Introduction
Ilex kudingcha C.J. Tseng (Kudingcha) is a
bitter tea of Chinese origin. Kudingcha has been consumed
traditionally as a type of herbal tea in China and South Eastern
Asia (1). Ilex kudingcha is
one of the main plants that produces Kudingcha in China. Certain
studies have investigated its chemical composition and
pharmaceutical functions, which demonstrated the numerous
functional compositions of Kudingcha and the functional effects of
those compositions, such as the antioxidant effect (2). It has been reported that Kudingcha is
rich in polyphenolic compounds and that it demonstrates potent
antioxidant activities in vitro(3,4). It
also has been demonstrated that the major phenolic compounds in
Kudingcha are caffeoylquinic acid (CQA) derivatives. CQA
derivatives are natural functional compounds isolated from a
variety of plants, which possess a broad range of pharmacological
properties, including antioxidant, hepatoprotectant, antibacterial,
antihistaminic, anticancer, neuroprotective and other biological
effects (5,6).
Apoptosis induction in cancer cells is initially
identified by morphological changes, including cell shrinkage,
membrane blebbing, chromatin condensation and nuclear fragmentation
(7). Apoptosis is an important
defense against cancer that involves the elimination of potentially
harmful cells. Numerous diseases have been associated with
dysregulated apoptotic processes that ultimately lead to the
inhibition of cell death and propagation of diseases, such as
cancer (8).
A previous epidemiological study showed that chronic
inflammation predisposes individuals to certain types of cancer
(9). Hallmarks of
inflammation-related cancers include the presence of inflammatory
cells and mediators in tumor tissues, tissue remodeling and
angiogenesis, similar to that observed during chronic inflammatory
responses and tissue repair. The study of mechanisms underlying
inflammation-related cancer has been focused on the early stages of
cancer (10).
Previously, Kudingcha was shown to demonstrate
strong in vitro anti-cancer effects in human nasopharyngeal
carcinoma cells (11). In the
present study, the anti-cancer and anti-metastatic effects of
Kudingcha were further examined. MCF-7 human breast adenocarcinoma
cells were treated with Kudingcha and the molecular mechanisms
underlying the consequent anticancer effects were studied. Changes
in the activities of Kudingcha were evaluated at different
concentrations and the anti-metastatic effects were assessed in
mice with tumors propagated by 26-M3.1 colon carcinoma cells.
Materials and methods
Preparation of Ilex kudingcha C. J. Tseng
(Kudingcha)
Kudingcha was purchased in Chongqing, China. The
Kudingcha was stored at −80°C and freeze-dried to produce a powder.
A 20-fold volume of boiling water was added to the powdered sample
and extracted twice. The water extract was evaporated using a
rotary evaporator (N-1100; Eywla, Tokyo, Japan), concentrated and
then dissolved in dimethylsulfoxide (DMSO; Amresco, Solon, OH, USA)
to adjust to the stock concentration (20%, w/v).
Cancer cell preparation
MCF-7 human breast adenocarcinoma cells obtained
from the American Type Culture Collection (ATCC; Manassas, VA, USA)
were used for the experiments. The cells were cultured in RPMI-1640
medium (Gibco Co., Birmingham, MI, USA) supplemented with 10% fetal
bovine serum (FBS; Gibco Co.) and 1% penicillin-streptomycin (Gibco
Co.) at 37°C in a humidified atmosphere containing 5%
CO2 (model, 311 S/N29035; Forma, Waltham, MA, USA). The
medium was changed two or three times each week.
3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyltetrazoliumbromide (MTT)
assay
The anticancer effects of Kudingcha were assessed by
MTT assay. The MCF-7 human breast adenocarcinoma cells were seeded
in a 96-well plate at a density of 2×104cells/ml in a
volume of 180 μl per well. Kudingcha solutions (20
μl) with concentrations of 50, 100 and 200 μg/ml were
added and then the cells were incubated at 37°C in 5%
CO2 for 48 h. An MTT solution (200 μl, 5 mg/ml;
Amresco) was added and the cells were cultured for a further 4 h
under the same conditions. Subsequent to removing the supernatant,
150 μl of DMSO was added per well and mixed for 30 min.
