Introduction
Gastric cancer (GC) is the fourth most common type
of cancer (989,000 cases, 7.8% of the total) and the second highest
cause of cancer mortality worldwide (738,000 mortalities, 9.7% of
the total) (1). GC rates have
decreased noticeably over the majority of the world, but remain a
burden to Eastern Asian countries (mainly China) (2). By the time symptoms occur, the cancer
has often reached an advanced stage and may have also
metastasized.
Clinicians are able to treat patients with
appropriate doses of chemoradiotherapy and schedules to achieve
logarithmic cancer cell death, while unavoidably killing normal
cells that undergo rapid division (3). Due to advances in the understanding of
the molecular and cellular basis of cancer, current therapeutic
strategies focus on inhibiting the molecular drivers of cancer.
What all these strategies, whether from the past or present, have
in common is that they treat cancer as a homogeneous, abnormal
entity. Therefore, drugs targeting molecular lesions should be
equally effective against all tumour cells, barring the emergence
of a resistant subpopulation (4).
Cancer cells are heterogeneous, not only in
morphology, but also in functionality (i.e., marker expression,
proliferation capacity and tumourigenicity). Only a minority of
tumour cells, termed cancer stem cells (CSCs), have the capacity to
regenerate the tumour and sustain its growth when injected into
immune-compromised mice (5). Based
on accumulating evidence, the American Association for Cancer
Research (AACR) made a consensus definition of CSCs in 2006 as
‘cells within a tumour that possess the capacity for self-renewal
and that can cause the heterogeneous lineages of cancer cells that
constitute the tumour’ (6).
Studies of gastric CSCs (GCSCs) began relatively
late compared with other solid tumours. In 2009, Takaishi et
al(7) screened a series of
potential stem cell markers in various human GC cell lines and
demonstrated for the first time that CD44 may be an appropriate
marker for stem cells. However, similar studies from clinical
research are rare. To date, the theory of CSCs as a subpopulation
with chemo/radioresistance has been verified in a number of solid
tumours with the exception of GC (8–12).
Furthermore, the role that GC stem-like cells play in cancer
invasion remains to be elucidated.
Materials and methods
Cells and animals
Poorly differentiated human GC cells were derived
from a 55-year-old female patient, who provided written informed
consent. The patient did not undergo chemoradiotherapy prior to
resection. A total of 30, 4-week-old Balb/cA nu/nu female mice were
obtained from the Shanghai Experimental Animal Centre of the
Chinese Academy of Science (Shanghai, China). The mice were
maintained in plastic cages (five mice/cage) in a room with a
constant temperature (22±1°C) and a dark-light cycle (12 h/12 h).
The animal experiments and human research were performed in
accordance with the ethics code approved by the Ethics Committee of
Chongqing Medical University, Chongqing, China.
Fluorescence-activated cell sorting
(FACS)
For the FACS, 80% confluent cells in a 100-mm cell
plate (5–10 million cells per plate) were harvested and incubated
for 30 min at room temperature, with a 10-fold dilution of the
following antibodies: Anti-CD44-fluorescein isothiocyanate rat
monoclonal antibody and anti-CD44-PE (eBioscience, San Diego, CA,
USA). The cells were then detected using a FACS-LSRII flow
cytometer (Becton Dickinson, Franklin Lakes, NJ, USA).
Spheroid colony formation assay
The CD44+ and the CD44−
fractions from the FACS-sorted GC cells were inoculated into each
well (20 cells per well) of the ultra-low-attachment 48-well plates
and supplemented with 300 μl Dulbecco’s modified Eagle’s
medium (DMEM) plus 40 ng/ml basic fibroblast growth factor (bFGF)
and 20 ng/ml epidermal growth factor (EGF; Invitrogen, Carlsbad,
CA, USA). After 4 weeks, each well was examined under a light
microscope (Olympus, Tokyo, Japan) and the total wells with
spheroid colonies were counted. Each experiment was performed at
least three times.
In vivo xenograft assay
The CD44+ and CD44− fractions
were maintained in sterile DMEM supplemented with 10% fetal bovine
serum (FBS). Both cell fractions were left to grow to be tested for
tumourigegnicity. The cells were harvested when they were
subconfluent and adjusted to the concentration of the cell
suspension to be inoculated to 5×104/ml
(CD44+) and 5×106/ml (CD44−) in
PBS. A total of 0.2 ml of the cell suspension was subcutaneously
injected into the left (CD44−) and right
(CD44+) hind limbs of the mice. The mice were observed
daily and inspected for tumour growth each week for 6 weeks.
