Introduction
Lipocalin 2, also known as neutrophil
gelatinase-associated lipocalin (NGAL), is a prominent member of
the lipocalin family. The lipocalins constitute a large group of
small, predominantly extracellular proteins previously regarded as
obscure transporters of hydrophobic ligands (1,2 and refs.
therein). In humans, NGAL was originally identified as a 25-kDa
protein covalently linked to matrix metalloproteinase-9 (MMP-9) in
human neutrophils (3), which
normally provide the main cellular source of circulating NGAL.
MMP-9, by degrading components of the extracellular matrix and thus
promoting the release of growth factors, is important in tumor
growth and tumorigenicity (4,5). By
forming the MMP-9/NGAL complex, NGAL protects MMP-9 from
proteolytic degradation, increasing the enzymatic activity of MMP-9
and subsequently enhancing tumoral invasiveness and diffusion
(6).
NGAL expression has been studied in several normal
tissues where it funtions to modulate oxidative stress and to
provide protection against bacterial infection. Its expression is
altered in several benign conditions, including inflammatory,
ischemic and metabolic disorders. With regard to the kidney, NGAL
is expressed in the epithelial cells and is involved in kidney
development, where it has been demonstrated to regulate epithelial
morphogenesis. Persistent high levels of NGAL lead to the
development of proliferative renal lesions and chronic kidney
disease via an epidermal growth factor receptor-dependent mechanism
(7). Recent data have demonstrated
that increased levels of NGAL are present in chronic kidney disease
and acute kidney damage (8,9). Furthermore, it is overexpressed in
numerous tumor types, including breast, thyroid, colorectal,
gastric and pancreatic cancer (8).
Observations in animal models and human subjects suggest that NGAL
is required for the development and/or progression of benign and
malignant disease, and its expression is associated with invasive
cancer progression. Several studies have investigated the level of
circulating NGAL in the blood as a potential marker for the
detection and prognostication of solid tumor and hematological
malignancies (8,10–13).
However, it is likely that the level of NGAL is largely
neoplasia-specific and influenced by tumor type.
In the present study, we measured serum and urinary
levels of NGAL, MMP-9 and MMP-9/NGAL complex in patients with
oncocytoma and clear cell renal cell carcinoma (ccRCC) in order to
verify whether these molecules may offer a potential non-invasive
biomarker to provide useful clinical information for kidney
carcinoma.
Materials and methods
Patients
Patients were selected for the study and samples of
their peripheral venous blood and first morning urine were
collected prior to surgical or other therapeutic intervention.
Specimens were obtained from patients who had undergone surgical
procedure. Diagnosis of the tumor type was performed by usual
clinical laboratory criteria and confirmed by histopathological
observations. The age of patients ranged between 40 and 73 years
(mean ± SD, 59.2±9.7) and in total, there were 11 males and 9
females. The tumors were classified by grade and stage according to
the pTNM classification (14). All
patients provided written informed consent. The study was approved
by the local ethics committee. Normal, healthy laboratory
volunteers provided their permission verbally. The healthy
volunteers had no concomitant illnesses, including no signs of
infection, gastrointestinal hepatic or renal disease. The values of
the basic laboratory parameters of these participants were within
the reference limits.
Serum
Peripheral venous blood samples were collected in
vacutainers, allowed to clot for 30 min at room temperature and
centrifuged at 1,600 g for 10 min at 4°C. The samples were then
divided into aliquots and stored at −20°C until used. Each aliquot
was used only once in order to prevent enzyme activation due to
freeze-thawing processes.
Urine sample preparation
Prior to analysis, urine samples were examined using
the Multistix Combur test (Roche Diagnostics GmbH, Mannheim,
Germany). Urine samples which tested positive for leukocytes were
excluded due to confounding leukocytic gelatinases. Microscopic
hematuria present in the majority of cancer samples was not
quantified, however, excessively hematuric samples were excluded.
Samples were frozen immediately following collection and stored at
−20°C until assay. The samples were thawed and an aliquot of each
sample (15 ml) was centrifuged at 1,000 × g for 10 min at 4°C.
Supernatant was collected and used to determine NGAL, MMP-9 and
MMP-9/NGAL by immunoassay.