Finally, the absorbance of each well was measured with an ELISA
reader (model 680; Bio-Rad, Hercules, CA, USA) at 540 nm (12).
RT-PCR to measure mRNA expression
Total RNA was isolated from the MCF-7 human breast
adenocarcinoma cells using TRIzol reagent (Invitrogen, Carlsbad,
CA, USA), according to the manufacturer’s instructions. The RNA was
digested with RNase-free DNase (Roche, Basel, Switzerland) for 15
min at 37°C and purified using an RNeasy kit (Qiagen, Hilden,
Germany), according to the manufacturer’s instructions. cDNA was
synthesized from 2 μg total RNA by incubation at 37°C for l
h with avian myeloblastosis virus (AMV) reverse transcriptase (GE
Healthcare, Little Chalfont, Buckinghamshire, UK) with random
hexanucleotides, according to the manufacturer’s instructions. The
sequences of the primers that were used to specifically amplify the
genes of interest are shown in Table
I. Amplification was performed in a thermal cycler (Eppendorf,
Hamburg, Germany). The PCR products were separated in 1.0% agarose
gels and visualized using ethidium bromide (EtBr) staining
(13).
 | Table ISequences of RT-PCR primers used in
the present study. |
Table I
Sequences of RT-PCR primers used in
the present study.
| Gene name | Sequence |
|---|
| Bax | Forward, 5′-AAG CTG
AGC GAG TGT CTC CGG CG-3′ |
| Reverse, 5′-CAG ATG
CCG GTT CAG GTA CTC AGT C-3′ |
| Bcl-2 | Forward, 5′-CTC GTC
GCT ACC GTC GTG ACT TGG-3′ |
| Reverse, 5′-CAG ATG
CCG GTT CAG GTA CTC AGT C-3′ |
| Caspase-3 | Forward, 5′-CAA ACT
TTT TCA GAG GGG ATC G-3′ |
| Reverse, 5′-GCA TAC
TGT TTC AGC ATG GCA-3′ |
| Caspase-9 | Forward, 5′-GGC CCT
TCC TCG CTT CAT CTC-3′ |
| Reverse, 5′-GGT CCT
TGG GCC TTC CTG GTA T-3′ |
| NF-κB | Forward, 5′-CAC TTA
TGG ACA ACT ATG AGG TCT CTG G-3′ |
| Reverse, 5′-CTG TCT
TGT GGA CAA CGC AGT GGA ATT TTA GG-3′ |
| IκB-α | Forward, 5′-GCT GAA
GAA GGA GCG GCT ACT-3′ |
| Reverse, 5′-TCG TAC
TCC TCG TCT TTC ATG GA-3′ |
| iNOS | Forward, 5′-AGA GAG
ATC GGG TTC ACA-3′ |
| Reverse, 5′-CAC AGA
ACT GAG GGT ACA-3′ |
| COX-2 | Forward, 5′-TTA AAA
TGA GAT TGT CCG AA-3′ |
| Reverse, 5′-AGA TCA
CCT CTG CCT GAG TA-3′ |
| GAPDH | Forward, 5′-CGG AGT
CAA CGG ATT TGG TC-3′ |
| Reverse, 5′-AGC CTT
CTC CAT GGT CGT GA-3′ |
Measurement of lung metastasis following
Kudingcha treatment in BALB/c mice bearing 26-M3.1 colon carcinoma
cell tumors
A quantity of 26-M3.1 colon carcinoma cells were
obtained from Professor Yoon at the Department of Food and
Nutrition, Yuhan University, Bucheon, South Korea. These highly
metastatic cells were maintained as monolayers in Eagle’s minimal
essential medium (EMEM; Gibco Co.) supplemented with 7.5% FBS, a
vitamin solution, sodium pyruvate, non-essential amino acids and
L-glutamine (Gibco Co.). The cultures were maintained in a
humidified atmosphere of 5% CO2 at 37°C. Experimental
lung metastasis was induced by injecting the colon 26-M3.1 cells
into the lateral tail vein of 6-week-old female Balb/c mice
(Experimental Animal Center of Chongqing Medical University,
Chongqing, China) (14). Kudingcha
solutions (400, 800 and 1,600 mg/kg) were subcutaneously injected
into the mice and after 2 days the animals were intravenously
inoculated with the 26-M-3.1 cells (2.5×104/mouse).
