Introduction
Evaluation of the KRAS mutational status is a
crucial step for the correct therapeutic approach in advanced
colorectal cancer. According to well-established criteria, a
molecular analysis of exon 2 (codons 12 and 13) is routinely
performed in formalin-fixed, paraffin-embedded (FFPE) tissue and
the identification of a wild-type (WT) KRAS tumor may lead to a
more tumor-specific and less toxic treatment for the patient.
Numerous studies have demonstrated significant
intratumoral heterogeneity, with spatially separated heterogeneous
somatic mutations and chromosomal imbalances (1). With regard to colorectal cancer,
several studies have highlighted the differences in the KRAS
mutational status between primary and metastatic tumors within
lymph nodes and visceral metastases or even different portions of
the primary lesion (2), thus
supporting the overall current theory of neoplastic heterogeneity
(1,3). However, the possibility of two or more
mutations in the same codon of the KRAS gene has seldom been
reported in colorectal cancer and the real clinical impact of
multiple mutations on patient prognosis has not yet been well
studied and clarified (4–7).
The present study reports an additional case of the
coexistence of two somatic mutations (p.G12D and p.G12V) in the
same codon (codon 12) of exon 2 of the KRAS gene in a female
patient affected by an advanced adenocarcinoma of the rectum. This
supports the possibility for two clonal origins of the tumor along
with concomitantly different mutations at the same genetic
level.
Case report
In March 2012, a 70-year-old female patient was
admitted to the IRCCS-CROB Hospital (Rionero In Vulture, Italy) due
to tenesmus and blood in the stool. The subsequent colonoscopy
examination showed a hyperemic flat lesion of the rectal mucosae
with rectal canal stenosis. A rectal biopsy supported the diagnosis
of poorly differentiated adenocarcinoma. Subsequent to a careful
clinical evaluation, including a total body computed tomography
(CT) scan, the patient was diagnosed as having locally advanced
rectal cancer cT3N1M0, stage IIIb.
Between April and June 2012, the patient received
neoadjuvant chemotherapy with 1,650 mg/m2 capecitabine
orally twice daily, plus concomitant radiotherapy (45 Gy in 5
weeks), followed 6 weeks later by surgery. In August 2012, the
patient underwent an anterior resection for rectal cancer. The
histological examination revealed a ypT3 adenocarcinoma, of tumor
regression grading (TRG) 3 with metastases in 1 out of 10 resected
lymph nodes (ypT3pN1Mx TRG3).
In October 2012, prior to starting the planned
post-operative chemotherapy, a CT scan revealed multiple liver
metastases, although the carcinoembryonic antigen (CEA) and
carbohydrate antigen (CA) 19.9 values were normal. The patient
began chemotherapy with 7.5 mg/kg bevacizumab daily and the XELOX
regimen (130 mg/m2 oxaliplatin daily + 2,000
mg/m2 capecitabine on days 1–14). This treatment is
ongoing.
Materials and methods
Prior to the mutation analysis, the patient provided
informed written consent. A tissue sample from the primary tumor
was obtained from the archives of the IRCCS-CROB Hospital. A
section of this specimen, stained with hematoxylin and eosin, was
observed by a pathologist to evaluate the percentage of cancer
cells prior to performing a manual dissection of the tumor area.
DNA extraction was performed using a QIAamp® DNA Mini
kit (Qiagen, Hilden, Germany), and exon 2 (codons 12 and 13) of the
KRAS gene was amplified by polymerase chain reaction (PCR) using
the following designed primers: forward,
5′-GGTGGAGTATTTGATAGTGTAT-3′ and reverse,
5′-AGAATGGTCCTGCACCAGTAA-3′. PCR was performed in a final volume of
25 μl under the following conditions: 1X buffer, 3 mmol/l
magnesium chloride, 200 μmol/l deoxyribonucleotide
phosphates (all from Applied Biosystems, manufactured by Roche,
Branchburg, NJ, USA), 12.5 pmol of each primer (Sigma Aldrich, St.