In vitro cell migration and invasion
assay
Matrigel was diluted in serum-free DMEM (5 mg/ml),
then 100 μl of this was introduced into the upper chamber of
a 24-well millicell with a 8 μm pore size insert (Millipore,
Billerica, MA, USA). The cells were harvested and resuspended at a
density of 1×104/ml in media containing 0.1% FBS. Next,
100 μl of the cell suspension was introduced onto the
matrigel. The lower chamber of the millicell was filled with 600
μl DMEM containing 10% FBS. The cells on the lower side of
the insert filter were then stained with a 1% crystal violet
solution for 20 min. The average number of stained cells was
counted on the lower side of the filter using an inverted
wide-field microscope (Olympus, Tokyo, Japan). Each experiment was
performed at least three times.
Cell treatment
The anti-cancer drug, 5-fluorouracil (5-FU;
Sigma-Aldrich, St. Loius, MO, USA), was used to assess the
responses of the sorted cells. To evaluate the cell viability, the
sorted cells were seeded in a flat-bottomed microculture 96-well
plate (2,000 cells/well) and allowed to adhere for 24 h. The cells
were then treated with 5-FU (10 nM; 30 μM) in phenol-free
DMEM medium for 72 h. To measure the reactive oxygen species (ROS)
accumulation, the sorted cells were plated in 6-well plates and
stimulated with 5-FU (50 μM).
MTT assay
Once the sorted cells had been treated with 5-FU for
72 h, MTT was added to a final concentration of 0.5 mg/ml and the
cells were incubated for 4 h at 37°C. The culture medium was then
removed and the remaining blue precipitate was solubilized in
dimethyl sulfoxide (DMSO). The absorbance at 570 nm was read using
a microplate reader (BioTek, Winooski, VT, USA). This reading was
divided by the adjusted absorbance reading of the untreated cells
in the control wells. The IC50 was calculated by
non-linear regression analysis using sigmoidal fitting from the
sigmoidal dose-response curve. For each concentration of 5-FU, five
wells were analysed. Each experiment was performed at least three
times.
ROS assay
A ROS assay kit was purchased from the Beyotime
Institute of Biotechnology (Haimen, Jiangsu, China) and used
according to the manufacturer’s instructions. Briefly, to load the
probe in situ, 2 ml 2′,7′-dichlorofluorescin-diacetate
(DCFH-DA; 1:1000) fluorescent probe diluted with serum-free DMEM
was loaded onto each well of the 6-well plate. The plates were
incubated at 37°C for 20 min and then washed with serum-free media
three times. The cells were stimulated with 5-FU (50 μM) for
2 h. Following this stimulation, dichlorofluorescein (DCF;
excitation 488/emission 525) fluorescence was assessed immediately
by flow cytometry.
Irradiation
For the colony forming assays, the sorted cells were
irradiated using a Varian Clinac iX linear accelerator (Varian,
Palo Alto, CA, USA) at a dose rate of 3 Gy/min for the time
required to generate dose curves of 0, 2, 4, 6 and 8 Gy. The
corresponding control was sham irradiated. Colony forming assays
were performed immediately after irradiation by plating the cells
into 6-well culture plates. After 20 days, the colonies containing
>50 cells were counted. A radiation survival curve was generated
using Albright’s method (13). For
the comet assays, the sorted cells were irradiated, using the
method previously stated, with 0, 2 and 4 Gy. The performance of
the comet assay was mainly based on the method described by Olive
et al(14). A total of 30
representative cells were investigated per slide. The ‘comets’ were
measured using the image analysis Comet Assay Software Project
(CASP; Free Software Foundation, Inc., Boston, MA, USA). The Olive
Tail Moment (OTM), used to quantify DNA damage, was calculated as
follows: Median DNA migration distance × relative amount of DNA in
the tail of the comet.
Real-time PCR
Quantitative real-time RT-PCR was performed using a
2X Maxima SYBR Green/ROX qPCR Master mix (Thermo Fisher Scientific,
Co., Ltd., Milford, MA, USA). Reactions were carried out using
iCycler (Bio-Rad, Hercules, CA, USA) and the results were evaluated
using the iCycler real-time detection system software. Relative
quantitation of target gene expression was evaluated using the
comparative Ct method.
Western blot
The immunoreagents used for the western blot
analysis were rabbit monoclonal antibodies against matrix
metalloproteinase-1 (MMP-1) and cyclooxygenase 2 (COX-2) (1:100)
and mouse monoclonal antibodies against MMP-2 and epidermal growth
factor receptor (EGFR; 1:100). Mouse polyclonal anti-actin
antibodies (1:1000; Santa Cruz Biotechnology, Inc., Santa Cruz, CA,
USA) were used as the loading control. The blots were developed
using a standard enhanced chemiluminescence (ECL) method (Pierce
Biotechnology, Inc., Rockford, IL, USA).