After 2 weeks the mice were sacrificed and their lungs were fixed
in Bouin’s solution (saturated picric acid:formalin:acetic acid,
15:5:1; v/v/v). The rate of metastasis was assessed by counting the
lung tumor colonies (tumors on the lung surface) as observed under
the naked eye using a digital camera (Canon D550, Tokyo, Japan).
The inhibitory rate of metastasis was assessed using the formula:
Inhibitory rate = (Number of control mouse metastatic tumors −
number of kudingcha mouse metastatic tumors) / number of control
mouse metastatic tumors × 100. The protocol for these experiments
was approved by the Animal Ethics Committee of Chongqing Medical
University.
Statistical analysis
Data are presented as the mean ± SD. Differences
between the mean values for individual groups were assessed using a
one-way ANOVA with Duncan’s multiple range test. P<0.05 was
considered to indicate a statistically significant difference. SAS
version 9.1 (SAS Institute Inc., Cary, NC, USA) was used for
statistical analysis.
Results
In vitro anticancer effect of Kudingcha
on MCF-7 cells
The anti-cancer effects of Kudingcha on the MCF-7
cells were evaluated using an MTT assay. The growth inhibitory
rates of the MCF-7 cells treated with the varying concentrations of
Kudingcha are shown in Table II.
When solutions of the Kudingcha were administered to the MCF-7
cells, the growth inhibitory rates observed at concentrations of
50, 100 and 200 μg/ml were 19, 58 and 81%, respectively
(P<0.05). These results demonstrated that Kudingcha had marked
anti-proliferative effects on the MCF-7 cells. In addition, it was
observed that the higher the concentration of Kudingcha, the
stronger the anticancer effects.
