Introduction
Neuroblastoma is a common extracranial childhood
solid tumor (1,2). The majority of neuroblastomas are
highly metastastic with poor clinical outcome despite the
application of intensive multimodal therapy (3). However, its oncogenesis is still
poorly understood. Our previous studies showed that environmental
endocrine disruptors (EEDs) may be one of the most important causes
of the carcinogenesis of neuroblastoma (4,5). A
recent multicenter case-control survey also showed that paternal
exposure to hydrocarbons was associated with an increased incidence
of neuroblastoma in children (6).
Therefore, specific antagonists blocking EED-dependent adverse
effects may potentially protect against neuroblastoma occurrence
and become a new option for the treatment of patients with
neuroblastoma in the clinical practice.
Isoflavones are natural compounds that commonly
exist in soy-based foods. They display a variety of biological
activities including suppression of activity of several enzymes
that regulate cell proliferation (7), prevention of cell-cycle progression
(8) and inhibition of tumor growth
(7). Genistein
4′,5,7-trihydroxyisoflavone, also known as genistein, is one of the
major soy isoflavones. Genistein is considered to be a promising
therapeutic candidate for various cancers (9–11).
However, the effects of genistein on neuroblastoma
cell growth, especially those induced by EED and its molecular
mechanisms, are rarely reported. In this study, we used SK-N-SH
human neuroblastoma cells as a cell model, and investigated the
effect of genistein on the bisphenol A (BPA)-and Di-2-ethylhexl
phthalate (DEHP)-induced proliferation of human neuroblastoma in
vitro.
Materials and methods
Cell line, reagents and antibodies
SK-N-SH, a human neuroblastoma cell line, was
purchased from Shanghai Institute for Biological Sciences, Chinese
Academy of Sciences (Shanghai, China). Roswell Park Memorial
Institute-1640 (RPMI-1640) medium, phenol red-free RPMI-1640 medium
and fetal bovine serum (FBS) were obtained from Gibco (Invitrogen,
Carlsbad, CA, USA). 17β-estradiol (E2), genistein,
sulphatase and dextran-coated charcoal were purchased from Sigma
(St. Louis, MO, USA). Cell counting kit-8 (CCK-8) was obtained from
Dojindo Laboratory (Kumamoto, Japan). BPA and DEHP were purchased
from Shanghai Chemical Reagents Company (Shanghai, China). Akt and
phospho-Akt (Ser473; p-Akt) antibodies were obtained from Cell
Signaling Technology (Beverly, MA, USA). E2, BPA, DEHP
and genistein were dissolved in Dimethyl Sulfoxide (DMSO), and then
diluted with phenol red-free RPMI-1640 medium containing
charcoal-dextran-stripped FBS (cd-FBS) to final concentration for
culturing at 1 ng/ml E2, 2 μg/ml BPA, 100
μM/l DEHP and 12.5 μM/l genistein, respectively. The
final solvent concentration in the culture did not exceed 0.1%.
Cell culture and treatment
SK-N-SH cells were cultured in 75-cm2
flasks with RPMI-1640 medium supplemented with 0.1 M L-glutamine,
10% (v/v) FBS, 100 U/ml of penicillin, 100 μg/ml of
streptomycin at 37°C with 5% CO2 in a fully humidified incubator.
Prior to treatments, cells were starved in phenol red-free
RPMI-1640 medium without FBS for 24 h. The study was approved by
the ethics committee of Children’s Hospital, Fudan University.
CCK-8 assay
SK-N-SH cells were seeded into 96-well flat-bottomed
microtiter plates at a density of 105/ml and the final
volume in each well was 100 μl. After being starved, cells
were then treated with 1 ng/ml E2, 2 μg/ml BPA,
or 100 μM/l DEHP with or without 12.5 μM/l genistein.
The number of viable cells was detected with CCK-8 assay as
described previously, at 0, 24, 48 and 72 h (4,5). The
treatment wells were in quadruplicates and the whole experiment was
repeated independently three times.