Measurement of NGAL, MMP-9 and
MMP-9/NGAL
NGAL and the MMP-9/NGAL complex levels were
determined by a solid-phase immunoassay using commercial kits
obtained from R&D Systems (Minneapolis, MN, USA). The NGAL
assay used two monoclonal antibodies specific for two different
epitopes of lipocalin 2. The MMP-9/NGAL complex assay used
monoclonal antibodies raised against recombinant human MMP-9 and
recombinant human NGAL, and is subsequently not capable of
detecting recombinant human MMP-9 or NGAL in their free forms.
MMP-9 was detected by immunoassay (ELISA) with a commercial kit
obtained from GE Healthcare (Buckinghamshire, UK). This assay is
based on a two-site sandwich format using two antibodies directed
against different epitopes of the molecule; the assay recognises
the precursor of MMP-9 (proMMP-9) and that complexed with TIMP-1
(proMMP-9/TIMP-1 complex). All analyses were performed according to
the manufacturer's instructions.
Statistical analysis
All statistical analyses were performed with the
statistical computing environment R (version 2.12.1; R Foundation
for Statistical Computing, Vienna, Austria). Results are summarized
as the mean ± standard deviation (SD). Fisher's exact test was
performed. P<0.05 was considered to indicate a statistically
significant difference.
Results
Patients
During a 1-year period, a total of 20 patients with
kidney disease were evaluated. Of these patients, 4 had oncocytoma
and 16 had ccRCC. For each of the patients, a venous blood sample
was collected, and for four of the patients with oncocytoma and 9
patients with ccRCC, first morning urine samples were obtained. All
three molecules, including NGAL, MMP-9 and their complex MMP-9/NGAL
were measured in serum and in urine samples. Since normal values
for these molecules were unavailable, we measured these molecules
in sera and in the urine of 53 healthy subjects, which was
considered as the control group.
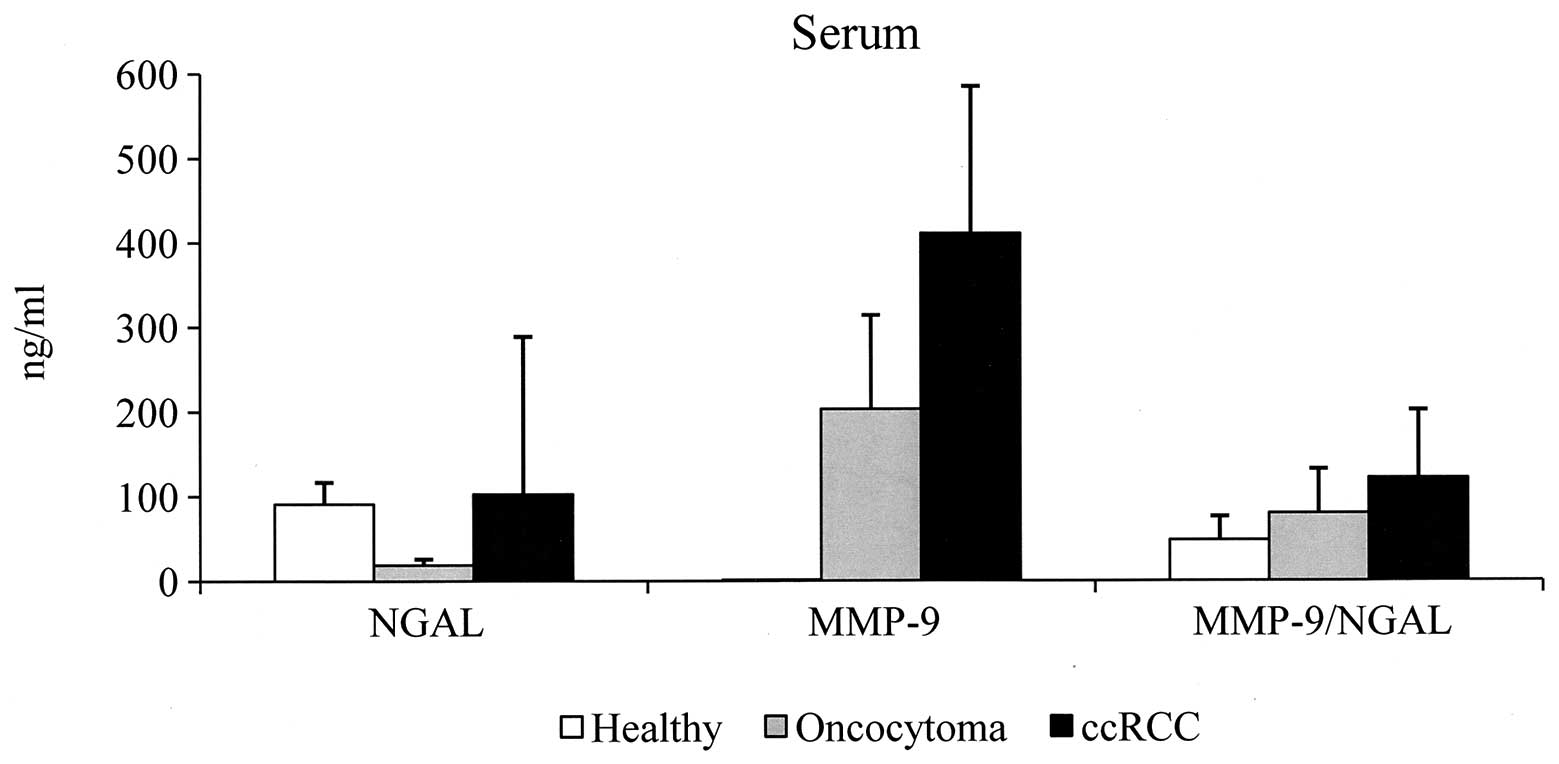
Serum samples
In sera of the control group, NGAL was detected in
all samples with a value ranging between 35 and 153 ng/ml (91±26).
MMP-9 was undetectable at levels equal to or below the sensitivity