Immunofluorescence staining
CD44+ cell spheroids were fixed and
blocked in PBS solution with 10% FBS. The primary antibodies
(rabbit anti-MMP-1 and mouse anti-MMP-2; 1:100) were incubated at
4°C overnight. The fluorescent secondary antibodies [goat
anti-rabbit IgG antibodies conjugated with fluorescein
isothiocyanate (FITC) and goat anti-mouse IgG antibodies conjugated
with tetramethylrhodamine isothiocyanate (TRITC); 1:100] were added
and incubated at 37°C for 30 min. In the negative controls, the
primary antibodies were substituted with PBS. The cell nuclei were
counter-stained with 4′,6-diamidino-2-phenylindole (DAPI).
Fluorescence was observed under a laser scan confocal microscope
(Leica Microsystems, Wetzlar, Germany).
Histological examination
Tumour tissues were fixed in 10% neutral-buffered
formalin, embedded in paraffin, sectioned and stained with
haematoxylin and eosin (HE). The histological differences were
examined under microscopy at magnification ×40.
Statistical analysis
Data are presented as the mean ± standard error of
the mean (SEM). GraphPad Prism 5.0 software (GraphPad Software,
Inc., San Diego, CA, USA) was used for statistical analysis.
Statistical differences in the IC50 were determined
using the F test. The remaining comparisons between the groups were
evaluated using an unpaired t-test. P≤0.05 was considered to
indicate a statistically significant difference.
Results
Confirmation of stemness properties of
CD44+cells
CSCs have been shown to form suspended cell
spheroids under rich growth factor, low-attachment and serum-free
culture conditions (15). In the
spheroid colony formation assay, the CD44+ cells formed
more spheroids than the CD44− cells (P<0.01)
(Table I). The diameter of the
CD44+ spheroids reached ∼120 μm, containing
∼1,000 cells (data not shown). The tumourigenic capacity of the
CSCs varied between 10- and 100-fold compared with that of the
non-stem-like counterparts in different cancer cells (16–18).
In the tumourigenicity assay, individual difference variables of
the nude mice were excluded by an injection of the two cell