Flow cytometry
Cells were cultured into 6-well plates at a density
of 3×105/ml, and then treated as described above. After
72 h incubation, 106 cells were fixed with 1 ml 70%
ethanol at 4°C for 30 min and then stained with staining solution
(2 mg/ml propidium iodide and 10 mg/ml RNase in PBS) at 37°C for 30
min. Stained cells were analyzed for fluorescence intensity with a
fluorescence-activated cell sorter (Becton Dickinson, San Jose, CA,
USA) equipped with an argon laser emitting at 488 nm, using the
CellQuest software (Becton Dickinson). A minimum of 10,000 events
were acquired for each determination. The percentages of cells in
G2/M phases were calculated by the ModFit program
(Becton Dickinson).
Western blot analysis
Neuroblastoma cells were plated in 100-mm dishes and
subsequently treated as described above for 72 h. Then cells were
collected in RIPA buffer (50 mmol/l Tris-HCl, pH 7.5, 150 mmol/l
NaCl, 0.5% deoxycholate, 0.1% sodium dodecyl sulfate) on ice for 15
min. Protein concentrations were determined by the Bradford method
and protein extracts (50 μg/lane) were loaded on an 8%
SDS-polyacrylamide gels. After gel separation, proteins were
transferred to a nitrocellulose membrane. The membrane was blocked
with 5% skimmed milk, then separately incubated with anti-Akt
antibody (1:1000 dilution), anti-p-Akt antibody (1:200 dilution).
Incubation with anti-β-actin antibody (1:5000 dilution) was
performed together as an internal control. The membranes were
visualized with an enhanced chemiluminescence system according to
the manufacturer’s instructions. The bands on the western blot
films were scanned by VersaDoc Image Analysis System (Bio-Rad,
Hercules, CA, USA), and analyzed with the QualityOne Image Analysis
software (Bio-Rad).
Statistical analysis
All results were analyzed using SPSS 11.5 software
(SPSS Inc., Chicago, IL, USA). Data were expressed as mean ±