of the assay. MMP-9/NGAL complex was detected in all specimens and
ranged between 9 and 145 ng/ml (48±28). We established the cut-off
value by calculating the mean + 2SD and the following cut-off
values were considered; 143 ng/ml for NGAL and 104 ng/ml for
MMP-9/NGAL. Samples with a value higher than that of the cut-off
were considered positive. The data obtained in sera from patients
with oncocytoma and ccRCC are shown in Tables I and II, respectively. In oncocytoma, NGAL
values were negative in all specimens analyzed, while in ccRCC,
values were positive in 2/16 (12.5%) of specimens (range, 6–750;
103±186). MMP-9 was detected in all specimens analyzed and with
values of 203±111 (range, 82–327) and 411±174 (range, 168–730)
observed in oncocytoma and ccRCC patients, respectively. Since
serum MMP-9 was undetectable in all healthy subjects, we considered
all pathological specimens positive as all samples possessed serum
MMP-9 values higher than the sensitivity of the assay (assay
sensitivity has been calculated by two standard deviations above
the zero dose binding of 80 determinations and was 0.8 ng/ml).
MMP-9/NGAL complex in oncocytoma was positive in 25% (1/4) of
samples (range, 18–145; 80±52), and was positive in 44% (7/16) of
ccRCC specimens (range, 14–306; 122±80). Considering the average
value of each molecule, we observed that the values are higher in
sera from ccRCC patients compared with those of oncocytoma
patients. In addition, the serum MMP-9 level is 2-fold higher in
ccRCC compared with oncocytoma specimens (Fig. 1).
 | Table ISerum levels of MMP-9, NGAL and
MMP-9/NGAL in oncocytoma patients. |
Table I
Serum levels of MMP-9, NGAL and
MMP-9/NGAL in oncocytoma patients.
| Case no. | Age (years) | Gender | Stage | Grade | NGAL (ng/ml) | MMP-9 (ng/ml) | MMP-9/NGAL
(ng/ml) |
|---|
| P1 | 42 | F | T1 N0 M0 | G1 | 11 | 259 | 145 |
| P2 | 66 | M | T1 N0 M0 | G1 | 26 | 142 | 71 |
| P3 | 59 | F | T2 N0 M0 | G1 | 16 | 82 | 18 |
| P4 | 59 | F | T1 N0 M0 | G1 | 22 | 327 | 85 |
 | Table IISerum levels of MMP-9, NGAL and
MMP-9/NGAL in clear cell renal cell carcinoma patients. |
Table II
Serum levels of MMP-9, NGAL and
MMP-9/NGAL in clear cell renal cell carcinoma patients.