Louis, MO, USA), 200 ng/μl DNA and 1.5 U Taq DNA polymerase
(Applied Biosystems manufactured by Roche). Following use of an
initial melting temperature of 94°C for 2 min, the reaction mixture
was subjected to 35 cycles of 94°C for 30 sec, 56°C for 30 sec and
65°C for 30 sec, followed by a final 72°C extension step for 2 min.
Prior to sequencing, the PCR products were stained with ethidium
bromide and visualized on a UV transilluminator following 2% gel
electrophoresis. The PCR products were then sequenced using the
BigDye Terminator v.1.1 sequencing kit and an ABI Prism 310 genetic
analyzer (both from Applied BioSystems, Foster City, CA, USA)
(8).
Results
The percentage of sample tumor cells was evaluated
by the pathologist and estimated to be ∼60%. The DNA quality was
evaluated by spectrophotometer analysis and the
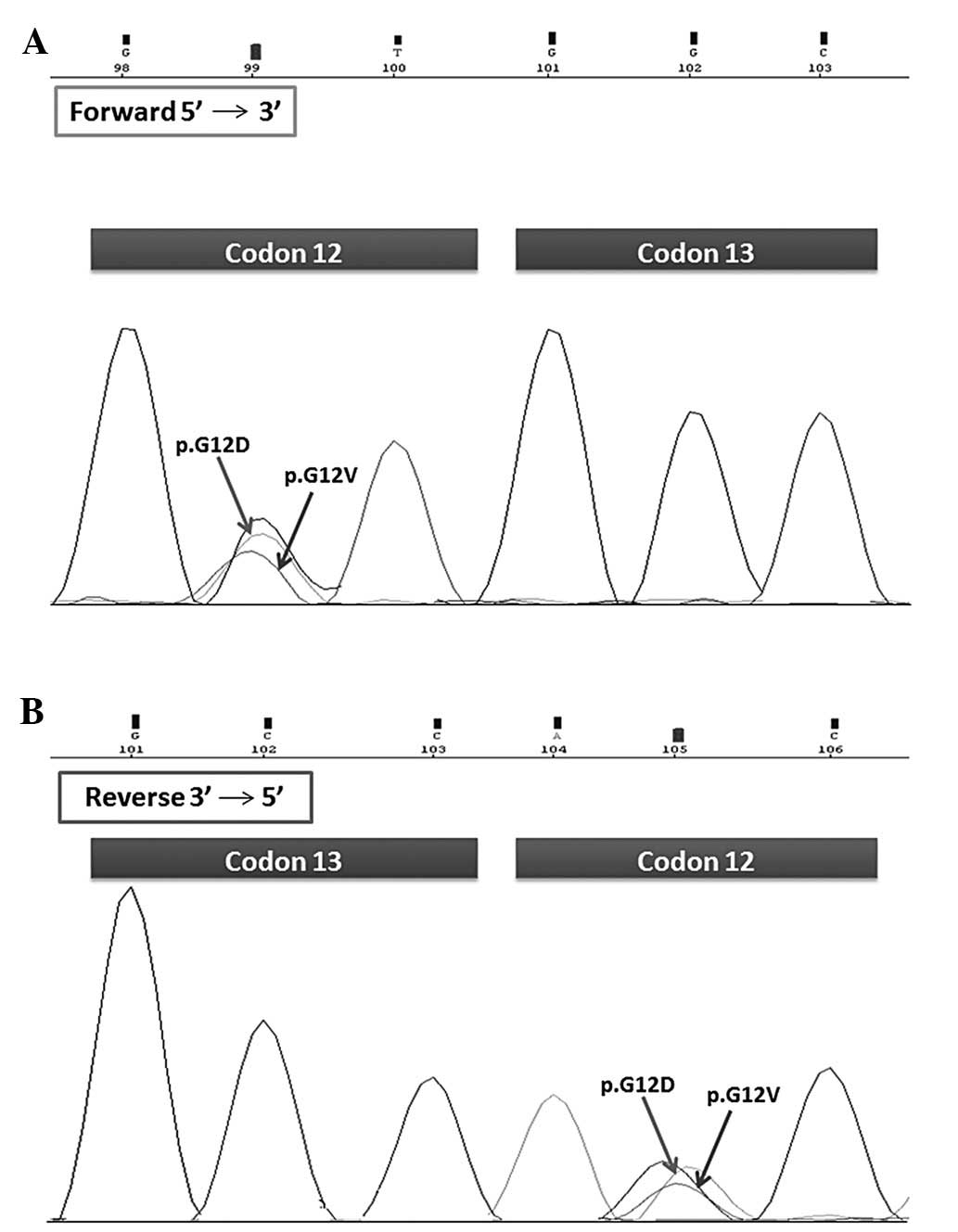
A260/A280 ratio was 1.8. The coexistence of
two mutations, p.G12D and p.G12V, in the same codon (codon 12) of
the KRAS gene was observed by molecular biologists in two
independent PCR products and demonstrated by sense and anti-sense
sequence analysis of the fragments (Fig. 1). The DNA amplified sequence of the
KRAS gene was compared with the wild-type KRAS sequence.
Discussion
Colorectal cancer is the third most commonly
diagnosed type of cancer and the third leading cause of cancer
mortality in males and females. With the development of drugs,
including irinotecan and oxaliplatin, and targeted therapies,
including cetuximab and bevacizumab therapy, the median survival
has increased to >20 months. Several studies have shown that
KRAS mutations in primary tumors predict resistance to anti-EGFR
antibodies (9–11) and thus, only patients with wild-type
KRAS tumors (∼60% of patients) are eligible for anti-EGFR
therapy.
Although the results of the KRAS mutational analysis
of the primary tumor usually match with the metastases, in a
minority of cases (5–10%), the KRAS mutational status is
heterogeneous between the primary tumor and metastases (2,12–15).
These observations may reflect the increased genetic instability in
cells that progressively acquire mutations or the presence of a
heterogeneous group of neoplastic cells inside the tumor (16,17).