 | Table IIGrowth inhibition of MCF-7 human
breast adenocarcinoma cells caused by varying concentrations of
Kudingcha, as evaluated by MTT assay at OD540. |
Table II
Growth inhibition of MCF-7 human
breast adenocarcinoma cells caused by varying concentrations of
Kudingcha, as evaluated by MTT assay at OD540.
| Concentration of
sample, μg/ml
|
|---|
| Treatment | 50 | 100 | 200 |
|---|
| Control
(untreated) | - | 0.531±0.005a | - |
| Kudingcha | 0.430±0.007b (19) | 0.223±0.009c (58) | 0.101±0.008d (81) |
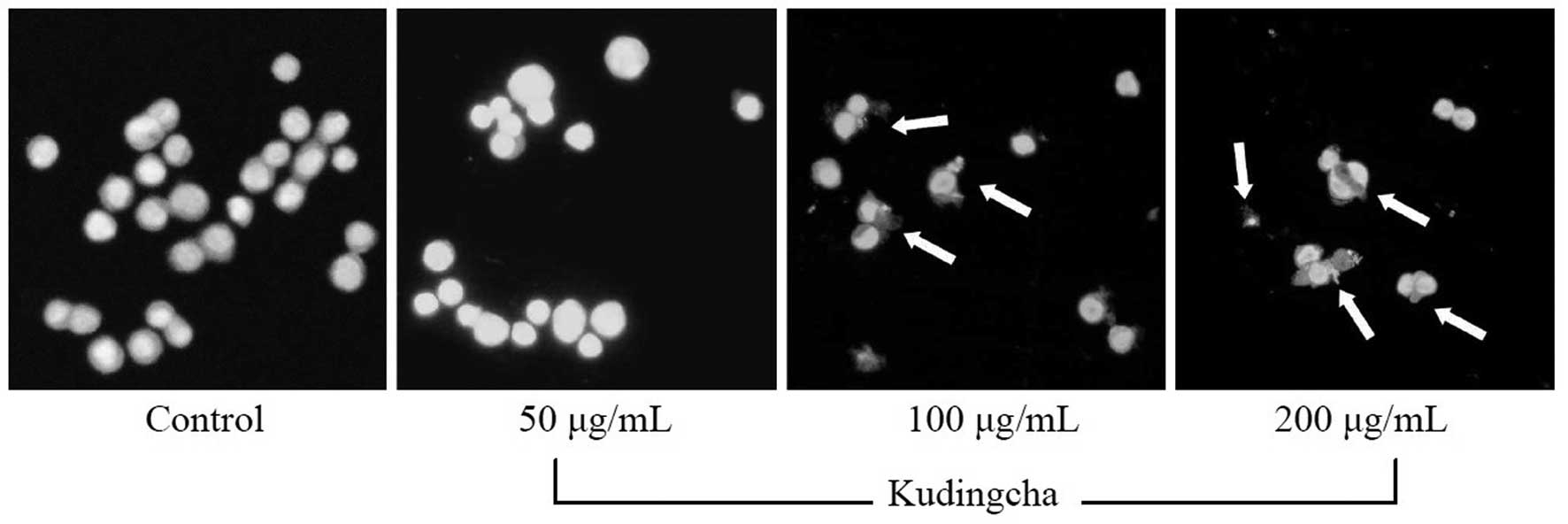
Induction of apoptosis by Kudingcha
In order to determine a possible mechanism
underlying the growth inhibitory activity of Kudingcha in the MCF-7
cancer cells, the induction of apoptosis was monitored. The extent
of chromatin condensation was analyzed by fluorescence microscopy
of cells stained with the DNA-binding fluorescent dye,
4,6-diamidino-2-phenylindole (DAPI). While the untreated MCF-7
cells presented nuclei with homogeneous chromatin distribution,
treatment with Kudingcha induced chromatin condensation and nuclear
fragmentation, suggesting the presence of apoptotic cells (Fig. 1).
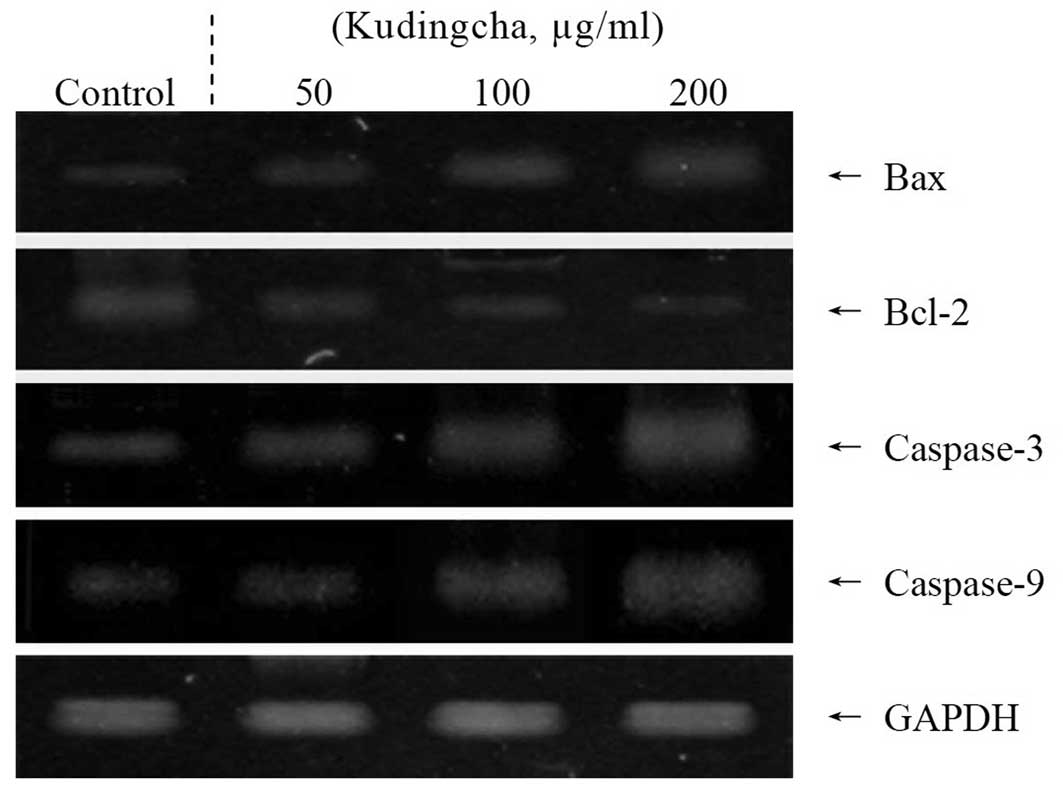
Apoptosis-related gene expression of Bax,
Bcl-2 and caspases
To elucidate the mechanisms underlying the
inhibition of cancer cell growth by Kudingcha, the expression of
Bax, Bcl-2, and caspase-3 and -9 was measured in the MCF-7 cells by
RT-PCR analysis following a 48-h incubation with the various
concentrations of Kudingcha solution. As shown in Fig. 2, the expression of pro-apoptotic Bax
and anti-apoptotic Bcl-2 showed significant changes in the presence