fractions in the same mouse. In general, an injection of
1×104 CD44+ cells gave rise to tumours with
80% incidence, with relatively short latency periods (<1 week)
in all mice. In contrast, an injection of 1×106
CD44− cells conferred a tumour formation with a very low
engraftment rate of 27% (P<0.01) (Table I). This may be as the
CD44+ cells exhibited the correct intrinsic properties
to form the tumours.
 | Table IComparative analysis of
CD44+ and CD44− GC cells in vivo and
in vitro. |
Table I
Comparative analysis of
CD44+ and CD44− GC cells in vivo and
in vitro.
| Assay | CD44+ GC
cells | CD44− GC
cells | P-value |
|---|
| Spheriod colony
formation | 26.33±2.906
(n=3) | 8.667±1.453
(n=3) | 0.0056 |
| Tumourigenic
capacity | 4.000±0.258
(n=6) | 1.500±0.223
(n=6) | <0.0001 |
| Matrigel invasion
assay | 74.33±8.988
(n=3) | 22.00±5.508
(n=3) | 0.0077 |
| ROS (intracellular
fluorescence value) | 58.67±3.930
(n=3) | 82.00±3.512
(n=3) | 0.0114 |
Responses of CD44+ stem-like
cells to chemoradiation
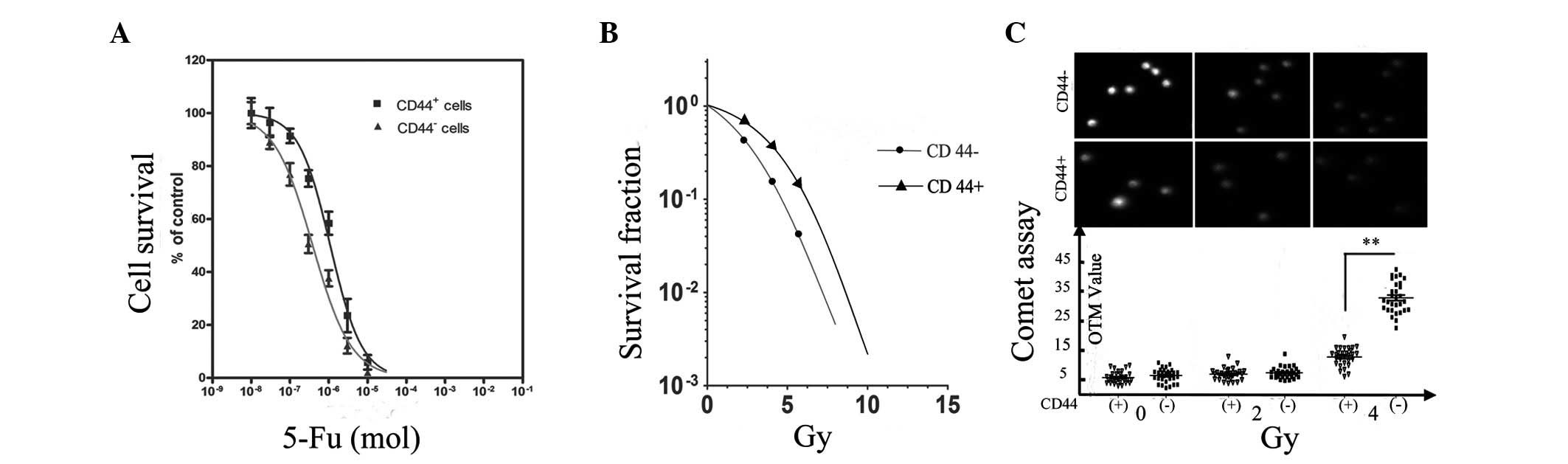
In an MTT assay based on the calculated
IC50 values, the responses of the two cell fractions to
5-FU varied significantly. The log IC50 of the
CD44+ and CD44− cells was −5.961±0.04566 and
−6.415±0.04231, respectively (P<0.05; Fig. 1A). A ROS assay was performed to
explore the underlying mechanism of chemoresistance. Fluorescence
enhancement was consequently observed in the two cell fractions.
However, the intracellular fluorescence value in the
CD44+ cells was considerably lower than that of the
CD44− cells (P<0.05; Table I).
Cytotoxic oxidative stress resulting from ROS may
damage DNA, RNA, proteins and lipid components, which may lead to
cell death. Most significantly, ROS-induced cell apoptosis occurs
in the early stages in response to chemotherapeutic drug treatment
(19). Therefore, we hypothesized
that the reduction in oxidatively-generated DNA damage in the
CD44+ cells may be due to their antioxidant ability.
Clonogenic and comet assays were conducted to independently
evaluate the proliferative death of the cells and to quantify the