In addition, few studies have observed the coexistence of more than
one mutation in the KRAS gene within the same colorectal tumor,
correlating this type of alteration with clinical and morphological
features (4–7).
The present study reports a case of the coexistence
of two mutations, p.G12D (GGT>GAT) and p.G12V (GGT>GTT), in
the same codon of the KRAS gene, in the same selected tumor area,
thus demonstrating the existence of intratumoral heterogeneity.
Based on data in the literature, multiple mutations in the KRAS
gene are infrequent, representing 2.1% of mutations in colorectal
cancer (18). The majority of
co-mutations in the KRAS gene affect only one codon (59%), mainly
codon 12, although co-mutations may affect codons 12 and 13
simultaneously (18). The most
frequently altered amino acid sequences involved in these
co-mutations are GAT (in codon 12) and GAC (in codon 13) (18).
Due to the scarcity of data in the literature, the
clinical implications and prognostic significance of multiple KRAS
mutations remain unknown, although associations with advanced
clinical stage and aggressive clinical course have been reported
(4–7,19). The
present case was characterized by an aggressive clinical course
with the development of early liver metastases despite the
administration of neoadjuvant chemo-radiotherapy. Whether this
aggressiveness was due to the coexistence of multiple mutations or
a specific single mutation is, however, questionable.
It has been shown that not all KRAS mutations have
the same prognostic relevance. A meta-analysis demonstrated that
the p.G12V mutation at codon 12 in the KRAS gene increases the risk
of recurrence or mortality in patients with colorectal cancer
(17,20–21),
unlike other KRAS mutations that have only a moderate,
non-significant effect on overall survival (22). These data are consistent with
experimental evidence showing that valine mutations produce
proteins with different behavior compared with other mutated KRAS
proteins (12). The lower affinity
of GTP to p.G12D allows p.G12D to escape from the oncogenic
GTP-bound state, whereas GTP that is tightly bound to p.G12V
generates a more persistent, potentially oncogenic signal.