of 200 μg/ml Kudingcha. These results suggest that Kudingcha
induced apoptosis in the MCF-7 cells via a Bax- and Bcl-2-dependent
pathway. The mRNA expressions levels of caspase-3 and -9 were
extremely low in the untreated control MCF-7 cells, but
significantly increased once the cells were treated with 200
μg/ml Kudingcha. With increased concentrations of the
Kudingcha treatment, the mRNA expression of caspase-9 and -3 was
gradually elevated. More specifically, the apoptotic induction
caused by Kudingcha was correlated with the upregulation of Bax,
caspase-3 and caspase-9, and the down-regulation of Bcl-2 in terms
of mRNA expression. The effects of 200 μg/ml Kudingcha were
greater than those of the 100 and 50 μg/ml Kudingcha
solutions.
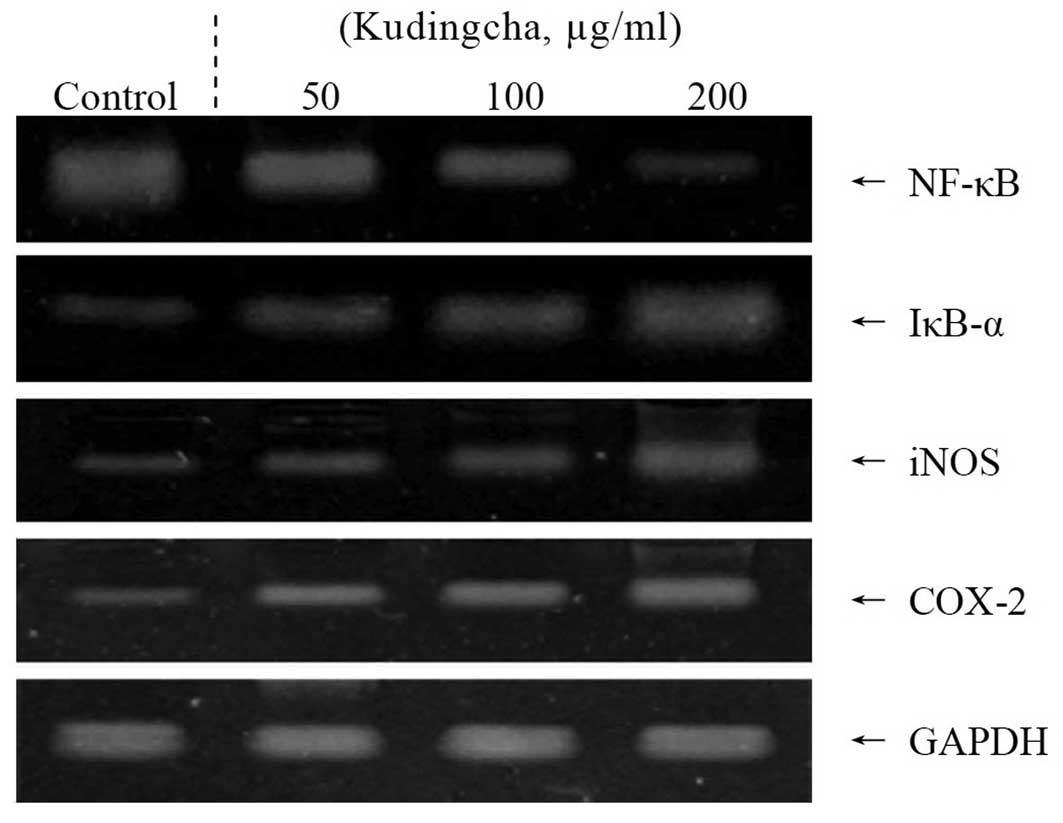
Inflammation-related gene expression of
NF-κB, IκB-α, iNOS and COX-2
The present study also determined whether the
anticancer actions of Kudingcha were associated with the inhibition
of NF-κB, IκB-α, iNOS and COX-2 gene expression. As shown in
Fig. 3, the mRNA expression of
NF-κB and IκB-α was reduced in the MCF-7 cells treated with 200
μg/ml Kudingcha solution. Kudingcha significantly modulated
the expression of the genes associated with inflammation. The mRNA
expression of NF-κB was decreased, while IκB-α mRNA expression
levels were increased. Additionally, the mRNA expression of COX-2
and iNOS was gradually decreased in the presence of Kudingcha
depending on the concentrations. These observations indicate that
Kudingcha may help prevent cancer in the early stages by increasing
anti-inflammatory activities. Overall, the results of this
experiment demonstrated that a higher concentration of Kudingcha
