Introduction
The function of ceramide in apoptosis and its
association with receptor-associated apoptotic signaling proteins
remain unresolved. It has previously been shown that TNF-α-induced
apoptosis is preceded by an increase in intracellular ceramide
levels (1). TNF-α and exogenous C6
ceramide interfere with the activation of Raf-1 and ERK by EGF and
down-regulate v-Src-induced Raf-1 kinase activity (1). Exogenous C6 ceramide induces endocytic
vesicle formation and results in enlarged late endosomes and
lysosomes in mouse fibroblasts (2).
Chemotherapeutic agents, including paclitaxel and
taxol, as well as physiological stimuli, such as TNF-α, stimulate
ceramide accumulation and increase oxidative stress in cancer
cells, and the upregulation of glucosylceramide synthase has been
hypothesized to contribute to chemoresistance (3). Notably, multidrug-resistant cancer
cells exhibit elevated levels of glucosylceramide (4–7).
Agents that block ceramide glycosylation potentiate the cellular
sensitivity to ceramide and chemotherapeutic agents, indicating
that the ceramide metabolic pathway is an important target for
anticancer drug development (8).
Paclitaxel has emerged as a valuable antimitotic
chemotherapy drug, particularly in breast and ovarian cancer
(9). Although cytotoxic mechanisms
are well understood, the efficacy of this drug cannot be explained
by microtubular interactions only. Paclitaxel-induced apoptosis has
been shown to be attributable, in part, to ceramide and sphingoid
bases, and the simultaneous treatment of Jurkat cells with
paclitaxel and ceramide has been demonstrated to enhance
paclitaxel-induced cell growth inhibition (10). Paclitaxel/ceramide combination
therapy has been actively studied (11) and the clinical use of paclitaxel
with ceramide-enhancing agents may maximize cytotoxic potential
(12).
Our previous studies have demonstrated that the
combination of paclitaxel and ceramide synergistically induced
pancreatic cancer cell death through differential modulation of
EGFR-mediated MAP kinases. EGFR and ERK inhibitors may further
enhance the effect of paclitaxel and ceramide (13). The combination of paclitaxel and
ceramide in biodegradable polymeric nanoparticles has been
identified as an extremely effective therapeutic strategy to
overcome drug resistance in ovarian cancer (14).
Additional studies have identified a ceramide
transport protein, COL4A3BP or CERT, which sensitizes cancer cells
to multiple cytotoxic agents when downregulated. COL4A3BP
expression is increased in drug-resistant cell lines and in
residual tumor cells following paclitaxel treatment of ovarian
cancer, indicating that it may be a target for
chemotherapy-resistant cancers (15–17).
Considering the rising functions of ceramide in
combinatorial therapies with other chemotherapeutic agents, and the
involvement of its modified form in chemoresistance, the entry of
exogenous C6 ceramide was analyzed in the present study using
fluorescently-labeled C6-NBD. C6 ceramide was observed to enter the
ovarian cancer cells in a polarized fashion. In addition to this,
paclitaxel was observed to induce vesicle formation and prevent the
polarized entry of C6 into the cancer cells, thus exhibiting a
synergistic effect on apoptosis.
Materials and methods
Chemicals and reagents
C6-NBD-ceramide was a gift from Avanti Polar Lipids,
Inc. (Alabaster, AL, USA). Filipin, taxol and doxorubicin were
obtained from Sigma-Aldrich (St. Louis, MO, USA). Hoechst 33342 was
purchased from Molecular Probes (Calsbad, CA, USA).
Cell culture
Human ovarian cancer cells (CaOV3 cells) were
maintained as described previously (18) in DMEM (Sigma-Aldrich) supplemented
with 10% fetal bovine serum, penicillin/streptomycin (1:100,
Sigma-Aldrich) and 4 mM L-glutamine, in a CO2 incubator
at 37°C.
Confocal microscopy
The cells were plated in eight-well chamber slides
(Lab-Tek; Nalge Nunc International, Naperville, IL, USA) and
treated with various reagents, including C6-NBD ceramide, taxol and
CuSO4. Next, the cells were either left untreated or
were fixed for 20 min in fresh 4% paraformaldehyde-PBS. The cell
nuclei were also stained with Hoechst (1 μg/ml in PBS) for
10 min. The slides were mounted with anti-fade (Life Technologies,
Grand Island, NY, USA) and stored in the dark until viewing. The
samples were observed under a confocal microscope and images were
captured by Zen 2009 Light Edition (Carl Zeiss AG, Oberkochen,
Germany).