DNA breaks. Notably, the radiation survival curve of the two cell
fractions had a shoulder that was characterized by a comparably
higher resistance at lower doses of radiation. The curve shoulder
of the CD44− cells disappeared at survival fraction (SF)
4 Gy. In contrast, the shoulder existed in the CD44+
stem-like cells until the radiation dose increased to 6 Gy
(Fig. 1B). In the comet assay,
irradiated cells with damaged DNA fragments formed a tail around
the DNA head following electrophoresis. The OTM value was
positively correlated with the extent of the damaged DNA.
Consistent with the clonogenic assay, the difference in the OTM
value was not significant when irradiated with 2 Gy (P>0.05). In
contrast, the difference became considerably larger when the
irradiation doses increased to 4 Gy (P<0.01; Fig. 1C).
Enhancement of invasion capacity of
CD44+ stem-like cells
The invasive and migratory capacities of the
CD44+ stem-like cells were much higher compared with the
CD44− cells (P<0.01; Table I). The CD44+ cells were
able to penetrate into and pass through the matrix in vitro,
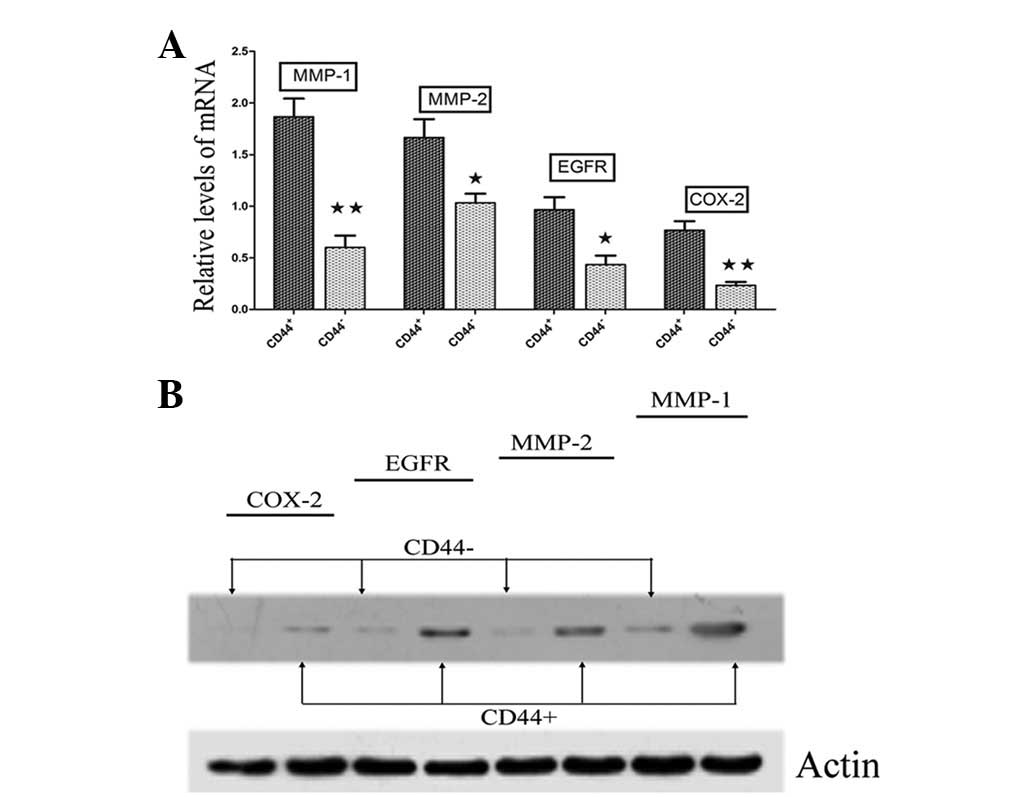
which indicated that they may synthesize more MMPs. A previous
study revealed four important cancer invasion-related genes, MMP-1,
MMP-2, EGFR and COX-2, in a variety of human cancers, including GC,
which may manipulate the migration of tumour cells and the
formation of new tumours by facilitating the release of tumour
cells into the circulation (20).
Therefore, the present study compared the expression profiles of
the four cancer invasion-related genes in the CD44+
stem-like cells and their non-stem-like counterparts. This process
contributed to the validation of the four genes, showing
considerably varied RNA (Fig. 2A)
and protein (Fig. 2B) expression
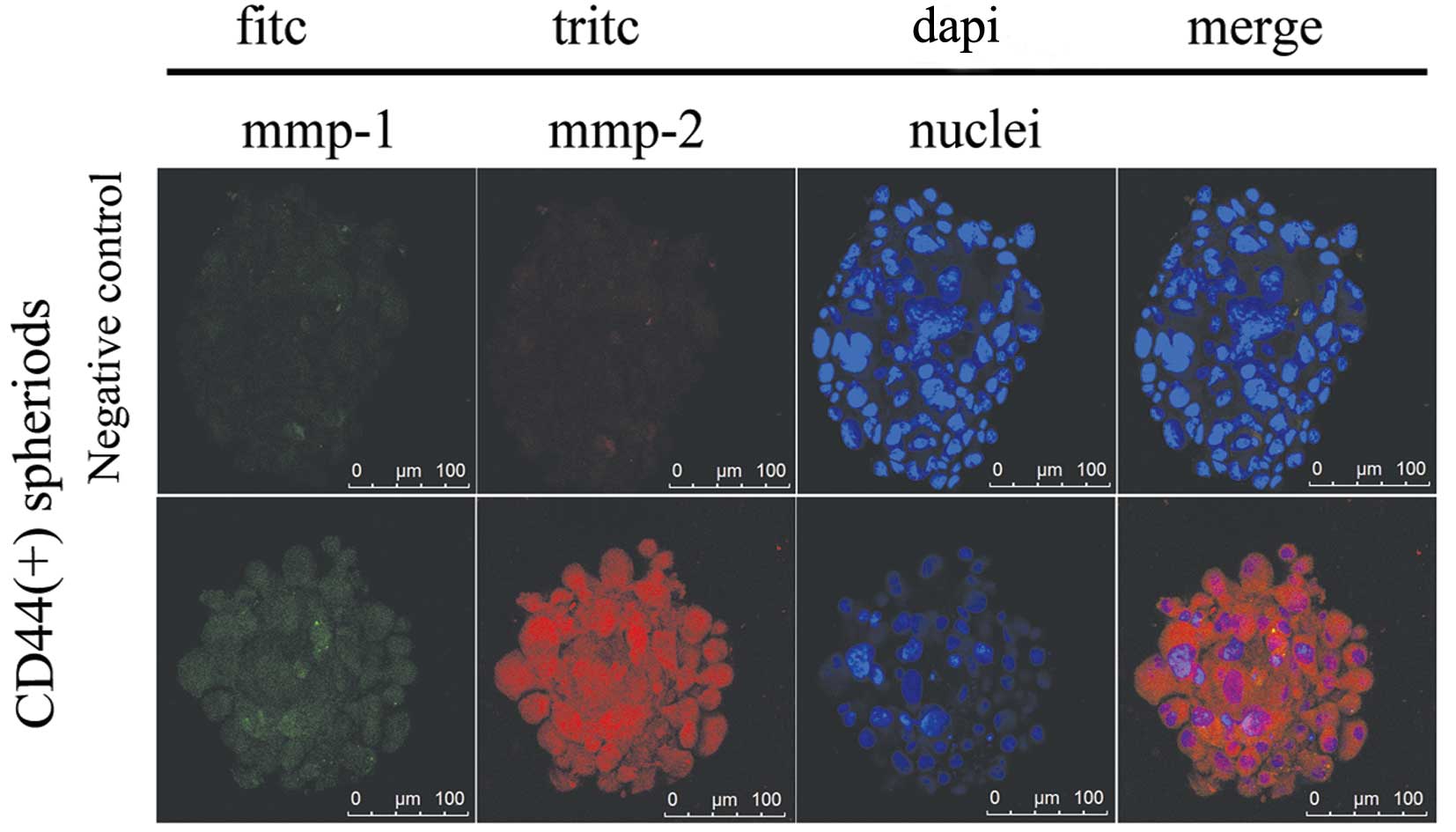
levels. Furthermore, immunofluorescence staining determined that
two representative genes, MMP-1 and MMP-2, were co-expressed in the
CD44+ cell spheroids. Strong immunoreactivity was
detected for each of the proteins localized in the whole cancer
cells (Fig. 3).