Furthermore, differences in the effector region of p.G12D and
p.G12V may modify interactions with downstream signaling molecules
(12).
In the present case the coexistence of these two
mutations, p.G12D and p.G12V, may have had an almost different
clinical relevance on patient prognosis (20,22).
The effect of various KRAS mutations on overall survival may be
explained by the fact that the heterogeneity of the various KRAS
mutations in colorectal cancer may differ in carcinogenic
potential. This may justify the selection of a new clone with a
p.G12V mutation and more aggressive behavior, along with the
pre-existing p.G12D clone.
Therefore, it should be a great challenge to detect
all mutations present in tumors. It has been suggested that a DNA
tumoral mix obtained from different tumor areas may increase the
detection rate of mutations, including multiple mutations (2,23).
This is consistent with the current theory that tumors show
significant intratumoral heterogeneity, characterized by separated
heterogeneous somatic mutations and chromosomal aberrations
(1). Such genetic heterogeneity may
also cause heterogeneitiy in terms of radiosensitivity (24), with a strong impact on the choice of
the most appropriate treatment option when the disease is treatable
with radiotherapy alone or combined with chemotherapy or biological
drugs (25,26).
In conclusion, the present study underlines the
intratumoral heterogeneity, as supported by the current data
(1,2) in which tumors may be polyclonal with a
mixture of cell populations harboring varying mutations. The
coexistence of distinct clones within a tumor may have profound
clinical implications for disease progression and therapeutic
responses. In particular, the present case appears to support the
hypothesis that the presence of multiple mutations in codon 12 is
associated with a more aggressive disease.
References
|
1
|
Gerlinger M, Rowan AJ, Horswell S, et al:
Intratumor heterogeneity and branched evolution revealed by
multiregion sequencing. N Engl J Med. 366:883–892. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Baldus SE, Schaefer KL, Engers R, Hartleb
D, et al: Prevalence and heterogeneity of KRAS, BRAF, and PIK3CA
mutations in primary colorectal adenocarcinomas and their
corresponding metastases. Clin Cancer Res. 16:790–799. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Marusyk A and Polyak K: Tumor
heterogeneity: causes and consequences. Biochim Biophys Acta.
1805:105–117. 2010.PubMed/NCBI
|
|
4
|
De Roock W, Piessevaux H, De Schutter J,
et al: KRAS wild-type state predicts survival and is associated to
early radiological response in metastatic colorectal cancer treated
with cetuximab. Ann Oncol. 19:508–515. 2008.PubMed/NCBI
|
|
5
|
Jönsson M, Ekstrand A, Edekling T, et al:
Experiences from treatment-predictive KRAS testing; high mutation
frequency in rectal cancers from females and concurrent mutations
in the same tumor. BMC Clin Pathol. 9:82009.PubMed/NCBI
|
|
6
|
Moerkerk P, Arends JW, van Driel M, et al:
Type and number of Ki-ras point mutations relate to stage of human
colorectal cancer. Cancer Res. 54:3376–3378. 1994.PubMed/NCBI
|
|
7
|
Sameer AS, ul Rehman S, Pandith AA, et al:
Molecular gate keepers succumb to gene aberrations in colorectal
cancer in Kashmiri population, revealing a high incidence area.
