Introduction
In human epithelial cancers, the PI3K-Akt signaling
pathway is frequently hyperactivated during cancer invasion, and
the progressive enhancement of PI3K-Akt coupled to efficient cell
migration is a hallmark of high metastatic potential (1–3).
Although many identified molecules have a role in breast cancer
progression and metastasis, the mechanisms involved are far from
clear (4,5). To date, few molecules exhibit a high
efficiency in predicting postoperative distant metastasis for
breast cancer.
In our recent study, Girdin was highly expressed in
breast cancer and was a potential biomarker for the initiation,
progression and differentiation of breast cancer tumors (6). Another study observed that the
expression of Girdin associated with PI3K-Akt signaling, actin
remodeling, motility and invasion varies among tumors, and the
majority of them have failed to make a transition into cancer
clinics as prognostic biomarkers (7). Girdin is a novel protein, found at the
crossroads of G protein signaling and tyrosine kinase receptor
signaling (8). When the epidermal
growth factor receptor signal is activated, Girdin is directly
activated by Akt (9). Recently,
Ghosh et al discovered a Girdin-Gαi molecular complex that
binds to the epidermal growth factor receptor and determines
whether cells migrate or proliferate (9). The authors also suggested that the
expression of Girdin predicts patient survival in colon cancer and
may serve as a useful adjunct to traditional staging strategies in
colorectal carcinoma (9).
Currently, studies addressing the function and
specific mechanism of Girdin and the PI3K-Akt signaling pathway in
regulating the biological behavior of breast cancer remain rare.
Moreover, the correlation between the expression status of Girdin
and PI3K proteins remains unclear. In this study, we investigated
the expression status of Girdin and PI3K proteins and the clinical
implications in the management of breast cancer.
Materials and methods
Patients and tissue specimens
In this study, we selected 820 patients who had
histologically confirmed breast cancer and had undergone radical
surgery in the Liaoning Province Tumor Hospital between January
2003 and December 2006. The inclusion criteria were: a) curative
surgery had been performed; b) resected specimens had been
pathologically examined; c) >15 lymph nodes had been
pathologically examined after surgery; and d) a complete medical
record was available. Of 820 enrolled patients, 591 had received
adjuvant chemotherapy and 165 had received adjuvant radiotherapy.
Of the 591 cases that had received adjuvant chemotherapy, 153
developed distant postoperative metastasis. Additionally, 52 of the
165 patients who received adjuvant radiotherapy also developed
postoperative metastasis. This study was approved by the Ethics
Committee of China Medical University, Shenyang, China.
Experiment materials
Polyclonal rabbit antihuman Girdin and PI3K
antibodies (dilution 1:100) were purchased from Santa Cruz
Biotechnology (Santa Cruz, CA, USA). The monoclonal mouse antihuman
ER, PR and anti-CerbB2 antibodies, all with a dilution factor of
1:100, were purchased from Dako (Carpinteria, CA, USA). CD24-PE and
CD44-FITC were purchased from BD Pharmingen (San Diego, CA, USA)
and the flow cytometer used in this study was a FACSVantage
obtained from BD Pharmingen.
Flow cytometry test
Cells were counted and transferred to a 5-ml tube,
washed twice with Hank’s balanced salt solution (HBSS) with 2%
heat-inactivated calf serum (HICS; 5 min at 1,000 rpm), then
resuspended in 100 μl (per 106 cells) of HBSS/2%
HICS (10). Then, 5 μl of
Sandoglobin solution (1 mg/ml) was added and incubated on ice for
10 min, after which the sample was washed twice with HBSS/2% HICS
and resuspended in 100 μl (per 106 cells) of
HBSS/2% HICS. Antibodies (appropriate dilution per antibody) were
added and incubated for 20 min on ice, and then washed twice with
HBSS/2% HICS and flow cytometry was performed. Cells were routinely
sorted twice, and then reanalyzed for purity, typically >95%.
Dead cells were removed using the viability dye 7AAD.
CD44+/CD24− tumor cells were selected by CD44
and CD24.