standard error of mean (SEM) of separate experiments (n≥3) and
compared by one-way analysis of variance (ANOVA). The difference
between two treatments was considered significant at P<0.05.
Results
Genistein suppressed SK-N-SH cell
proliferation induced by E2, BPA and DEHP
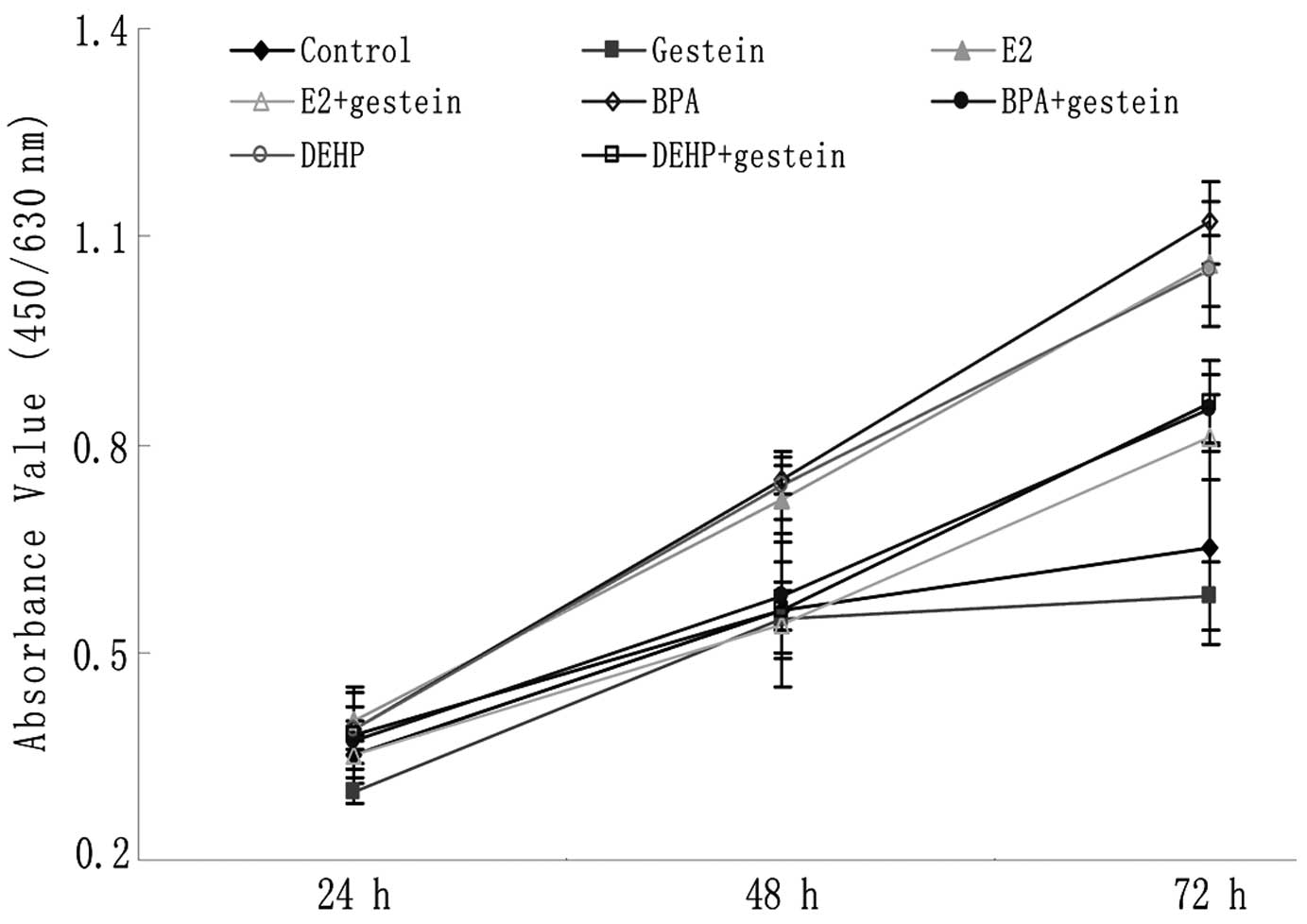
The effect of genistein on the proliferation of
neuroblastoma cells was assessed using CCK-8 proliferation assay.
Time- and dose-dependent experiments showed that genistein
inhibited the SK-N-SH cell growth in a dose-dependent manner (data
not shown). After 24 h treatment, absorbance values (AVs) among all
the groups showed no significant difference. Then at 48 h, the AVs
of the E2, BPA and DEHP groups (0.72±0.06, 0.75±0.02,
0.74±0.05, respectively) were significantly higher than those of
the control group (0.56±0.03, P<0.001; Fig. 1). However, there was not a similar
increase of AV in groups treated with E2, BPA or DEHP
with additional genistein treatment. The AVs in these groups were
not significantly higher than those in the control group
(P>0.05), but were evidently lower than those in the groups
treated with E2, BPA and DEHP alone (P<0.001;
Fig. 1). At 72 h, a similar
phenomenon was noted (Fig. 1).
Genistein arrested SK-N-SH cells at
G2/M phase of cell cycle
After 72 h treatment, flow cytometric analysis was
also applied to investigate whether the effect of genistein
inhibition against neuroblastoma cell proliferation occurred in a
cell cycle-dependent manner. When cells were treated with
additional genistein, the percentage of cells in the
G2/M phase significantly increased. Cells were arrested
at the G2/M phase of the cell cycle (P>0.05 vs. the
control group; P<0.01 vs. E2, BPA or DEHP groups;
Table I).
 | Table IG2/M phase analysis of
SK-N-SH cells at 72 h. |
Table I
G2/M phase analysis of
SK-N-SH cells at 72 h.