had a stronger anti-inflammatory effect on the human breast
adenocarcinoma cells than lower concentration solutions.
In vivo anti-metastatic effect of
Kudingcha
The prophylactic inhibition of tumor metastasis by
Kudingcha was evaluated using an experimental mouse metastasis
model (Table III). All
Kudingcha-treated mice had significantly fewer lung metastatic
colonies than those of the control mice (number of metastatic
tumors, 57±6, n=10; P<0.05). Kudingcha was most effective at
inhibiting lung metastasis at a concentration of 1600 mg/kg. This
concentration (inhibitory rate, 33.3%; number of metastatic tumors,
38±6) inhibited tumor formation and lung metastasis to a greater
degree than the 800 mg/kg (inhibitory rate, 22.8%; number of
metastatic tumors, 44±6) and 4000 mg/kg solutions (inhibitory rate,
8.8%; number of metastatic tumors, 52±6).
 | Table IIIInhibitory effects of Kudingcha on the
metastasis of tumors produced by colon 26-M3.1 cells in Balb/c
mice. |
Table III
Inhibitory effects of Kudingcha on the
metastasis of tumors produced by colon 26-M3.1 cells in Balb/c
mice.
| Group | Number of metastatic
tumors | Inhibitory rate
(%) |
|---|
| Control | 57±6a,b | - |
| Kudingcha | | |
| 400 mg/kg | 52±6c | 8.8 |
| 800 mg/kg | 44±6d | 22.8 |
| 1,600 mg/kg | 38±6e | 33.3 |
Discussion
Apoptosis is a fundamental cellular event, and
understanding its mechanisms of action will aid in harnessing this
process for use in tumor diagnosis and therapy (15). In a healthy cell, the anti-apoptotic
protein Bcl-2 is expressed on the outer mitochondrial membrane
surface (16). Apoptosis results
from the activation of caspase family members that act as
aspartate-specific proteases (17).