Western blot analysis
For western blot analysis, cells were lysed with the
buffer [0.1% SDS, 50 mmol/l Tris-HCl (pH 7.6), 1% NP-40, 150 mmol/l
NaCl, 2 mg/ml aprotinin, 2 mg/ml leupeptin and 7 mg/ml PMSF]
(10). Protein concentrations were
determined using a BCA Protein Assay kit (Pierce Biotechnology,
Inc., Rockford, IL, USA). Proteins (30 μg) were separated on
10% SDS-PAGE gels and transferred to a PVDF membrane. After
blocking, the membrane was incubated with the primary antibody
(1:500, Biorbyt Ltd., Cambridge, UK) at 4°C overnight. After
washing, the membrane was incubated with a secondary antibody at a
dilution of 1:2,000 at room temperature for 1 h. Proteins were
detected using an ECL kit (Varsal Instruments, Beijing, China), and
anti-β-actin antibody (Sigma-Aldrich, St. Louis, MO, USA) was used
as a loading control. Densitometry was performed using Gel-pro
Analyzer software (Media Cybernetics, Silver Spring, MD, USA).
Immunohistochemistry experimental
procedures
We fixed thin slices of tumor tissue from all the
cases we received in our histopathology unit in 4% formaldehyde
solution (pH 7.0) for <24 h (11). The tissues were processed routinely
for paraffin embedding, and 4-μm-thick sections were cut and
placed on glass slides coated with (3-aminopropyl)triethoxysilane
for immunohistochemistry. Tissue samples were stained with
hematoxylin and eosin to determine histological type and tumor
grade.
Breast tumor tissues and non-neoplastic breast
tissues were cut at a thickness of 4 μm using a cryostat.
The sections were mounted on microscope slides, air-dried and fixed
in a mixture of 50% acetone and 50% methanol. The sections were
de-waxed with xylene, gradually hydrated with gradient alcohol and
washed with phosphate-buffered saline (PBS). Sections were then
incubated for 60 min with the primary antibody. Following washing
with PBS, the sections were incubated for 30 min in the secondary
biotinylated antibody (multilink swine anti-goat/mouse/rabbit
immunoglobulin; Dako). Following washing, avidin-biotin complex
(1:1,000 dilution; Vector Laboratories, Burlingame, CA, USA) was
applied to the sections for 30–60 min at room temperature. The
immunoreactive products were visualized by the catalysis of
3,3′-diaminobenzidine (DAB) using horseradish peroxidase in the
presence of H2O2 following extensive washing.
Sections were counterstained in Gill’s hematoxylin and dehydrated
in ascending grades of methanol prior to being cleared in xylene
and mounted under a coverslip.
Girdin and PI3K expression was classified
semi-quantitatively according to the following criteria: 0 if
<1% of the neoplastic cells discretely expressed Girdin in their
cytoplasm; 1+ if ≥1 but <10% of morphologically unequivocal
neoplastic cells discretely expressed Girdin in their cytoplasm;
and 2+ if ≥10% of morphologically unequivocal neoplastic cells
discretely expressed Girdin in their cytoplasm. We considered
samples scored as either 1+ or 2+ as positive.
Statistical analysis
All data were analyzed using SPSS statistical
software (Version 13.0, Chicago, IL, USA). Correlations between
tumor markers and other parameters were studied using the
Chi-square test and Fisher’s test or an independent t-test.
Disease-specific survival was analyzed using the Kaplan-Meier
method. The log-rank test was used to analyze differences in
survival. Multivariate analysis was performed using the Cox
proportional hazards model selected in forward stepwise. P<0.05
was considered to be statistically significant.