| Groups | G2/M
(%) | Groups | G2/M
(%) |
|---|
| Control | 9.15±1.08 | Genistein | 16.58±2.71c |
| E2 | 11.70±1.14a | E2+genistein | 15.56±0.58b |
| BPA | 11.94±0.06a | BPA+genistein | 17.76±1.45c |
| DEHP | 11.64±0.39a | DEHP+genistein | 15.99±0.98c |
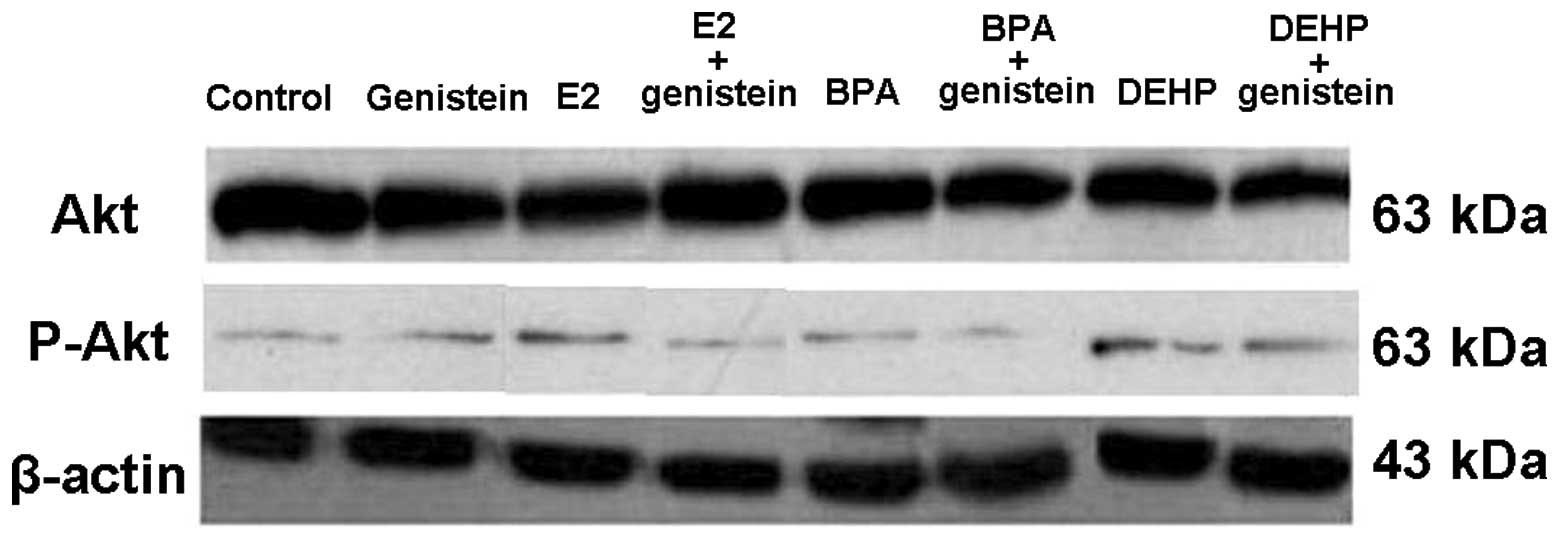
Genistein modulated p-Akt (Ser473)
protein expression
Akt and p-Akt expression levels were further
detected by western blot analysis to determine the principal
signaling mechanism of genistein inhibiting SK-N-SH cell growth at
72 h after drug treatments. The expression of p-Akt was abundant
when treated with E2, BPA or DEHP only (Fig. 2). By contrast, there was no increase
in the expression of p-Akt protein in the genistein-treated groups
(Fig. 2). Akt protein expression
had no significant change in any of the groups.
Discussion
During the last decade, soy isoflavones mainly
derived from soybean received much attention as dietary components
having inhibitory effects on cancers. The lower risk of breast and
prostate cancers in Asians, who consume 20–50 times more soy than
Americans, has raised the question whether compounds in the soy
diet act as a natural chemopreventive agent. Indeed, a
cross-national study involving 59 countries identified that soy
products have a highly significant effect against the development
of prostate cancer (12). Elevated
levels of soy isoflavones in the micromolar range have been
detected in the serum, urine, prostatic fluid and prostate tissue
in vegetarians and Asian men who consume a soy-rich diet and have
low risk of prostate cancer (13).
