Introduction
Giant cell tumours (GCTs) account for ∼8% of all
bone tumours (1,2), commonly arising from the
meta-epiphyseal region of the knee (condyles and tibial plateau),
proximal humerus and distal radius (3,4). Wide
resection of these tumours lowers the local recurrence rate but
often results in a loss of function due to extensive joint
resection (2,3). Therefore, extended intralesional
curettage has become the recommended treatment (5,6).
Combined with various adjuvant therapies, including phenolisation,
ethanolisation, rinsing with H2O2, heat
(electric cauterisation or cementation) cryosurgery, burring and
argon beam coagulation, recurrence rates vary from 5–50% (7–12).
Among the additional adjuvant therapies, phenolisation (chemical
cauterisation) and cementation (thermic cauterisation and
stabilisation of the bone defect) are common treatments (8,11,13–15).
After curettage of the tumour a phenol solution is instilled and
should cover the whole cavity. Phenol is highly toxic and supposed
to eliminate the majority of the remaining tumour cells by
denaturation (16). In this
context, little is known concerning the necessary concentration,
the duration of exposure and the depth of tissue penetration of the
phenol solution. Commonly used phenol concentrations for the
treatment of GCT are either low (5–6%) or very high (60–80%)
(8,14,15).
Therefore, we exposed GCT specimens to various concentrations of
phenol. The time-dependent depth of tissue penetration and
denaturation of cells were evaluated using transmission electron
microscopy.
Materials and methods
Samples
Histologically determined GCTs of 3 patients (2
proximal tibia and 1 metatarsal bone) were surgically removed at
the Department of Orthopaedic Surgery, Tuebingen, Germany.
Additionally, 6% phenol instillation and cementation were
performed. Viable solid tumour tissue specimens (∼0.5 cm in
diameter) of the removed GCTs were obtained and tested in
vitro. All patients provided informed consent to partake in the
study. The study was approved by the local ethics committee (Nr.
605/2011BO2).
Preparation of specimens
Phenol solution (6, 60 or 80%) was added to the
surface of the tumour specimens for either 1 or 3 min in
vitro. Following washing with 0.9% NaCl solution, specimens
were immediately embedded in paraffin, sliced and stained. In
addition, following phenolisation, each specimen was examined by
transmission electron microscopy. Briefly, tissues were fixed with
2.5% glutaraldehyde (Paesel and Lorei; Frankfurt, Germany) buffered
in 0.1 mol/l cacodylate buffer (pH 7.4). Thereafter, the tissues
were postfixed in the same fixative as used previously for an
additional 4 h, then post-fixed in 1% OsO4 in 0.1 mol/l
cacodylate buffer and dehydrated in an ethanol series (50, 70, 96
and 100%). The 70% ethanol solution was saturated with uranyl
acetate for contrast enhancement. Dehydration was completed in
propylene oxide. The specimens were embedded in Araldite (Serva;
Heidelberg, Germany). Semi- and ultra-thin sections were produced
on an FCR Reichert Ultracut ultramicrotome (Leica, Bensheim,
Germany). The semi-thin sections were stained with toluidine blue
for inspection, while the ultra-thin sections were mounted on
pioloform-coated copper grids, contrasted with lead citrate, and
analysed and documented with an EM10A electron microscope (Carl
Zeiss; Oberkochen, Germany). The penetration depth of phenol was
observed to be dependent on the destruction of cell organelles in
the deeper cell layers.