| Case no. | Age (years) | Gender | Stage | Grade | NGAL (ng/ml) | MMP-9 (ng/ml) | MMP-9/NGAL
(ng/ml) |
|---|
| P5 | 69 | M | T1 N0 M0 | G1 | 31 | 168 | 56 |
| P6 | 54 | M | T1 N0 M0 | G1 | 54 | 355 | 114 |
| P7 | 53 | M | T1 N0 M0 | G2 | 750 | 173 | 62 |
| P8 | 60 | F | T1 N0 M0 | G2 | 306 | 609 | 42 |
| P9 | 51 | F | T1 N0 M0 | G2 | 39 | 356 | 160 |
| P10 | 63 | F | T1 N0 M0 | G2 | 18 | 428 | 92 |
| P11 | 63 | M | T2 N0 M0 | G2 | 67 | 312 | 102 |
| P12 | 60 | F | T2 N0 M0 | G2 | 37 | 575 | 237 |
| P13 | 40 | M | T2 N0 M0 | G3 | 32 | 291 | 84 |
| P14 | 73 | M | T2 N0 M0 | G3 | 80 | 202 | 14 |
| P15 | 70 | M | T2 N0 M0 | G3 | 6 | 497 | 117 |
| P16 | 61 | M | T2 N0 M0 | G3 | 58 | 681 | 306 |
| P17 | 73 | F | T2 N0 M0 | G3 | 70 | 730 | 229 |
| P18 | 43 | M | T2 N0 M0 | G3 | 60 | 499 | 177 |
| P19 | 67 | M | T3 N0 M1 | G3 | 15 | 309 | 69 |
| P20 | 57 | F | T3b N0 M1 | G3 | 18 | 393 | 90 |
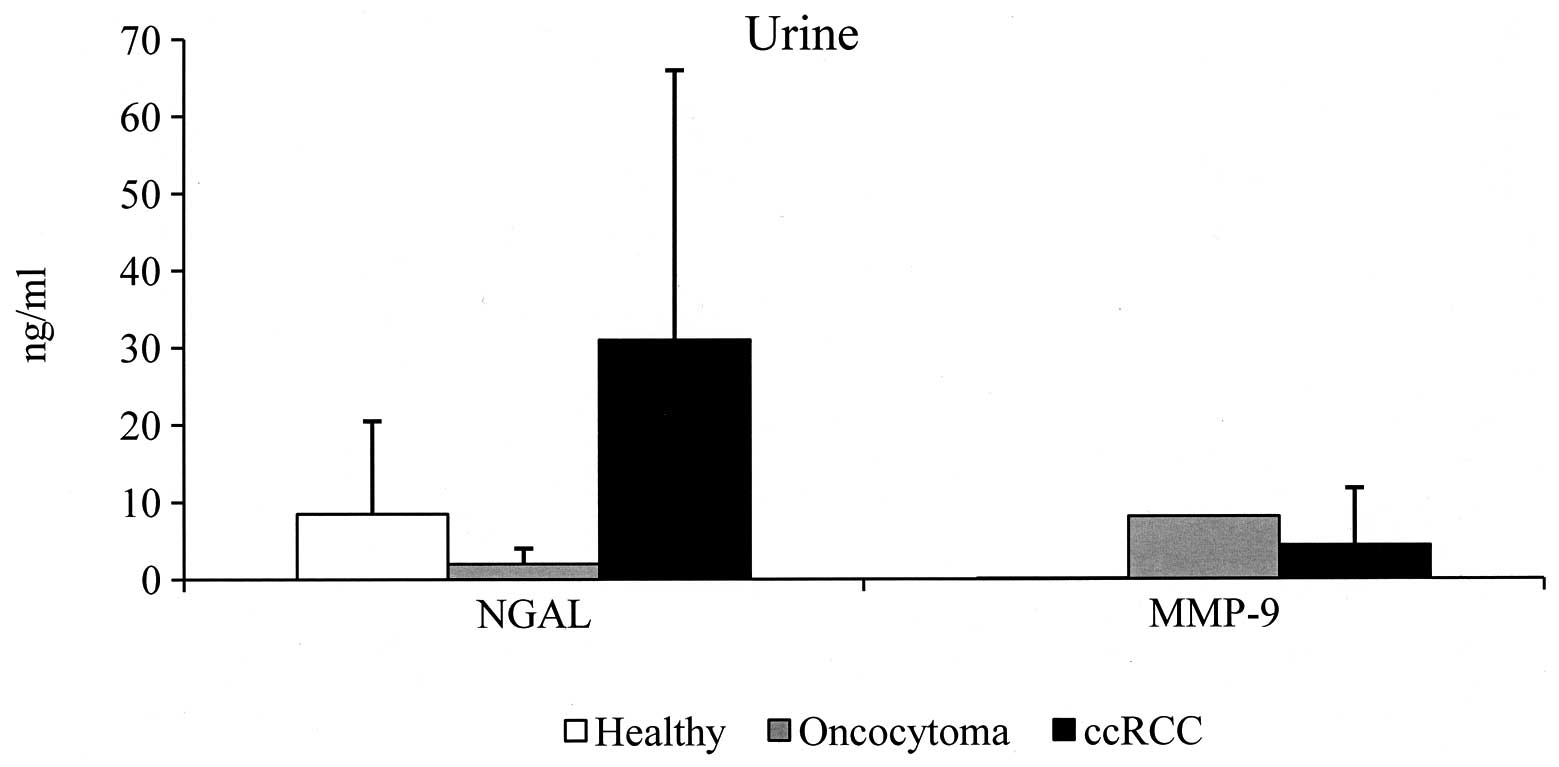
Urine samples
With regard to urine specimens, NGAL was detected in
all urine samples of the control group with a value ranging between
0.1 and 52 ng/ml (8.5±12), with 32.5 ng/ml being considered as the
cut-off value. By contrast, no cut-off value for urinary MMP-9 and
urinary MMP-9/NGAL complex was established since MMP-9 was
undetectable in all specimens analyzed and the MMP-9/NGAL complex
was detectable in only 10% of urine samples from normal healthy
subjects. In oncocytoma, urinary NGAL values were negative in all
specimens analyzed, whereas 44% (4/9) of ccRCC specimens were
