regulation of certain important processes, such as
the cell cycle and cell death, resistance to carcinogenesis and the
development of metastases (26,38).
Other potential mechanisms have also been suggested with regard to
the role of LF in the process of human carcinogenesis. These
include induction of programmed cell death, prevention of
angiogenesis and regulation of cell cycle protein expression
(39,40). LF is able to trigger the apoptotic
process by the activation of caspase-3 and -8 as well as the FAS
signaling pathway (41,42). By contrast, LF has also been
demonstrated to inhibit tumour-initiated angiogenesis in
vitro and in vivo, possibly by blocking endothelial
function and inducing IL-18 production (39,43,44).
In addition, it has been demonstrated that LF promoted growth
arrest either at the G1-S transition in breast cancer cells
(43) as well as at the G0-G1
checkpoint in oral and neck cancer cells (44). However, regardless of the mechanism
of action of LF in human malignant tumours, we have identified LF
immunohistochemical reproducibility at primary and metastatic
sites. Therefore, we hypothesise that the appearance of LF in
native neoplastic carcinomatous clones is maintained in secondary
bone metastatic deposits. However, additional investigations are
required, mainly regarding the potential for new applications of LF
in cancer treatment, due to its nutraceutical function and its
ability to potentiate chemotherapy.
References
|
1
|
González-Chávez SA, Arévalo-Gallegos S and
Rascón-Cruz Q: Lactoferrin: structure, function and applications.
Int J Antimicrob Agents. 33:301.e1–e.8. 2009.
|
|
2
|
Steijns JM and van Hooijdonk AC:
Occurrence, structure, biochemical properties and technological
characteristics of lactoferrin. Br J Nutr. 84(Suppl 1): S11–S17.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
van der Strate BW, Beljaars L, Molema G,
Harmsen MC and Meijer DK: Antiviral activities of lactoferrin.
Antiviral Res. 52:225–239. 2001.
|
|
4
|
Öztaş Yeşim ER and Özgüneş N: Lactoferrin:
a multifunctional protein. Adv Mol Med. 1:149–154. 2005.
|
|
5
|
Pierce A, Legrand D and Mazurier J:
Lactoferrin: a multifunctional protein. Med Sci. 25:361–369.
2009.
|
|
6
|
de Mejia EG and Dia VP: The role of
nutraceutical proteins and peptides in apoptosis, angiogenesis, and
metastasis of cancer cells. Cancer Metastasis Rev. 29:511–528.
2010.PubMed/NCBI
|
|
7
|
Mason DY and Taylor CR: Distribution of
transferrin, ferritin, and lactoferrin in human tissues. J Clin
Pathol. 31:316–327. 1978. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Reitamo S, Konttinen YT, Dodd S and
Adinolfi M: Distribution of lactoferrin in human fetal tissues.
Acta Paediatr Scand. 70:395–398. 1981. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ieni A, Barresi V, Grosso M and Tuccari G:
Immunohistochemical evidence of lactoferrin in human embryo-fetal
bone and cartilage tissues. Cell Biol Int. 34:845–849. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Cornish J: Lactoferrin promotes bone
growth. Biometals. 17:331–335. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Naot D, Grey A, Reid IR and Cornish J:
Lactoferrin a novel bone growth factor. Clin Med Res. 3:93–101.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Cornish J and Naot D: Lactoferrin as an
effector molecule in the skeleton. Biometals. 23:425–430. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ieni A, Barresi V, Grosso M, Rosa MA and
Tuccari G: Lactoferrin immuno-expression in human normal and
neoplastic bone tissue. J Bone Miner Metab. 27:364–371. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ieni A, Barresi V, Grosso M, Rosa MA and
Tuccari G: Immunolocalization of lactoferrin in cartilage-forming
neoplasms. J Orthop Sci. 14:732–737. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ieni A, Barresi V, Grosso M, Speciale G,
Rosa MA and Tuccari G: Does lactoferrin behave as an
immunohistochemical oncofetal marker in bone and cartilage human
neoplasms? Pathol Oncol Res. 17:287–293. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Tuccari G, Villari D, Giuffrè G, Simone A,
Squadrito G, Raimondo G and Barresi G: Immunohistochemical evidence
of lactoferrin in hepatic biopsies of patients with viral or
cryptogenetic chronic liver disease. Histol Histopathol.
17:1077–1083. 2002.PubMed/NCBI
|
|
17
|
Blais A, Malet A, Mikogami T, Martin-Rouas
C and Tomé D: Oral bovine lactoferrin improves bone status of
ovariectomized mice. Am J Physiol Endocrinol Metab. 296:1281–1288.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Guo HY, Jiang L, Ibrahim SA, Zhang L,
Zhang H, Zhang M and Ren FZ: Orally administered lactoferrin
preserves bone mass and microarchitecture in ovariectomized rats. J
Nutr. 139:958–964. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Takayama Y and Mizumachi K: Effect of
lactoferrin-embedded collagen membrane on osteogenic
differentiation of human osteoblast-like cells. J Biosci Bioeng.
107:191–195. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Naot D, Chhana A, Matthews BG, Callon KE,
Tong PC, Lin JM, Costa JL, Watson M, Grey AB and Cornish J:
Molecular mechanisms involved in the mitogenic effect of
lactoferrin in osteoblasts. Bone. 49:217–224. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Tuccari G and Barresi G: Lactoferrin in
human tumours: immunohistochemical investigations during more than
25 years. Biometals. 24:775–784. 2011.PubMed/NCBI
|
|
22
|
Penco S, Scarfì S and Giovine M:
Identification of an import signal for, and the nuclear
localization of, human lactoferrin. Biotechnol Appl Biochem.