Results
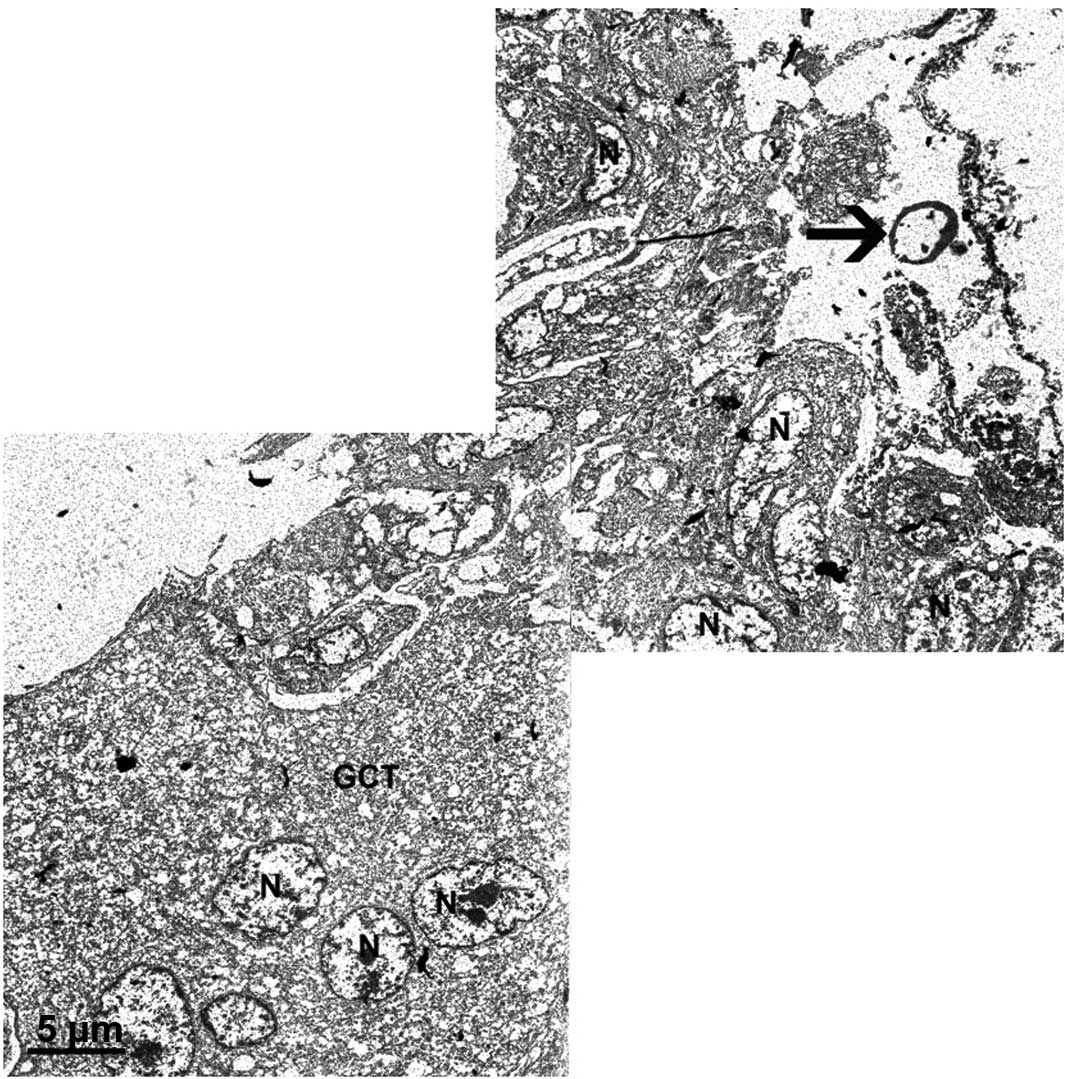
Incubation with 6% phenol solution for 1 min
resulted in a damage to only the uppermost cell layers (10–20
μm; Figs. 1 and 2). Damage was represented as a complete
coagulation of the cytoplasm and in particular the nucleoplasm. No
complete loss of any of the cell substructures was observed. The
outlines of the organelles were simply converted to black. After 3