positive (range, 0.9–116; 31±35.5). Urinary MMP-9 was detected in
only one oncocytoma specimen with a value of 8.13 ng/ml, and in 67%
(6/9) of ccRCC patients (range, 0.55–22.8; 4.38±7.4). MMP-9/NGAL
complex was undetectable in all urine samples analyzed from
oncocytoma and ccRCC patients (Tables
III and IV). The average value
of NGAL and MMP-9 of the positive urine specimens is shown in
Fig. 2. It is evident that the most
abundant substance in ccRCC patients is NGAL and that the mean
value is 3.6-fold higher than that detected in the urine samples of
the control group. Secondly, the mean value of urinary MMP-9 is
less than that of sera. Finally, specimens P19 (T3N0M1, G3) and P20
(T3bN0M1, G3) demonstrated metastasis of the bone. We demonstrated
that serum and urine values of NGAL in these patients were not
increased compared with the mean values of healthy subjects.
 | Table IIIUrine levels of MMP-9, NGAL and
MMP-9/NGAL in oncocytoma patients. |
Table III
Urine levels of MMP-9, NGAL and
MMP-9/NGAL in oncocytoma patients.
| Case no. | Age (years) | Gender | Stage | Grade | NGAL (ng/ml) | MMP-9 (ng/ml) | MMP-9/NGAL
(ng/ml) |
|---|
| P1 | 42 | F | T1 N0 M0 | G1 | 4.7 | 8.13 | N.D. |
| P2 | 66 | M | T1 N0 M0 | G1 | 0.1 | N.D. | N.D. |
| P3 | 59 | F | T2 N0 M0 | G1 | 3.2 | N.D. | N.D. |
| P4 | 59 | F | T1 N0 M0 | G1 | 0.1 | N.D. | N.D. |
 | Table IVUrine levels of MMP-9, NGAL and
MMP-9/NGAL in clear cell renal cell carcinoma patients. |
Table IV
Urine levels of MMP-9, NGAL and
MMP-9/NGAL in clear cell renal cell carcinoma patients.