34:151–159. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Barresi G and Tuccari G: Lactoferrin in
benign hypertrophy and carcinomas of the prostatic gland. Virchows
Arch A Pathol Anat Histopathol. 403:59–66. 1984. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Tuccari G, Rizzo A, Crisafulli C and
Barresi G: Iron-binding proteins in human colorectal adenomas and
carcinomas: an immunocytochemical investigation. Histol
Histopathol. 7:543–547. 1992.PubMed/NCBI
|
|
25
|
Giuffrè G, Arena F, Scarfì R, Simone A,
Todaro P and Tuccari G: Lactoferrin immunoexpression in endometrial
carcinomas: relationships with sex steroid hormone receptors (ER
and PR), proliferation indices (Ki-67 and AgNOR) and survival.
Oncol Rep. 16:257–263. 2006.PubMed/NCBI
|
|
26
|
Giuffrè G, Barresi V, Skliros C, Barresi G
and Tuccari G: Immunoexpression of lactoferrin in human sporadic
renal cell carcinomas. Oncol Rep. 17:1021–1026. 2007.PubMed/NCBI
|
|
27
|
Wurster K, Heberling D and Rapp W:
Carcinoembryogenic antigen (CEA) and lactoferrin (LF) in benign and
malignant disease of the breast. A contribution to the
immuno-histological demonstration of marker substances.
Geburtshilfe Frauenheilkd. 40:412–422. 1980.(In German).
|
|
28
|
Charpin C, Lachard A, Pourreau-Schneider
N, et al: Localization of lactoferrin and nonspecific
cross-reacting antigen in human breast carcinomas. An
immunohistochemical study using the avidin-biotin-peroxidase
complex method. Cancer. 55:2612–2617. 1985. View Article : Google Scholar
|
|
29
|
Wrba F, Reiner A, Markis-Ritzinger E,
Holzner JH, Reiner G and Spona J: Prognostic significance of
immunohistochemical parameters in breast carcinomas. Pathol Res
Pract. 183:277–283. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Caselitz J, Jaup T and Seifert G:
Lactoferrin and lysozyme in carcinomas of the parotid gland.
Virchows Arch A Pathol Anat Histol. 394:61–73. 1981. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Cabaret V, Vilain MO, Delobelle-Deroide A
and Vanseymortier L: Immunohistochemical demonstration of
ceruloplasmin and lactoferrin in a series of 59 thyroid tumors. Ann
Pathol. 12:347–352. 1992.(In French).
|
|
32
|
Weinberg ED: Iron withholding: a defense
against infection and neoplasia. Physiol Rev. 64:65–102.
1984.PubMed/NCBI
|
|
33
|
Ye XY, Wang HX, Liu F and Ng TB:
Ribonuclease, cell-free translation-inhibitory and superoxide
radical scavenging activities of the iron-binding protein
lactoferrin from bovine milk. Int J Biochem Cell Biol. 32:235–241.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Tuccari G, Rossiello R and Barresi G: Iron
binding proteins in gallbladder carcinomas. An immunocytochemical
investigation. Histol Histopathol. 12:671–676. 1997.PubMed/NCBI
|
|
35
|
Tuccari G, Giuffrè G, Scarf R, Simone A,
Todaro P and Barresi G: Immunolocalization of lactoferrin in
surgically resected pigmented skin lesions. Eur J Histochem.
49:33–38. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
36
|
Roiron D, Amouric M, Marvaldi J and
Figarella C: Lactoferrin-binding sites at the syurface of HT29-D4
cells. Comparison with transferrin. Eur J Biochem. 186:367–373.
1989. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Suzuki YA, Lopez V and Lönnerdal B:
Mammalian lactoferrin receptors: structure and function. Cell Mol
Life Sci. 62:2560–2575. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Ward PP, Paz E and Conneely OM:
Multifunctional roles of lactoferrin: a critical overview. Cell Mol
Life Sci. 62:2540–2548. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Rodrigues L, Teixeira J, Schmitt F,
Paulsson M and Månsson HL: Lactoferrin and cancer disease
prevention. Crit Rev Food Sci Nutr. 49:203–217. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Fujita K, Matsuda E, Sekine K, Iigo M and
Tsuda H: Lactoferrin enhances Fas expression and apoptosis in the
colon muciosa of azoxymethane-treated rats. Carcinogenesis.
25:1961–1966. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Norrby K: Human apo-lactoferrin enhances
angiogenesis mediated by vascular endothelial growth factor A in
vivo. J Vasc Res. 41:293–304. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Shimamura M, Yamamoto Y, Ashino H, Oikawa
T, Hazato T, Tsuda H and Iigo M: Bovine lactoferrin inhibits tumor
induced angiogenesis. Int J Cancer. 111:111–116. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Damiens E, El Yazidi I, Mazurier J, et al:
Lactoferrin inhibits G1 cyclin-dependent kinases during growth
arrest of human breast carcinoma cells. J Cell Biochem. 74:486–498.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Xiao Y, Monitto CL, Minhas KM and
Sidransky D: Lactoferrin down-regulates G1 cyclin-dependent kinases
during growth arrest of head and neck cancer cells. Clin Cancer
Res. 10:8683–8686. 2004. View Article : Google Scholar : PubMed/NCBI
|