min, the penetration depth increased to ∼200 μm. Incubation
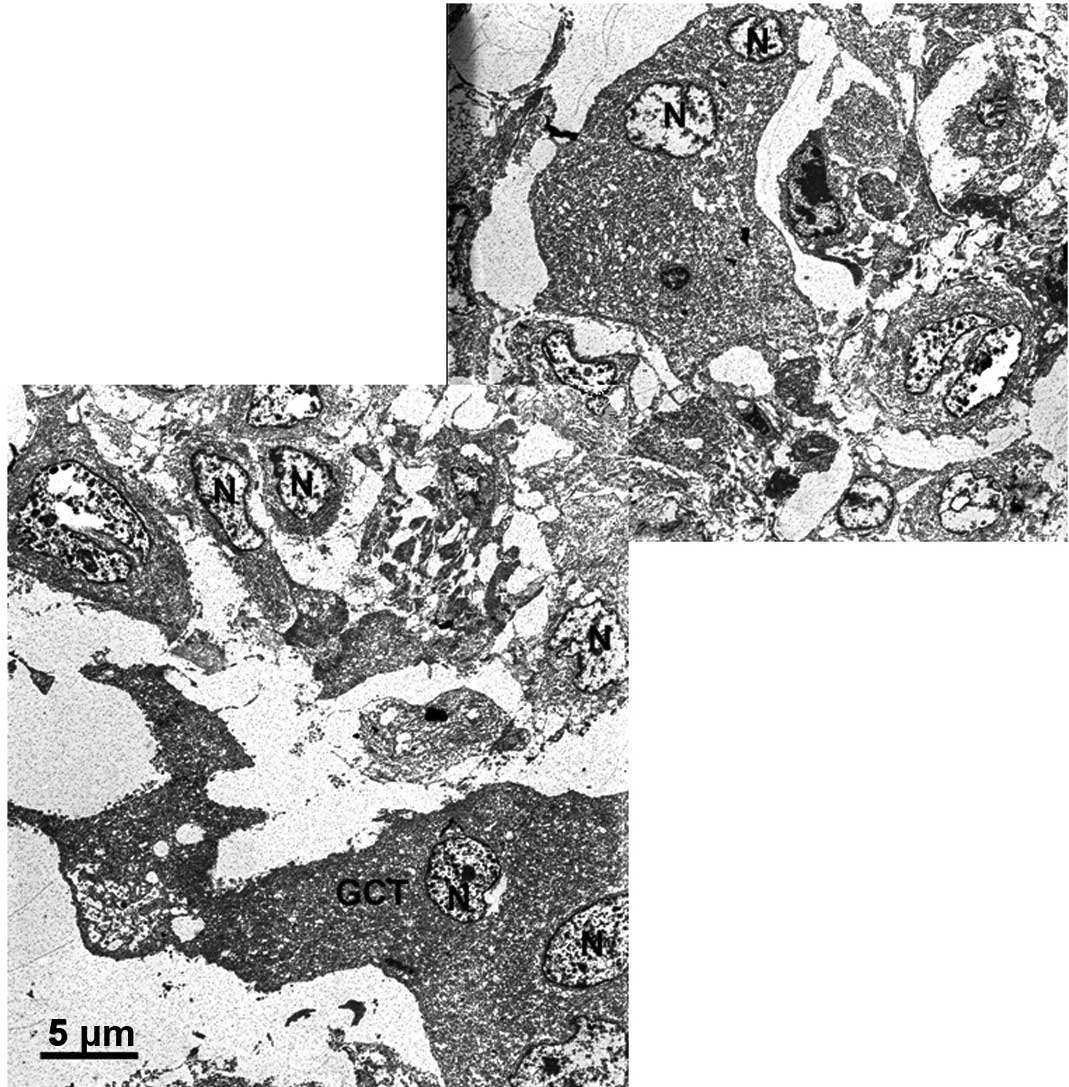
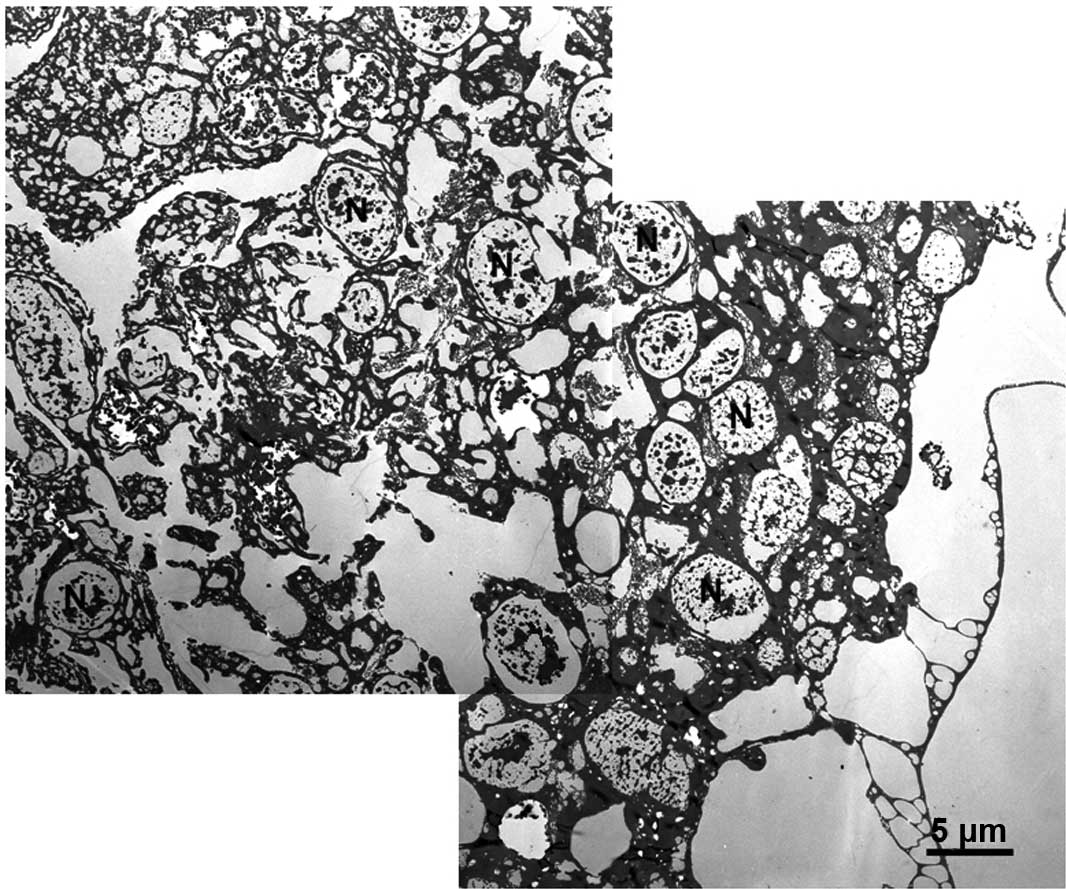
with the 60 and 80% phenol solution for 1 min resulted in the
destruction of 10 cell layers and a penetration depth of ∼100
μm. After 3 min of 60 and 80% phenol exposure, neither
additional tissue damage or an increase in the penetration depth
were observed (Fig. 3 and Table I).