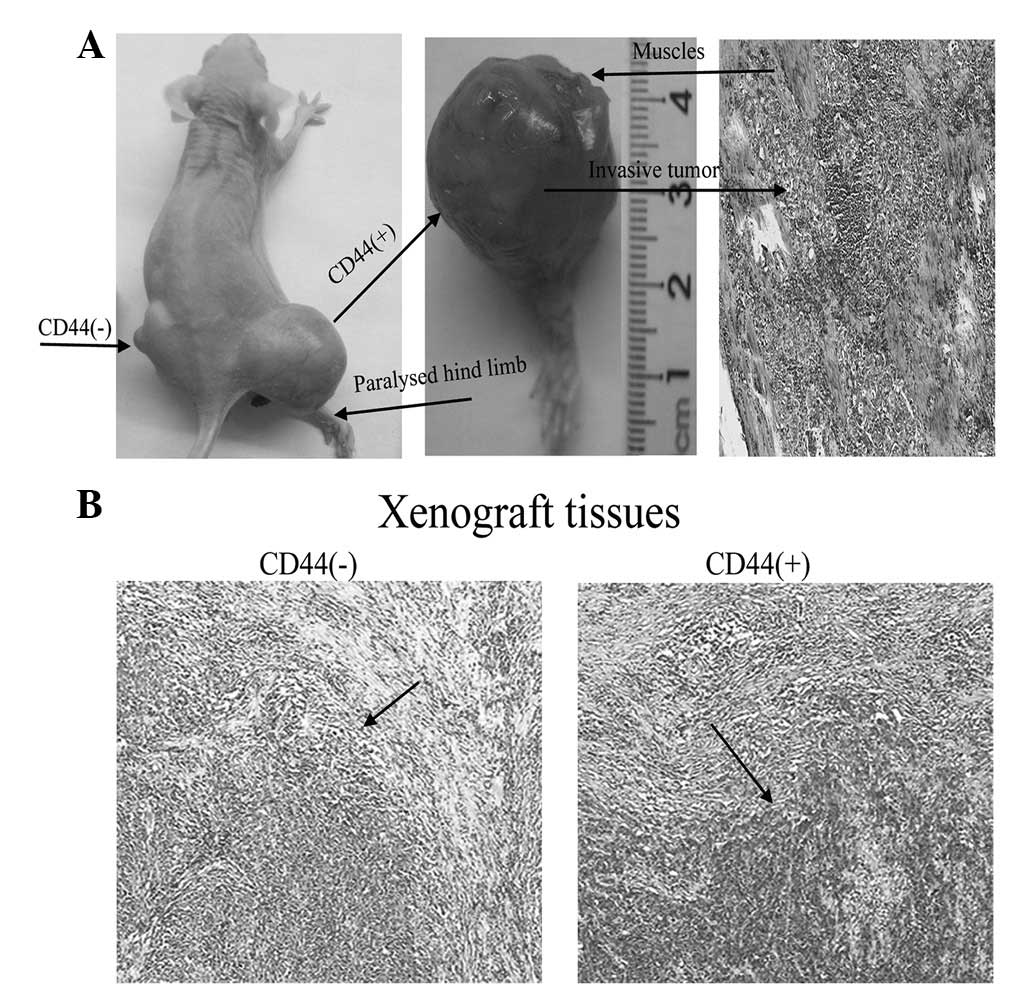
The CD44+ GC stem-like cells that
recorded a positive score in the migration and invasion assay in
vitro formed invasive tumours in vivo. Fig. 4A shows a representative mouse with
progressive muscle invasion by a tumour that paralysed its hind
limb. HE histological staining of the mass identified an invasive
tumour within the muscle that was consistent with a poorly
differentiated adenocarcinoma. Moreover, histological differences
were observed between the CD44+ and CD44−
tumours. The CD44+ tumours with larger, irregular and
hyperchromatic nuclei were more heterogeneous (Fig. 4B).