Results
Girdin and PI3K protein expression status
in breast cancer stem cells
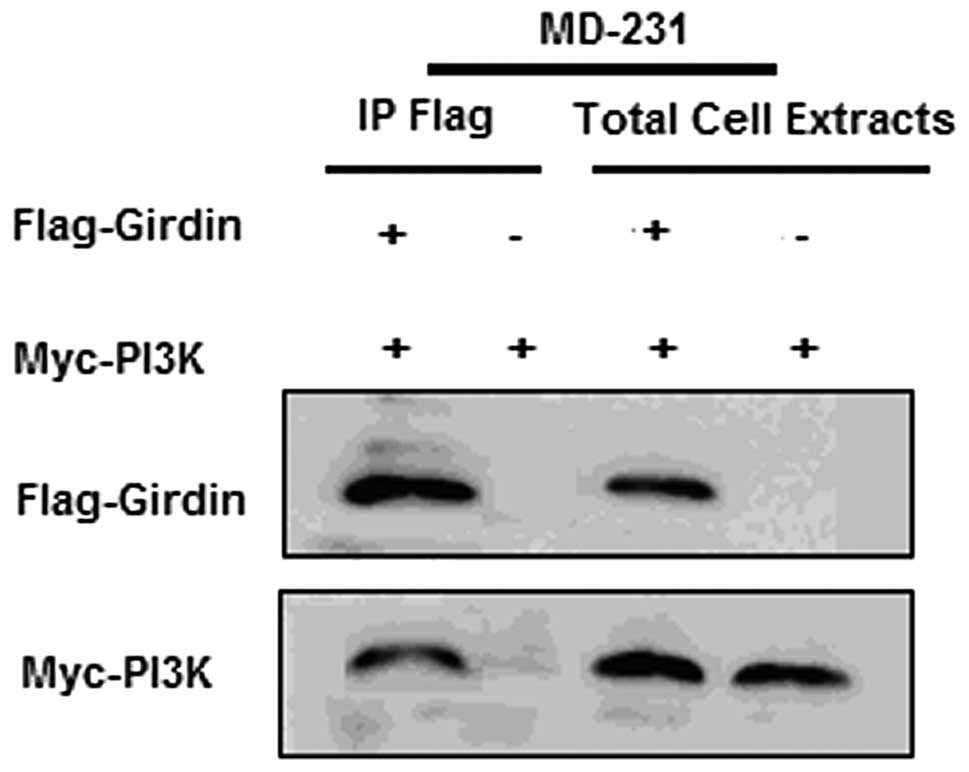
The CD44+/CD24− tumor cells
from the MD-231 cell line were sorted by flow cytometry. After
seven days of serum-free suspension culture, single-cell
suspensions of cancer stem cells that were separated from the solid
tumors produced viable mammospheres (20–100 μm). After
western blot analysis, Girdin and PI3K proteins were expressed at a
higher level in cancer stem cells compared to the control cells
(Fig. 1A). Furthermore, Girdin and
PI3K protein co-immuno-precipitation was observed (Fig. 1B).
Patient characteristics
Of the 820 enrolled breast cancer patients, Girdin
and PI3K proteins were expressed positively in 295 (35.98%) and 492
(60%) cases, respectively. In 162 (19.76%) cases, Girdin and PI3K
proteins were co-expressed (Table
I). After univariate analysis, the co-expression of Girdin and
PI3K protein was correlated with histological type, metastatic
nodes and distant metastasis (P=0.001, 0.001 and 0.001,
respectively). In order to exclude the influence of confounding
factors, we compared the age, tumor size, histological grade and
adjuvant treatment between the patients with distant metastasis and
those without. No difference was observed between the patients with
distant metastasis and those without.
 | Table ICorrelations between Girdin and PI3K
co-expression and clinicopathological features. |
Table I
Correlations between Girdin and PI3K
co-expression and clinicopathological features.