 | Table IPenetration depth of phenol in GCT
specimens incubated with different concentrations of phenol
soution. |
Table I
Penetration depth of phenol in GCT
specimens incubated with different concentrations of phenol
soution.
| | Penetration depth
(μm)
|
|---|
| Phenol concentration
(%) | Exposure time
(min) | Specimen 1 | Specimen 2 | Specimen 3 |
|---|
| 6 | 1 | 15 | 10 | 25 |
| 6 | 3 | 180–200 | No data | 200 |
| 60 | 1 | 160 | 80 | 100 |
| 60 | 3 | 80 | 100 | No data |
| 80 | 1 | 80 | 80–90 | 60 |
| 80 | 3 | 80–100 | 100 | 100 |
Discussion
The greatest penetration depth and tissue
destruction in GCT were observed when 6% phenol solution and a
contact time of ≥3 min were employed. This type of destruction
cannot be described as cell death in the sense of necrosis or
apoptosis, as such types of cellular destruction imply a reaction
(necrosis) or an active execution (apoptosis) as a consequence of
toxic treatment. Higher concentrations of phenol led to extreme
destruction of the uppermost cell layers, but not to a deeper
infiltration of the tissue. Superficial denaturised tissues were
considered to act as a barrier preventing further penetration of
phenol.
Due to the complex investigation of each single cell
layer with transmission electron microscopy and the achievement of
consistent results in all three tumours, we did not extend the
study. Nevertheless, it is possible that a deeper tissue
penetration depth may be achieved by 6% (or a slightly higher
concentrated) phenol solution with an incubation time >3 min.
This requires further investigation.
The penetration depth of various concentrations of
phenol in GCT has not yet been investigated. According to the
literature, phenol concentrations used in the treatment of GCTs
vary from 5–75% (8,14,15).
The incubation time is often not mentioned. Quint et al
investigated the cytotoxic effect of different phenol
concentrations on single-layer sarcoma cell lines and recommend the
use of a phenol concentration of 5% (17). Evaluation of the penetration depth
was not possible with this setting. Lack et al investigated
the denaturising effect of a 75% phenol solution on normal tissue,
tumours and chondromatous tissue, using light microscopy (18). The penetration depth in soft tissue
varied from 40–500 μm. In chondromatous tissue, no cytotoxic
effect was evaluated. This suggests that phenol has no toxic effect
on bone.
High concentrations of phenol are capable of causing
local chemical burs; contact of the healthy surrounding tissue with
the solution ought to be avoided. Phenol is locally absorbed and
excreted in the urine. In this regard, Quint et al described
a low risk for humans depending on the quantity of 5% phenol
solution used (16).
The results of the present study suggest that an
instillation of 6% phenol solution for ≥3 min is the most effective
method for denaturising as many of the remaining tumour cells as
possible. High phenol concentrations did not demonstrate a benefit,
and they increased the risk of bone necrosis and systemic
intoxication. The determined optimal phenol concentration and
incubation time for GCT are not transferable to the treatment of
other tumours.
Phenol instillation (6% for ≥3 min) may be used for
the denaturation of small, scattered, cellular debris following
intralesional curettage of GCT; however, due to the relatively low
tissue penetration of 200 μm, it is not suitable for
treatment of the remaining solid tumour tissue. Adequate surgical
removal of the tumour remains to be the most important predictive
factor in preventing recurrence of GCT of the bone.