| Case no. | Age (years) | Gender | Stage | Grade | NGAL (ng/ml) | MMP-9 (ng/ml) | MMP-9/NGAL
(ng/ml) |
|---|
| P5 | 69 | M | T1 N0 M0 | G1 | 34.1 | 6.93 | N.D. |
| P6 | 54 | M | T1 N0 M0 | G1 | 20.2 | N.D. | N.D. |
| P7 | 53 | M | T1 N0 M0 | G2 | 116 | 0.55 | N.D. |
| P9 | 51 | F | T1 N0 M0 | G2 | 41.1 | N.D. | N.D. |
| P13 | 40 | M | T2 N0 M0 | G3 | 0.9 | 1.68 | N.D. |
| P14 | 73 | M | T2 N0 M0 | G3 | 26.5 | N.D. | N.D. |
| P15 | 70 | M | T2 N0 M0 | G3 | 34.4 | 22.80 | N.D. |
| P19 | 67 | M | T3 N0 M1 | G3 | 3.8 | 6.13 | N.D. |
| P20 | 57 | F | T3b N0 M1 | G3 | 1.9 | 1.35 | N.D. |
Discussion
Renal cancer is generally silent and frequently
fatal. The majority of kidney tumors are discovered co-incidentally
during abdominal imaging performed for unrelated diagnostic
reasons. Currently, no diagnostic modality for the early detection
of renal cancer exists, other than incidental radiological
discovery. In addition, no method capable of monitoring recurrence
currently exists. Thus, there is a great interest in identifying
‘biomarkers’ that are capable of improving this situation. Several
tumor markers have been examined previously, however, there are no
definitive biomarkers available for such purposes (15). Among protein markers, MMP-2 and
MMP-9 have been investigated with variable results (16 and refs.
therein). By gelatin zymography, we have previously demonstrated
that the most abundant serum lytic activity was at 92 kDa (MMP-9)
and that MMP-9 activity was slightly enhanced in sera from ccRCC
patients compared with oncocytoma patients. In the present study,
we obtained identical results using ELISA. However, there was a
broad overlap of the data and we identified no correlation to the
type of carcinoma, pathological TNM stage or histological
grading.
NGAL is a biomarker of tubular injury, is expressed
in several histotypes of renal tumors and its high expression is
associated with a higher histological grade of ccRCC and papillary
RCC, whereas oncocytoma and urothelial carcinoma exhibit lower
expression levels (17). By
immunohistochemistry, Perrin et al showed that NGAL was
expressed by neutrophils infiltrating ccRCC and that the density of
NGAL-expressing neutrophils was associated with poor outcomes
(18). Furthermore, they reported
that high NGAL concentrations in serum were also associated with
shorter progression-free survival (18). In patients treated with sunitinib,
Porta et al demonstrated that serum levels of NGAL were
significant predictors of progression-free survival (19). NGAL appears to protect MMP-9 from
autodegradation, increasing its activity by binding and forming
MMP-9/NGAL complexes. Tumor cells excrete elevated levels of NGAL
resulting in an increase of the local concentration of MMP-9, which
is capable of affecting various aspects of tumor progression. High
concentrations of MMP-9/NGAL complex in serum have been associated
with a shorter progression-free survival and poor overall survival
(18). The results outlined in the
present study indicate that the mean values of serum NGAL and
MMP-9/NGAL complex are higher in ccRCC patients compared with
oncocytoma patients. However, there was a broad overlap of the data
and we observed no correlation with kidney disease severity.
Following localized tumors or monitoring drug-based
therapy results by analyzing tumor-specific markers in the
available excretory product of the kidney is highly desirable.
However, to the best of our knowledge, there is only limited
literature available with regard to urine markers for RCC.
Concerning NGAL, it is evident that any NGAL systematically
released from malignantly transformed cells would be freely
filtered by the kidney glomerulus, however, may be expected to be
largely reabsorbed by efficient endocytic mechanisms in the
proximal tubules. Therefore, urinary excretion of NGAL is more
likely to be present in tandem with a concomitant renal tubular
injury that increases de novo NGAL synthesis and/or
precludes NGAL reabsorption. We demonstrated that, in ccRCC
patients, the mean value of urinary NGAL is higher than that
observed in the urine of the control group. Our data support the
findings of Morrisssey et al(20). Meanwhile, like Morrissey et
al, we identified no correlation with tumor size or stage. With
regard to MMP-9/NGAL complex, recent evidence suggests that urinary
detection of the complex may represent a new biomarker for the
prediction of cancer disease (21–23).