In contrast, serum concentration of genistein in Americans and
Europeans is in the nanomolar range (13).
Soy isoflavones include genistein, daidzein and
glycitein. However, genistein is the principal isoflavone in soy
that has been demonstrated to be responsible for reducing the
incidence of hormone-related cancers. In laboratory in vitro
experiments, genistein has been found to inhibit the growth of
various cancer cell lines including prostate and breast cancer
cells (14). Moreover, the evidence
from in vitro studies has demonstrated that genistein exerts
its inhibitory effects on the development of cancers, cancer cell
growth, cancer progression, cancer cell invasion, metastasis and
angiogenesis (14).
The direct anti-tumor activity, action mechanisms
and therapeutic potential of genistein on neuroblastoma cells have
been investigated (8,15). We have previously reported that
environmental endocrine disruptor BPA promoted the proliferation of
SK-N-SH cells in vitro and in vivo(4,5).
However, the effects of genistein on the growth-promoted action of
BPA and DEHP on human neuroblastoma cells and its action mechanisms
remain poorly understood. In the current study, we hypothesized
that genistein may also inhibit the human neuroblastoma cell
proliferation induced by EED and estrodiol in vitro.
Our results showed that the proliferation of
neuroblastoma cells is enhanced by estrogen and certain
environmental estrogen-like contaminants. When genistein was used
in combination with E2, BPA or DEHP, cell growth was
inhibited effectively. This is in agreement with other studies
involving several types of cells (16–18).
The present results demonstrated that genistein suppressed SK-N-SH
cell proliferation induced by BPA and DEHP.
In addition, genistein prevented the SK-N-SH cells
from entering the G2/M phase during the cell cycle,
suggesting that the observed growth-inhibitory effect of genistein
was mediated through modulation of cell cycle progression in
SK-N-SH cells. Our results are in line with others which showed
that genistein exerts its anti-tumor effects by arresting SK-N-SH
cells in G2/M phase and inhibiting cancer cell death
(19).
The phosphatidylinositol-3 kinase/Akt (PI3K/Akt)
signaling pathway plays a critical role in cell survival and
apoptosis. Inhibition of the PI3K/Akt pathway has been considered
as a therapeutic target for cancer where PI3K/Akt activation is a
causative factor. It has also been reported that genistein inhibits
the activation of the Akt signaling pathway in prostate and breast
cancer cell lines (20,21). In our study, the expression of p-Akt
was abundant in the E2, BPA and DEHP groups, but
genistein caused a decrease in active p-Akt levels.
In summary, BPA and DEHP have growth-promoting
effects on human neuroblastoma SK-N-SH cells in vitro, which
can be inhibited by genistein. Genistein significantly inhibited
the growth of neuroblastoma cells through modulation of cell cycle
progression and the PI3K/Akt pathway. The results of our study
provide the experimental basis for the application of genistein in
additional preventive or therapeutic strategies in
neuroblastoma.
Acknowledgements
These results were presented at the
42nd Annual Meeting of the Pacific Association of Pediatric
Surgeons, May 9 to May 14, 2009, Hongkong, China. We thank the
Institute of Biochemistry and Cell Biology, Shanghai Institutes for
Biological Sciences, and the Chinese Academy of Sciences for their
help with the flow cytometric analysis. This study was supported by
the National Natural Science Foundation Commission (30801198/C1611)
and the Doctoral Fund of Ministry of Education of China.
References
|
1
|
van Noesel MM and Versteeg R: Pediatric
neuroblastomas: genetic and epigenetic ‘danse macabre’. Gene.
325:1–15. 2004.
|
|
2
|
Izbicka E and Izbicki T: Therapeutic
strategies for the treatment of neuroblastoma. Curr Opin Investig
Drugs. 6:1200–1214. 2005.PubMed/NCBI
|
|
3
|
DuBois SG, Kalika Y, Lukens JN, et al:
Metastatic sites in stage IV and IVS neuroblastoma correlate with
age, tumor biology, and survival. Journal of pediatric
hematology/oncology. 21:181–189. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zheng J, Xiao X, Liu J, Zheng S, Yin Q and
Yu Y: Growth-promoting effect of environmental endocrine disruptors
on human neuroblastoma SK-N-SH cells. Environ Toxicol Pharmacol.