| Variables | n |
Girdin+/PI3K+
[n(%)] | χ2
value | P-value |
|---|
| Age(years) | | | 0.135 | 0.713 |
| <35 | 124 | 26 (20.97) | | |
| >35 | 696 | 136 (19.54) | | |
| Tumor size | | | 1.778 | 0.409 |
| T1 | 167 | 27 (16.17) | | |
| T2 | 509 | 104 (20.43) | | |
| T3 | 144 | 31 (14.58) | | |
| Histological
grade | | | 62.002 | 0.001 |
| I | 104 | 11 (10.58) | | |
| II | 520 | 94 (18.08) | | |
| III | 196 | 57 (29.08) | | |
| Metastatic nodes | | | 11.656 | 0.001 |
| Negative | 392 | 58 (14.80) | | |
| Positive | 428 | 104 (24.30) | | |
| Distant
metastasis | | | 40.863 | 0.001 |
| Negative | 599 | 86 (14.36) | | |
| Positive | 221 | 76 (34.39) | | |
| Triple-negative
breast cancer | | | 0.257 | 0.612 |
| Yes | 131 | 28 (21.37) | | |
| No | 689 | 134 (19.45) | | |
Correlation between Girdin and PI3K
protein co-expression and clinicopathological characteristics
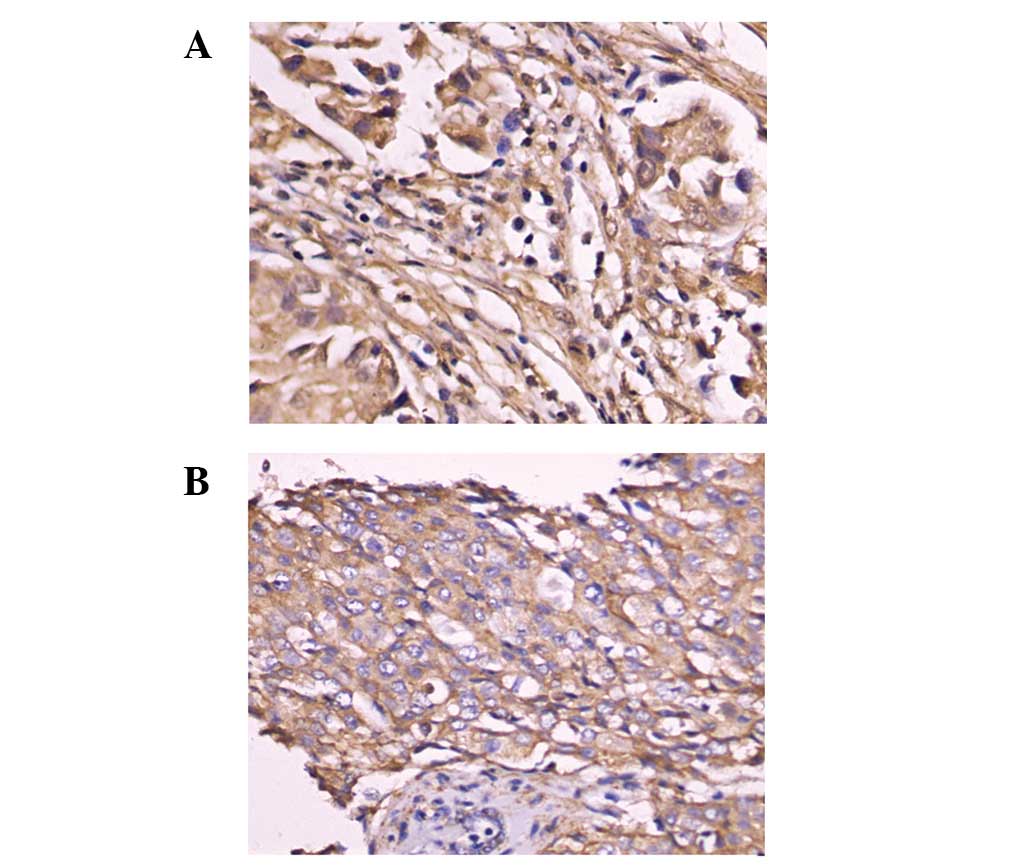
Immunohistochemical examination demonstrated that
Girdin and PI3K proteins were located at the cytoplasm and membrane
of breast cancer cells (Fig. 2).
Moreover, we observed that Girdin and PI3K protein co-expression
was related to histological type, meta-static nodes and distant
metastasis (P=0.001, 0.001, and 0.001, respectively). Following
multivariate analysis, Girdin and PI3K protein co-expression was
shown to be related to histological type, metastatic nodes and
distant metastasis (P=0.01, 0.001 and 0.001, respectively)
(Table II).
 | Table IIMultivariate analysis of the factors
related to Girdin and PI3K co-expression. |
Table II
Multivariate analysis of the factors
related to Girdin and PI3K co-expression.