In particular, MMP-9/NGAL enzymatic activity was observed in the
urine of breast cancer patients but not in healthy controls
(21), and in 50% of urine samples
from prostate cancer patients and in 49% of the urine from bladder
cancer patients (22). Evaluation
of MMP-9 and MMP-9/NGAL complex in urine of patients with brain
tumors revealed significantly higher expression levels compared
with controls (23), which was also
confirmed in tumor tissue. Following tumor resection, clearing of
biomarkers was observed. These findings have led to the suggestion
that the urinary detection of MMP-9/NGAL complex may represent a
novel biomarker with potential for generalized application in
cancer diagnostics and prognostics. However, no studies of urinary
MMP-9/NGAL complex in kidney carcinoma are currently available. By
ELISA, we detected no MMP-9/NGAL in urine specimens of oncocytoma
and ccRCC patients and only in 10% of urine specimens from healthy
individuals. The source of the MMP-9/NGAL complex in urine remains
unknown. In fact, due to its large size, it seems unlikely that the
MMP-9/NGAL complex is capable of being directly filtered from the
serum to the urine. Instead, it is likely that MMP-9 and NGAL are
mainly secreted into the blood by neutrophils infiltrating the
tumors and are separately excreted in urine where they subsequently
from the complex (6).
In spite of recent evidence and interest among
biologists and oncologists, the serum and urine levels of MMP-9,
NGAL and their complex (MMP-9/NGAL) appear to not provide an
adequate test to identify kidney cancer. Nevertheless, due to the
small number of patients included in the studies, the conclusion
may not be transferable to the general population and therefore
need further evaluation for validating diagnostic and prognostic
utility.
References
|
1
|
Flower DR: The lipocalin protein family:
structure and function. Biochem J. 318:1–14. 1996.
|
|
2
|
Di Carlo A: The enigmatic role of
lipocalin 2 in human cancer. The multifunctional protein neutrophil
gelatinase-associated lipocalin (NGAL) and its ambiguous role in
human neoplasias. Prevent Res. 2: View Article : Google Scholar
|
|
3
|
Triebel S, Bläser J, Reinke H and
Tschesche H: A 25 kDa alpha 2-microglobulin-related protein is a
component of the 125 kDa form of human gelatinase. FEBS Lett.
314:386–388. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Egeblad M and Werb Z: New functions for
the matrix metalloproteinases in cancer progression. Nat Rev
Cancer. 2:161–174. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
5
|
Björklund M and Koivunen E:
Gelatinase-mediated migration and invasion of cancer cells. Biochim
Biophys Acta. 1755:37–69. 2005.PubMed/NCBI
|
|
6
|
Yan L, Borregaard N, Kjeldsen L and Moses
MA; The high molecular weight urinary matrix metalloproteinase
(MMP) activity is a complex of gelatinase B/MMP and neutrophil
gelatinase-associated lipocalin (NGAL): Modulation of MMP-9
activity by NGAL. J Biol Chem. 276:37258–37265. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Viau A, El Karoui K, Laouari D, Burtin M,
Nguyen C, Mori K, Pillebout E, Bertger T, Mak TW, Knebelmann B,
Friedlander G, Barasch J and Terzi F: Lipocalin 2 is essential for
chronic kidney disease progression in mice and humans. J Clin
Invest. 120:4065–4076. 2010. View
Article : Google Scholar : PubMed/NCBI
|
|
8
|
Chakraborty S, Kaur S, Guha S and Batra
SK: The multifaceted roles of neutrophil gelatinase associated
lipocalin (NGAL) in inflammation and cancer. Biochim Biophys Acta.
1826:129–169. 2012.PubMed/NCBI
|
|
9
|
Bolignano D, Donato V, Coppolino G, Campo
S, Buemi A, Lacquaniti A and Buemi M: Neutrophil
gelatinase-associated lipocalin (NGAL) as a marker of kidney
damage. Am J Kidney Dis. 52:595–605. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Cho H and Kim JH: Lipocalin2 expressions
correlate significantly with tumor differentiation in epithelial
ovarian cancer. J Histochem Cytochem. 57:513–521. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wang HJ, He XJ, Ma YY, Jiang XT, Xia YJ,
Ye ZY, Zhao ZS and Tao HQ: Expression of neutrophil
gelatinase-associated lipocalin in gastric cancer: a potential
biomarker for prognosis and ancillary diagnostic test. Anat Rec
(Hoboken). 293:1855–1863. 2010. View
Article : Google Scholar : PubMed/NCBI
|
|
12
|
Provatopoulou X, Gounaris A, Kalogera E,
Zagouri F, Flessas I, Goussetis E, Nonni A, Papassotiriou I and
Zografos G: Circulating levels of matrix metalloproteinase-9
(MMP-9), neutrophil gelatinase-associated lipocalin (NGAL) and
their complex MMP-9/NGAL in breast cancer disease. BMC Cancer.