Discussion
Numerous studies have particularly focussed on the
signalling pathways that may mediate the resistance of CSCs
(21,22). The present study contributes to the
understanding of the antioxidant ability of CSCs as another
important mechanism in relation to chemoradioresistance. The
expression of CD44 in GC cells enhances tumourigenicity. This
observation is in accordance with the significance of
CD44+ cancer cells in the tumourigenicity of other
cancers (7,23–25).
The most unique and significant observation of the present study
was the increased invasion capacity of the CD44+
stem-like cells in vivo and in vitro. Furthermore,
the present study verified the expression of the cancer
invasion-related genes, MMP-1, MMP-2, EGFR and COX-2, which were
upregulated in the CD44+ GC cells, indicating a capacity
to manipulate cancer invasion and even metastasis.
These observations may have clinical implications.
An examination of the CD44+ cells in primary GC may
predict the development of distant metastasis. This may facilitate
patient selection for adjuvant chemoradiotherapy to reduce the
chance of recurrence following resection of primary GC. During the
write up of the present study, another study group successfully
isolated GCSCs from the peripheral blood of cancer patients using
CD44 surface markers (26). This
demonstrated that GCSCs have the ability to be transferred to any
of the organs of the body through the blood circulation. In total,
∼106 cancer cells per gram of cancer tissue are shed
into the bloodstream daily (27).
However, this is not a reflection of the amount of distant
metastasis found. Most cancer patients, even in their late-stage,
still have very few distant metastasis. Thus, we speculated that
only a small minority of cancer cells with a metastatic capacity
(i.e., the CSCs) are responsible for cancer invasiveness and
metastasis.
At least two important features of CD44 make it a
suitable CSC marker. CD44 is a receptor for hyaluronic acid and is
also able to interact with other ligands, including collagens and
MMPs (28). Therefore, the CSCs
that express CD44 may manipulate cell invasion, migration and
adhesion to the matrix (29).
Furthermore, CD44+ cancer cells are considered to be
slow-cycling, therefore insensitive to chemoradiotherapies
(30). The present study also
observed that sequentially-irradiated GC cells with low X-ray doses
resulted in the inhibition of DNA synthesis and the accumulation of
CD44+ GC cells in the G0/G1 phase
of the cell cycle (data not shown). This revealed that the cell
cycle of CD44+ GC cells may be effectively regulated to
avoid DNA damage by external stimuli.
Acknowledgements
The authors would like to express
gratitude to Hui-Ming Yang for her collection of the xenograft
tissues and Jian-Ye Xu for his technical assistance.
References
|
1
|
Ferlay J, Shin HR, Bray F, Forman D,
Mathers C and Parkin DM: Estimates of worldwide burden of cancer in
2008: GLOBOCAN 2008. Int J Cancer. 127:2893–2917. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Yeh JM, Kuntz KM, Ezzati M, Hur C, Kong CY
and Goldie SJ: Development of an empirically calibrated model of
gastric cancer in two high-risk countries. Cancer Epidemiol
Biomarkers Prev. 17:1179–1187. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Remesh A: Toxicities of anticancer drugs
and its management. Int J Basic Clin Pharmacol. 1:2–12. 2012.
View Article : Google Scholar
|
|
4
|
Dick JE: Stem cell concepts renew cancer
research. Blood. 112:4793–4807. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ward RJ and Dirks PB: Cancer stem cells:
at the headwaters of tumor development. Annu Rev Pathol. 2:175–189.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Clarke MF, Dick JE, Dirks PB, et al:
Cancer stem cells - perspectives on current status and future
directions: AACR Workshop on cancer stem cells. Cancer Res.
66:9339–9344. 2006. View Article : Google Scholar
|
|
7
|
Takaishi S, Okumura T, Tu S, et al:
Identification of gastric cancer stem cells using the cell surface
marker CD44. Stem Cells. 27:1006–1020. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhang Q, Shi S, Yen Y, Brown J, Ta JQ and
Le AD: A subpopulation of CD133(+) cancer stem-like cells
characterized in human oral squamous cell carcinoma confer
resistance to chemotherapy. Cancer Lett. 289:151–160. 2010.
|
|
9
|
Tomuleasa C, Soritau O, Kacso G, et al:
Arsenic trioxide sensitizes cancer stem cells to chemoradiotherapy.