Acknowledgements
The authors would like to thank
Professor Aicher and Mrs. Kienzle, Center for Regenerative Biology
and Medicine (ZRM, Eberhard-Karls-University Tuebingen), for their
assistance and technical support in performing GCT fixation and
staining.
References
|
1
|
Schajowicz F: Tumors and tumorlike lesions
of bone - pathology, radiology and treatment. 2nd edition.
Springer-Verlag; Berlin, Heidelberg, New York: pp. 257–299. 1994,
View Article : Google Scholar
|
|
2
|
Szendröi M: Giant-cell tumour of bone. J
Bone Joint Surg Br. 86:5–12. 2004.
|
|
3
|
Campanacci M, Baldini N, Boriani S and
Sudanese A: Giant-cell tumor of bone. J Bone Joint Surg Am.
69:106–114. 1987.PubMed/NCBI
|
|
4
|
Lee MJ, Sallomi DF, Munk PL, Janzen DL,
Connell DG, O’Conell JX, Logan PM and Masri BA: Pictorial review:
giant cell tumours of bone. Clin Radiol. 53:481–489. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Lackmann RD, Hosalkar HS, Ogilvie CM,
Torbert JT and Fox EJ: Intralesional curettage for grades II and
III giant cell tumors of bone. Clin Orthop Relat Res. 438:123–127.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Turcotte RE, Wunder JS, Isler MH, Bell RS,
Schachar N, Masri BA, Moreau G and Davis AM: Canadian Sarcoma
Group: Giant cell tumor of long bone: a Canadian Sarcoma Group
study. Clin Orthop Relat Res. 397:248–258. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Balke M, Schremper L, Gebert C, Ahrens H,
Streitburger A, Koehler G, Hardes J and Gosheger G: Giant cell
tumor of bone: treatment and outcome of 214 cases. J Cancer Res
Clin Oncol. 134:969–978. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Dürr HR, Maier M, Jansson V, Baur A and
Refior HJ: Phenol as an adjuvant for local control in the treatment
of giant cell tumour of the bone. Eur J Surg Oncol. 25:610–618.
1999.PubMed/NCBI
|
|
9
|
Lin WH, Lan TY, Chen CY, Wu K and Yang RS:
Similar Local Control between Phenol- and Ethanol-treated Giant
Cell Tumors of Bone. Clin Orthop Relat Res. 469:3200–3208. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Malawer MM, Bickels J, Meller I, Buch RG,
Henshaw R and Kollender Y: Cryosurgery in the treatment of giant
cell tumor: a long-term followup study. Clin Orthop Relat Res.
359:176–188. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Remedios D, Saifuddin A and Pringle J:
Radiological and clinical recurrence of giant-cell tumour of bone
after use of cement. J Bone Joint Surg Br. 79:26–30. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Willert HG: Clinical results of the
temporary acrylic bone cement plug in the treatment of bone tumors:
a multicentric study. In: Limb-sparing Surgery in Musculoskeletal
Oncology. Enneking WF: Churchill Livingstone; New York: pp.
445–458. 1987
|
|
13
|
O’Donell RJ, Springfield DS, Motwani HK,
Ready JE, Gebhardt MC and Mankin HJ: Recurrence of giant-cell
tumors of the long bones after curettage and packing with cement. J
Bone Joint Surg Am. 76:1827–1833. 1994.PubMed/NCBI
|
|
14
|
Schiller C, Ritschl P, Windhager R, Kropej
D and Kotz R: The incidence of recurrence in phenol treated and
non-phenol treated bone cavities following intralesional resection
of non-malignant bone tumors. Z Orthop Ihre Grenzgeb. 127:398–401.
1989.(In German).
|
|
15
|
Szendröi M: Adjuvant therapy (phenol, bone
cements) in giant cell tumors. Z Orthop Ihre Grenzgeb. 130:95–98.
1992.(In German).
|
|
16
|
Quint U, Müller RT and Müller G:
Characteristics of phenol. Arch Orthop Trauma Surg. 117:43–46.
1998. View Article : Google Scholar
|
|
17
|
Quint U, Vanhöfer U, Harstrick A and
Müller RT: Cytotoxicity of penol to musculoskeletal tumours. J Bone
Joint Surg Br. 78:984–985. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lack W, Lang S and Brand G: Necrotising
effect of phenol on normal tissues and on tumors. Acta Orthop
Scand. 65:351–354. 1994. View Article : Google Scholar : PubMed/NCBI
|