Cytochrome c and procaspase-9 processing is highly dependent
on caspase-3, thus, this caspase is in a central position as a
regulator of the essential apoptotic pathways in cancer cells
(18). Caspase-3 has also been
reported to play a role as an amplifier of apoptotic signals (i.e.,
by cleaving Bcl-2) (19).
Additionally, anticancer mechanisms underlying the
effect of Kudingcha on human cancer cells involve the induction of
apoptosis by increasing the number of apoptotic bodies, regulating
the mRNA expression of Bax and Bcl-2 and promoting
anti-inflammatory effects by downregulating iNOS and COX-2 gene
expression. COX-2 has been suggested to play an significant role in
colon carcinogenesis, and NOS, along with iNOS, may be a good
target for the chemoprevention of colon cancer (20). NF-κB is one of the most ubiquitous
transcription factors that regulates the expression of genes
required for cellular proliferation, inflammatory responses and
cell adhesion (21). These
mechanisms may be involved in the anticancer effects of Kudingcha
in cancer cells. Based on the results of the MTT assay and the
expression patterns of pro-apoptotic genes observed in the present
study, we concluded that cancer cells treated with Kudingcha
underwent apoptosis. With similar results to these findings, the
anticancer effects of Kudingcha in human nasopharyngeal carcinoma
cells were evaluated in a previous study using MTT assay and RT-PCR
analysis (11).
Metastasis is defined as the spread of cancer cells
from one organ or area to another adjacent organ or location
(22). Malignant tumor cells are
considered to have the capacity to metastasize. Cancer occurs once
cells in a tissue are genetically damaged in a progressive manner,
resulting in cancer stem cells possessing a malignant phenotype.
Once the tumor cells come to rest in another site, they penetrate
the vessel walls, continue to multiply and eventually form another
tumor. Colon 26-M3.1 carcinoma cells have been used to evaluate
anti-metastasis effects in vivo(23).
In conclusion, the present study used various in
vitro experimental methods, including MTT assays, DAPI staining
and RT-PCR assays, to evaluate the anticancer effects of Kudingcha.
A mouse model bearing tumors produced by 26-M3.1 colon carcinoma
cells was also assessed to study the in vivo effects of
Kudingcha. Overall, Kudingcha demonstrated potent in vitro
and in vivo anti-cancer activities, particularly for
combating in vivo tumor metastasis. The functional contents
of Kudingcha are important for augmenting these anticancer effects.
A high concentration solution of Kudingcha increased the anticancer
properties observed in the present study. However, the active
compounds of Kudingcha require identification and evaluation in
future studies.
References
|
1
|
Ye GS: Kuding Tea. Spec Econ Anim Plant.
5:262002.(In Chinese).
|
|
2
|
Sun Y, Xu WQ, Zhang WQ, Hu QH and Zeng XX:
Optimizing the extraction of phenolic antioxidants from kudingcha
made from Ilex kudingcha C.J. Tseng by using response
surface methodology. Sep Purif Technol. 78:311–320. 2011.
View Article : Google Scholar
|
|
3
|
Bravo L, Goya L and Lecumberri E: LC/MS
characterization of phenolic constituents of mate (Ilex
paraguariensis, St. Hil) and its antioxidant activity compared
to commonly consumed beverages. Food Res Int. 40:393–405. 2007.
|
|
4
|
Filip R, López P, Giberti G, Coussio J and
Ferraro G: Phenolic compounds in seven South American Ilex
species. Fitoterapia. 72:774–778. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Nakajima Y, Shimazawa M, Mishima S and
Hara H: Water extract of propolis and its main constituents,
caffeoylquinic acid derivatives, exert neuroprotective effects via
antioxidant actions. Life Sci. 80:370–377. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Han J, Miyamae Y, Shigemori H and Isoda H:
Neuroprotective effect of 3,5-di-O-caffeoylquinic acid on SH-SY5Y
cells and senescence-accelerated-prone mice 8 through the
up-regulation of phosphoglycerate kinase-1. Neuroscience.