| Characteristic | Exp (B) | 95% CI for Exp
(B) | P-value |
|---|
| Age | 0.528 | 0.378–1.408 | 0.270 |
| Tumor size | 1.074 | 0.542–1.860 | 0.164 |
| Histological
type | 2.612 | 1.264–4.105 | 0.01 |
| Metastatic node | 3.765 | 1.059–2.114 | 0.001 |
| Distant
metastasis | 4.156 | 1.958–6.426 | 0.001 |
| Triple-negative
breast cancer | 1.285 | 0.836–1.610 | 0.082 |
| Constant | 0.032 | | |
Prognostic analysis
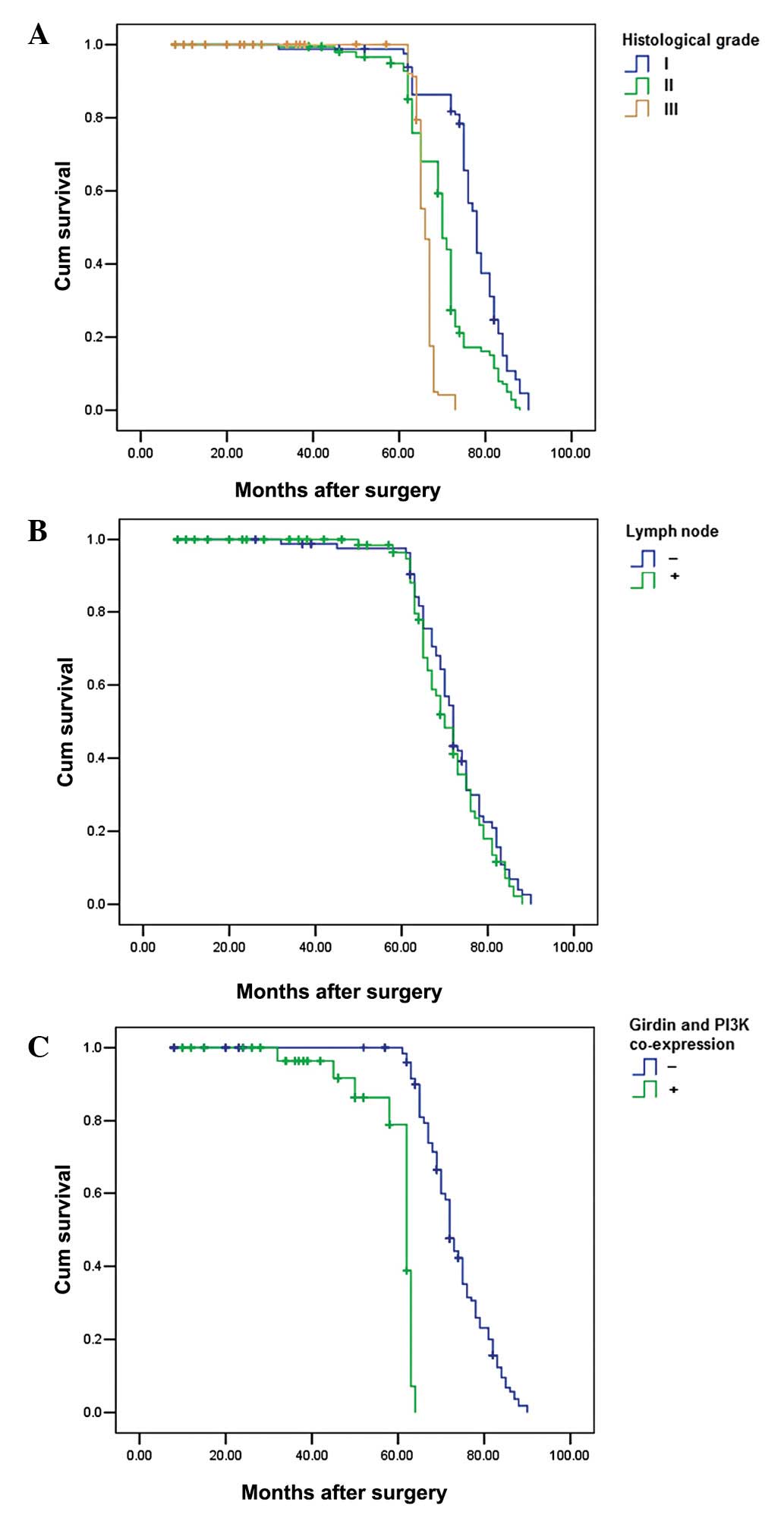
After analyzing survival rates, cases with Girdin
and PI3K co-expression were shown to have a significantly increased
distant metastasis rate and poorer postoperative, disease-specific
survival than those with Girdin and PI3K co-expression (P=0.001)
(Fig 3). In addition, histological
grade and lymph node metastasis were also significantly correlated
with the postoperative survival (P=0.01 and 0.001) (Fig. 3). In the Cox regression test, Girdin
and PI3K co-expression was detected as an independent prognostic
factor (P=0.001; Table III).