Saudi J Gastroenterol. 15:244–252. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Sanger F, Nicklen S and Coulson AR: DNA
sequencing with chain-terminating inhibitors. Proc Natl Acad Sci
USA. 74:5463–5467. 1977. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lièvre A, Bachet JB, Le Corre D, et al:
KRAS mutation status is predictive of response to cetuximab therapy
in colorectal cancer. Cancer Res. 66:3992–3995. 2006.PubMed/NCBI
|
|
10
|
Amado RG, Wolf M, Peeters M, et al:
Wild-type KRAS is required for panitumumab efficacy in patients
with metastatic colorectal cancer. J Clin Oncol. 26:1626–1634.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Karapetis CS, Khambata-Ford S, Jonker DJ,
et al: K-ras mutations and benefit from cetuximab in advanced
colorectal cancer. N Engl J Med. 359:1757–1765. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Al-Mulla F, Going JJ, Sowden ET, et al:
Heterogeneity of mutant versus wild-type Ki-ras in primary and
metastatic colorectal carcinomas, and association of codon-12
valine with early mortality. J Pathol. 185:130–138. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Albanese I, Scibetta AG, Migliavacca M, et
al: Heterogeneity within and between primary colorectal carcinomas
and matched metastases as revealed by analysis of Ki-ras and p53
mutations. Biochem Biophys Res Commun. 325:784–791. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Mancuso A, Sollami R, Recine F, et al:
Patient with colorectal cancer with heterogeneous KRAS molecular
status responding to cetuximab-based chemotherapy. J Clin Oncol.
28:e756–e758. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lamy A, Blanchard F, Le Pessot F, et al:
Metastatic colorectal cancer KRAS genotyping in routine practice:
results and pitfalls. Mod Pathol. 24:1090–1100. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Bouchahda M, Karaboué A, Saffroy R, et al:
Acquired KRAS mutations during progression of colorectal cancer
metastases: possible implications for therapy and prognosis. Cancer
Chemother Pharmacol. 66:605–609. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Andreyev HJ, Norman AR, Cunningham D, et
al: Kirsten ras mutations in patients with colorectal cancer: the
multicenter ‘RASCAL’ study. J Natl Cancer Inst. 90:675–684.
1998.
|
|
18
|
Macedo MP, Andrade Lde B, Coudry R, et al:
Multiple mutations in the Kras gene in colorectal cancer: review of
the literature with two case reports. Int J Colorectal Dis.
26:1241–1248. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Perou CM, Sørlie T, Eisen MB, et al:
Molecular portraits of human breast tumours. Nature. 406:747–752.
2000. View
Article : Google Scholar : PubMed/NCBI
|
|
20
|
Sørlie T, Perou CM, Tibshirani R, et al:
Gene expression patterns of breast carcinomas distinguish tumor
subclasses with clinical implications. Proc Natl Acad Sci USA.
98:10869–10874. 2001.PubMed/NCBI
|
|
21
|
Fialkow PJ: Clonal origin of human tumors.
Annu Rev Med. 30:135–143. 1979. View Article : Google Scholar
|
|
22
|
Winder T, Mündlein A, Rhomberg S, et al:
Different types of K-Ras mutations are conversely associated with
overall survival in patients with colorectal cancer. Oncol Rep.
21:1283–1287. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Richman SD, Chambers P, Seymour MT, et al:
Intra-tumoral heterogeneity of KRAS and BRAF mutation status in
patients with advanced colorectal cancer (aCRC) and
cost-effectiveness of multiple sample testing. Anal Cell Pathol
(Amst). 34:61–66. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Spitzner M, Emons G, Kramer F, et al: A
gene expression signature for chemoradiosensitivity of colorectal
cancer cells. Int J Radiat Oncol Biol Phys. 78:1184–1192. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Pedicini P, Nappi A, Strigari L, et al:
Correlation between EGFr expression and accelerated proliferation
during radiotherapy of head and neck squamous cell carcinoma.
Radiat Oncol. 7:1432012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Pedicini P, Caivano R, Jereczek-Fossa BA,
et al: Modelling the correlation between EGFr expression and tumor
cell radiosensitivity, and combined treatments of radiation and
monoclonal antibody EGFr inhibitors. Theor Biol Med Model.
9:232012. View Article : Google Scholar
|