169:1039–1045. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Lowe SW and Lin AW: Apoptosis in cancer.
Carcinogenesis. 21:485–495. 2000. View Article : Google Scholar
|
|
8
|
Koo JY, Kim HJ, Jung KO and Park KY:
Curcumin inhibits the growth of AGS human gastric carcinoma cells
in vitro and shows synergism with 5-fluorouracil. J Med Food.
7:117–121. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Balkwill F and Mantovani A: Inflammation
and cancer: back to Virchow? Lancet. 357:539–545. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Mantovani A, Allavena P, Sica A and
Balkwill F: Cancer-related inflammation. Nature. 454:436–444. 2008.
View Article : Google Scholar
|
|
11
|
Huang CJ, Nong CZ, Gu GL, Wei SY, Huang ZH
and Nong SY: Expression of Survivin and APC in Kuding tea ursolic
acid induced human nasopharyngeal carcinoma cell apoptosis.
Guangdong Med J. 32:830–832. 2011.
|
|
12
|
Zhao X, Song JL, Lee JH, Kim SY and Park
KY: Antioxidation and cancer cell (HT-29) antiproliferation effects
of Rubus coreanus Miquel bamboo salt. J Korean Assoc Cancer
Prev. 15:306–312. 2010.(In Korean).
|
|
13
|
Bak SS, Kong CS, Rhee SH, Rho CW, Kim NK,
Choi KL and Park KY: Effect of sulfur enriched young radish kimchi
on the induction of apoptosis in AGS human gastric adenocarcinoma
cells. J Food Sci Nutr. 12:79–83. 2007. View Article : Google Scholar
|
|
14
|
Jung KO, Park SY and Park KY: Longer aging
time increases the anticancer and antimetastatic properties of
doenjang. Nutrition. 22:539–545. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Milanezi F, Leitão D, Ricardo S, Augusto I
and Schmitt F: Evaluation of HER2 in breast cancer: reality and
expectations. Expert Opin Med Diagn. 3:607–620. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Chao DT and Korsmeyer SJ: Bcl-2 family:
regulators of cell death. Annu Rev Immunol. 16:395–419. 1998.
View Article : Google Scholar
|
|
17
|
Kidd VJ: Proteolytic activities that
mediate apoptosis. Annu Rev Physiol. 60:533–573. 1998. View Article : Google Scholar
|
|
18
|
Blanc C, Deveraux QL, Krajewski S, Jänicke
RU, Porter AG, Reed JC, Jaggi R and Marti A: Caspase-3 is essential
for procaspase-9 processing and cisplatin-induced apoptosis of
MCF-7 breast cancer cells. Cancer Res. 60:4386–4390.
2000.PubMed/NCBI
|
|
19
|
Kirsch DG, Doseff A, Chau BN, Lim DS, de
Souza-Pinto NC, Hansford R, Kastan MB, Lazebnik YA and Hardwick JM:
Caspase-3-dependent cleavage of Bcl-2 promotes release of
cytochrome c. J Biol Chem. 274:21155–21161. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Delić R and Stefanović M: Optimal
laboratory panel for predicting preeclampsia. J Matern Fetal
Neonatal Med. 23:96–102. 2010.PubMed/NCBI
|
|
21
|
Baeuerle PA: IkappaB-NF-kappaB structures:
at the interface of inflammation control. Cell. 95:729–731.
1998.PubMed/NCBI
|
|
22
|
Klein CA: Cancer. The metastasis cascade
Science. 321:1785–1787. 2008.PubMed/NCBI
|
|
23
|
Ha ES, Hwang SH, Shin KS, Yu KW, Lee KH,
Choi JS, Park WM and Yoon TJ: Anti-metastatic activity of
glycoprotein fractionated from Acanthopanax senticosus,
involvement of NK-cell and macrophage activation. Arch Pharm Res.
27:217–224. 2004. View Article : Google Scholar : PubMed/NCBI
|