 | Table IIICox model regression analysis of the
colorectal cancer prognostic factors. |
Table III
Cox model regression analysis of the
colorectal cancer prognostic factors.
| Variables | OR | 95% CI for OR | P-value |
|---|
| Age | 1.182 | 0.725–1.884 | 0.261 |
| Tumor size | 1.403 | 0.654–2.071 | 0.154 |
| Histological
type | 1.628 | 1.319–3.166 | 0.020 |
| Metastatic node | 2.135 | 1.508–3.967 | 0.010 |
| Triple-negative
breast cancer | 2.753 | 1.472–4.324 | 0.001 |
| Girdin and PI3K
co-expression | 3.420 | 1.563–5.182 | 0.001 |
Discussion
Currently, the expression status of Girdin protein
in breast cancer and its correlation with the biological behavior
of breast cancer remains unclear (12). Furthermore, few studies have
addressed Girdin expression in breast cancer and its correlation
with the prognosis of breast cancer (13).
In our recent study, the Girdin protein was found to
be related to histological grade and distant metastasis of breast
cancer, and Girdin expression was significantly related to both
CerbB2 and Ki67 expression (6).
Dunkel et al reported that GIV is a metastasis-related
protein, serving as both a therapeutic target and a prognostic
biomarker in cancer patients, and Girdin is a direct target of the
transcription factor signal transducer and activator of
transcription-3 (STAT3) (14). In
another study, Girdin was considered to be a stem cell gene related
to the histological grade and distant metastasis of colorectal
cancer. Cases with high Girdin protein expression levels were shown
to attain a significantly higher rate of liver metastasis and
poorer postoperative, disease-specific survival than those with no
or low levels of Girdin protein expression in colorectal cancer
(15).
In this study, it was observed that Girdin and PI3K
proteins were highly expressed in breast cancer tumor stem cells
and Girdin and PI3K proteins co-immunoprecipitated in the MD-231
cell line. The results indicated that Girdin may have an important
role in breast cancer stem cells. We further investigated the
correlation between Girdin and PI3K co-expression and the
biological behavior of breast cancer. Finally, co-expression of
Girdin and PI3K protein was related to histological type,
metastatic nodes and distant metastasis and the cases with Girdin
and PI3K co-expression attained a poor postoperative,
disease-specific survival. In the Cox regression test, Girdin and
PI3K co-expression was detected to be an independent prognostic
factor.
In a recent study, Natsume et al discovered
that Girdin is highly expressed in human glioblastoma. Stable
Girdin knockdown in isolated glioblastoma stem cells resulted in
the decreased expression of stem cell markers, including CD133,
induced multilineage neural differentiation, and inhibited in
vitro cell motility, ex vivo invasion, the capacity for
sphere-formation and in vivo tumor formation (16). The outcome of the study indicated
that Girdin is required for glioblastoma-initiating stem cells to
sustain their stemness and invasive properties. Girdin has been
reported as a multidomain signaling molecule that enhances PI3K-Akt
signals downstream of both G protein-coupled and growth factor
receptors (17). However, there has
been no study based on the correlation between Girdin and PI3K in
breast cancer, although it was reported as a novel substrate of
Akt. The outcomes of this study illustrated that Girdin and PI3K
have a linear correlation in breast cancer and they may be
necessary to the PI3K/Akt/mTOR pathway. However, the specific
mechanism involved requires further investigation.
Acknowledgements
This study was supported by grants
from the China National Natural Science Foundation (No. 81102029
and 81172047).