24:189–193. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zhu H, Xiao X, Zheng J, Zheng S, Dong K
and Yu Y: Growth-promoting effect of bisphenol A on neuroblastoma
in vitro and in vivo. J Pediatr Surg. 44:672–680. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
De Roos AJ, Olshan AF, Teschke K, et al:
Parental occupational exposures to chemicals and incidence of
neuroblastoma in offspring. Am J Epidemiol. 154:106–114.
2001.PubMed/NCBI
|
|
7
|
Formica JV and Regelson W: Review of the
biology of Quercetin and related bioflavonoids. Food Chem Toxicol.
33:1061–1080. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ismail IA, Kang KS, Lee HA, Kim JW and
Sohn YK: Genistein-induced neuronal apoptosis and G2/M cell cycle
arrest is associated with MDC1 up-regulation and PLK1
down-regulation. Eur J Pharmacol. 575:12–20. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Park OJ and Surh YJ: Chemopreventive
potential of epigallocatechin gallate and genistein: evidence from
epidemiological and laboratory studies. Toxicol Lett. 150:43–56.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Magee PJ and Rowland IR: Phyto-oestrogens,
their mechanism of action: current evidence for a role in breast
and prostate cancer. Br J Nutr. 91:513–531. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Sarkar FH and Li Y: Soy isoflavones and
cancer prevention. Cancer investigation. 21:744–757. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hebert JR, Hurley TG, Olendzki BC, Teas J,
Ma Y and Hampl JS: Nutritional and socioeconomic factors in
relation to prostate cancer mortality: a cross-national study. J
Natl Cancer Inst. 90:1637–1647. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Rannikko A, Petas A, Rannikko S and
Adlercreutz H: Plasma and prostate phytoestrogen concentrations in
prostate cancer patients after oral phytoestogen supplementation.
The Prostate. 66:82–87. 2006. View Article : Google Scholar
|
|
14
|
Li Y and Sarkar FH: Inhibition of nuclear
factor kappaB activation in PC3 cells by genistein is mediated via
Akt signaling pathway. Clin Cancer Res. 8:2369–2377.
2002.PubMed/NCBI
|
|
15
|
Lo FH, Mak NK and Leung KN: Studies on the
anti-tumor activities of the soy isoflavone daidzein on murine
neuroblastoma cells. Biomed Pharmacother. 61:591–595. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Linford NJ, Yang Y, Cook DG and Dorsa DM:
Neuronal apoptosis resulting from high doses of the isoflavone
genistein: role for calcium and p42/44 mitogen-activated protein
kinase. J Pharmacol Exp Ther. 299:67–75. 2001.PubMed/NCBI
|
|
17
|
Hewitt AL and Singletary KW: Soy extract
inhibits mammary adenocarcinoma growth in a syngeneic mouse model.
Cancer Lett. 192:133–143. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Chang KL, Kung ML, Chow NH and Su SJ:
Genistein arrests hepatoma cells at G2/M phase: involvement of ATM
activation and upregulation of p21waf1/cip1 and Wee1. Biochem
Pharmacol. 67:717–726. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Sarkar FH, Adsule S, Padhye S, Kulkarni S
and Li Y: The role of genistein and synthetic derivatives of
isoflavone in cancer prevention and therapy. Mini reviews in
medicinal chemistry. 6:401–407. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Gong L, Li Y, Nedeljkovic-Kurepa A and
Sarkar FH: Inactivation of NF-kappaB by genistein is mediated via
Akt signaling pathway in breast cancer cells. Oncogene.
22:4702–4709. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Park SS, Kim YN, Jeon YK, et al:
Genistein-induced apoptosis via Akt signaling pathway in anaplastic
large-cell lymphoma. Cancer chemother Pharmacol. 56:271–278. 2005.
View Article : Google Scholar : PubMed/NCBI
|