A new approach in the treatment of inoperable glioblastoma
multiforme. J BUON. 15:758–762. 2010.PubMed/NCBI
|
|
10
|
Guddati AK: Ovarian cancer stem cells:
elusive targets for chemotherapy. Med Oncol. 29:3400–3408. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Du Z, Qin R, Wei C, et al: Pancreatic
cancer cells resistant to chemoradiotherapy rich in
‘stem-cell-like’ tumor cells. Dig Dis Sci. 56:741–750.
2011.PubMed/NCBI
|
|
12
|
Croker AK and Allan AL: Inhibition of
aldehyde dehydrogenase (ALDH) activity reduces chemotherapy and
radiation resistance of stem-like ALDHhiCD44+ human
breast cancer cells. Breast Cancer Res Treat. 133:75–87. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Albright N: Computer programs for the
analysis of cellular survival data. Radiat Res. 112:331–340. 1987.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Olive PL, Banáth JP and Durand RE:
Heterogeneity in radiation-induced DNA damage and repair in tumor
and normal cells measured using the ‘comet’ assay. 1990. Radiat
Res. 178:AV35–AV42. 2012.
|
|
15
|
Shankar S, Nall D, Tang SN, et al:
Resveratrol inhibits pancreatic cancer stem cell characteristics in
human and KrasG12D transgenic mice by inhibiting pluripotency
maintaining factors and epithelial-mesenchymal transition. PLoS
One. 6:e165302011. View Article : Google Scholar
|
|
16
|
Walter D, Satheesha S, Albrecht P, et al
CWS Study Group: CD133 positive embryonal rhabdomyosarcoma
stem-like cell population is enriched in rhabdospheres. PLoS One.
6:e195062011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhou J, Wang H, Cannon V, Wolcott KM, Song
H and Yates C: Side population rather than CD133(+) cells
distinguishes enriched tumorigenicity in hTERT-immortalized primary
prostate cancer cells. Mol Cancer. 10:1122011.
|
|
18
|
Patrawala L, Calhoun T,
Schneider-Broussard R, et al: Highly purified CD44+ prostate cancer
cells from xenograft human tumors are enriched in tumorigenic and
metastatic progenitor cells. Oncogene. 25:1696–1708. 2006.
|
|
19
|
England K, Driscoll CO and Cotter TG: ROS
and protein oxidation in early stages of cytotoxic drug induced
apoptosis. Free Radic Res. 40:1124–1137. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Gupta GP, Nguyen DX, Chiang AC, et al:
Mediators of vascular remodelling co-opted for sequential steps in
lung metastasis. Nature. 446:765–770. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Chen MS, Woodward WA, Behbod F, et al:
Wnt/beta-catenin mediates radiation resistance of Sca1+ progenitors
in an immortalized mammary gland cell line. J Cell Sci.
120:468–477. 2007.PubMed/NCBI
|
|
22
|
Song Z, Yue W, Wei B, et al: Sonic
hedgehog pathway is essential for maintenance of cancer stem-like
cells in human gastric cancer. PLoS One. 6:e176872011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Dalerba P, Dylla SJ, Park IK, et al:
Phenotypic characterization of human colorectal cancer stem cells.
Proc Natl Acad Sci USA. 104:10158–10163. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Collins AT, Berry PA, Hyde C, Stower MJ
and Maitland NJ: Prospective identification of tumorigenic prostate
cancer stem cells. Cancer Res. 65:10946–10951. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Al-Hajj M, Wicha MS, Benito-Hernandez A,
Morrison SJ and Clarke MF: Prospective identification of
tumorigenic breast cancer cells. Proc Natl Acad Sci USA.
100:3983–3988. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Chen T, Yang K, Yu J, et al:
Identification and expansion of cancer stem cells in tumor tissues
and peripheral blood derived from gastric adenocarcinoma patients.
Cell Res. 22:248–258. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zetter BR: Angiogenesis and tumor
metastasis. Annu Rev Med. 49:407–424. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Yu Q and Stamenkovic I: Localization of
matrix metalloproteinase 9 to the cell surface provides a mechanism
for CD44-mediated tumor invasion. Genes Dev. 13:35–48. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Pongcharoen P, Jinawath A and Tohtong R:
Silencing of CD44 by siRNA suppressed invasion, migration and
adhesion to matrix, but not secretion of MMPs, of
cholangiocarcinoma cells. Clin Exp Metastasis. 28:827–839. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Ishimoto T, Oshima H, Oshima M, et al:
CD44+ slow-cycling tumor cell expansion is triggered by cooperative
actions of Wnt and prostaglandin E2 in gastric tumorigenesis.
Cancer Sci. 101:673–678. 2010.
|