9:3902009. View Article : Google Scholar
|
|
13
|
Moniaux N, Chakraborty S, Yalniz M,
Gonzalez J, Shostrom VK, Standop J, Lele SM, Ouellette M, Pour PM,
Sasson AR, Brand RE, Hollingsworth MA, Jain M and Batra SK: Early
diagnosis of pancreatic cancer: neutrophil gelatinase-associated
lipocalin as a marker of pancreatic intraepithelial neoplasia. Br J
Cancer. 98:1540–1547. 2008. View Article : Google Scholar
|
|
14
|
Sobin LH and Wittekind CH; International
Union Against Cancer (UICC): TNM Classification Of Malignant
Tumors. 6th edition. New York, NY: Wiley-Liss; pp. 193–195.
2002
|
|
15
|
Kashyap MK, Kumar A, Emelianenko N,
Kashyap A, Kaushik R, Huang R, Khullar M, Sharma SK, Singh SK,
Bhargave AK and Upadhyaya SK: Biochemical and molecular markers in
renal cell carcinoma: an update and future prospects. Biomarkers.
10:258–294. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Di Carlo A: Matrix metalloproteinase-2 and
-9 in the sera and in the urine of human oncocytoma and renal cell
carcinoma. Oncol Rep. 28:1051–1056. 2012.PubMed/NCBI
|
|
17
|
Barresi V, Ieni A, Bolignano D, Magno C,
Buemi M and Barresi G: Neutrophil gelatinase-associated lipocalin
immunoexpression in renal tumors: Correlation with histotype and
histological grade. Oncol Rep. 24:305–310. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Perrin C, Patard JJ, Jouan F, Collet N,
Théoleyre S, Edeline J, Zerrouki S, Laguerre B, Bellaud-Roturaud
MA, Rioux-Leclercq N and Vigneau C: The neutrophil
gelatinase-associated lipocalin, or LCN 2, marker of aggressiveness
in clear cell renal carcinoma. Prog Urol. 21:851–858. 2011.In
French.
|
|
19
|
Porta C, Paglino C, De Amici M, Quaglini
S, Sacchi L, Imarisio I and Canipari C: Predictive value of
baseline serum vascular endothelial growth factor and neutrophil
gelatinase-associated lipocalin in advanced kidney cancer patients
receiving sunitinib. Kidney Int. 77:809–815. 2010. View Article : Google Scholar
|
|
20
|
Morrissey JJ, London AN, Lambert MC and
Kharasch ED: Sensitivity and specificity of urinary neutrophil
gelatinase-associated lipocalin and kidney injury molecule-1 for
the diagnosis of renal cell carcinoma. Am J Nephrol. 34:391–398.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Fernández CA, Yan L, Louis G, Yang J,
Kutok JL and Moses MA: The matrix metalloproteinase-9/neutrophil
gelatinase-associated lipocalin complex plays a role in breast
tumor growth and is present in the urine of breast cancer patients.
Clin Cancer Res. 11:5390–5395. 2005.
|
|
22
|
Roy R, Louis G, Loughlin KR, Wiederschain
D, Kilroy SM, Lamb CC, Zurakowski D and Moses MA: Tumor-specific
urinary matrix metalloproteinase fingerprinting: identification of
high molecular weight urinary matrix metalloproteinase species.
Clin Cancer Res. 14:6610–6617. 2008. View Article : Google Scholar
|
|
23
|
Smith ER, Zurakowski D, Saad A, Scott RM
and Moses MA: Urinary biomarkers predict brain tumor presence and
response to therapy. Clin Cancer Res. 14:2378–2386. 2008.
View Article : Google Scholar : PubMed/NCBI
|