References
|
1
|
Gaikwad SM and Ray P: Non-invasive imaging
of PI3K/Akt/mTOR signalling in cancer. Am J Nucl Med Mol Imaging.
2:418–431. 2012.PubMed/NCBI
|
|
2
|
Xu HY, Chen ZW, Hou JC, Du FX and Liu JC:
Jolkinolide B induces apoptosis in MCF-7 cells through inhibition
of the PI3K/Akt/mTOR signaling pathway. Oncol Rep. 29:212–8.
2013.PubMed/NCBI
|
|
3
|
Slomovitz BM and Coleman RL: The
PI3K/AKT/mTOR pathway as a therapeutic target in endometrial
cancer. Clin Cancer Res. 18:5856–5864. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kim H, Choi JA, Park GS and Kim JH: BLT2
up-regulates interleukin-8 production and promotes the invasiveness
of breast cancer cells. PLoS One. 7:e491862012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Bao R, Christova T, Song S, Angers S, Yan
X and Attisano L: Inhibition of tankyrases induces Axin
stabilization and blocks Wnt signalling in breast cancer cells.
PLoS One. 7:e486702012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Liu C, Zhang Y, Xu H, Zhang R, Li H, Lu P
and Jin F: Girdin protein: a new potential distant metastasis
predictor of breast cancer. Med Oncol. 29:1554–1560. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Mittal Y, Pavlova Y, Garcia-Marcos M and
Ghosh P: Src homology domain 2-containing protein-tyrosine
phosphatase-1 (SHP-1) binds and dephosphorylates
G(alpha)-interacting, vesicle-associated protein (GIV)/Girdin and
attenuates the GIV-phosphatidylinositol 3-kinase (PI3K)-Akt
signaling pathway. J Biol Chem. 286:32404–32415. 2011. View Article : Google Scholar
|
|
8
|
Jiang P, Enomoto A, Jijiwa M, Kato T,
Hasegawa T, Ishida M, Sato T, Asai N, Murakumo Y and Takahashi M:
An actin-binding protein Girdin regulates the motility of breast
cancer cells. Cancer Res. 68:1310–1318. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ghosh P, Garcia-Marcos M and Farquhar MG:
GIV/Girdin is a rheostat that fine-tunes growth factor signals
during tumor progression. Cell Adh Migr. 5:237–248. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Xu D, Xu H, Ren Y, Liu C, Wang X, Zhang H
and Lu P: Cancer stem cell-related gene periostin: a novel
prognostic marker for breast cancer. PLoS One. 7:e466702012.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Liu C, Chen B, Zhu J, Zhang R, Yao F, Jin
F, Xu H and Lu P: Clinical implications for nestin protein
expression in breast cancer. Cancer Sci. 101:815–819. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ling Y, Jiang P, Cui SP, Ren YL, Zhu SN,
Yang JP, Du J, Zhang Y, Liu JY and Zhang B: Clinical implications
for girdin protein expression in breast cancer. Cancer Invest.
29:405–410. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wang J, Fu L, Gu F and Ma YJ: A new
protein Girdin in tumor metastasis. Chin Med J (Engl).
123:1786–1788. 2010.PubMed/NCBI
|
|
14
|
Dunkel Y, Ong A, Notani D, Mittal Y, Lam
M, Mi X and Ghosh P: STAT3 upregulates GIV/Girdin expression, and
GIV enhances STAT3 activation in a positive feedback loop during
wound healing and tumor invasion/metastasis. J Biol Chem.
287:41667–41683. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Liu C, Xue H, Lu Y and Chi B: Stem cell
gene Girdin: a potential early liver metastasis predictor of
colorectal cancer. Mol Biol Rep. 39:8717–22. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Natsume A, Kato T, Kinjo S, Enomoto A,
Toda H, Shimato S, Ohka F, Motomura K, Kondo Y, Miyata T, Takahashi
M and Wakabayashi T: Girdin maintains the stemness of glioblastoma
stem cells. Oncogene. 31:2715–2724. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ghosh P, Garcia-Marcos M and Farquhar MG:
GIV/Girdin is a rheostat that fine-tunes growth factor signals
during tumor progression. Cell Adh Migr. 5:237–248. 2011.
View Article : Google Scholar : PubMed/NCBI
|