Results and Discussion
C6 ceramide enters cells in a polarized
manner
The use of a combination of several chemotherapeutic
agents is well accepted clinically, as it enables drugs to be
administered at relatively low doses with an improved efficacy. Our
previous studies demonstrated that C6 ceramide functions
synergistically with taxol to inhibit cell proliferation and cell
migration in cultured ovarian cancer cells (18). However, the molecular mechanism of
this synergism remains unknown, and the entry of membrane-permeable
C6 ceramide into the cells remains uncharacterized. To investigate
the pattern of C6 entry into the cells, fluorescently-labeled
C6-NBD was used. Ovarian cancer cells were treated with C6 ceramide
and the resultant fluorescence signal was observed with or without
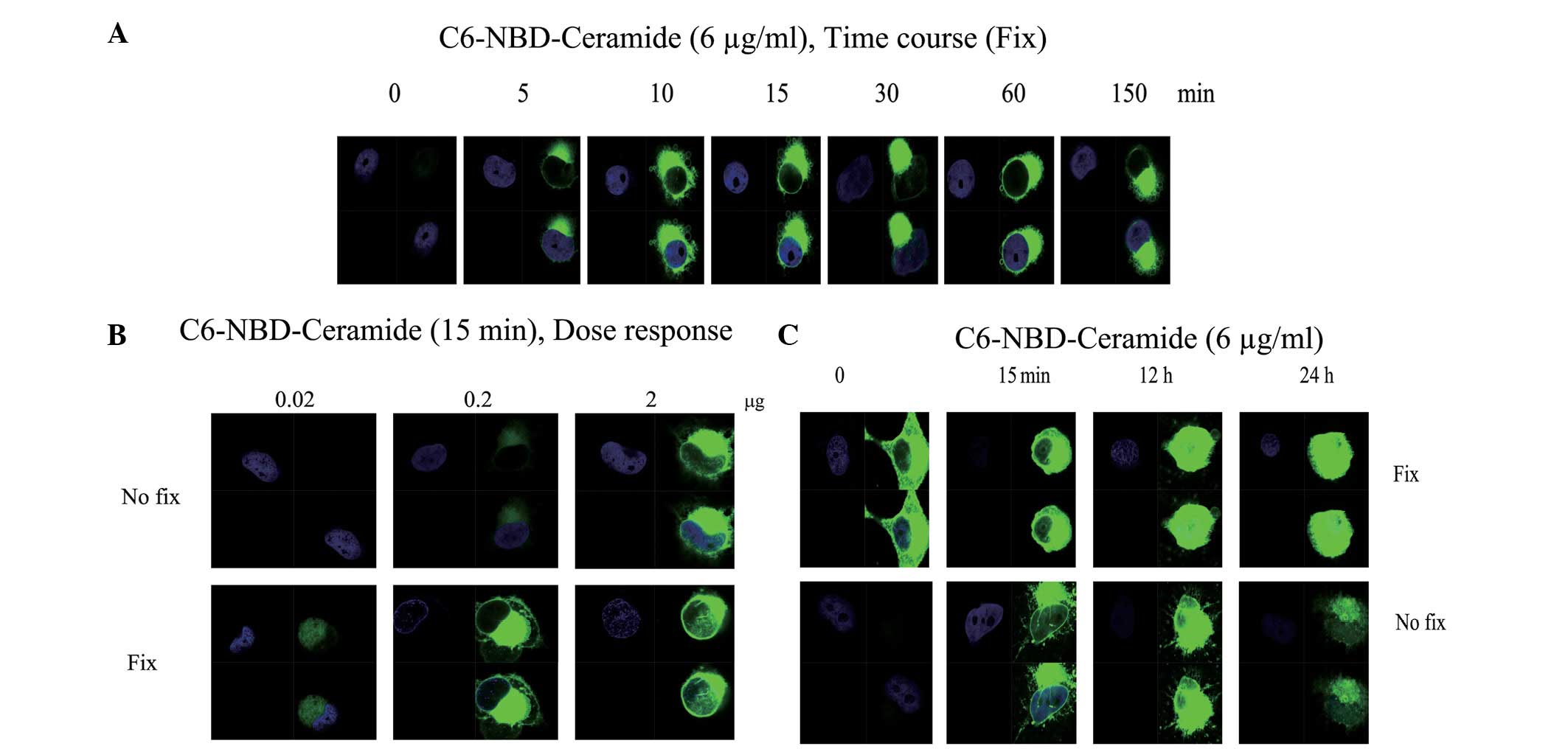
fixation. The results indicated that C6 ceramide enters the cells
in a time- (Fig. 1A) and
dose-dependent (Fig. 1B) manner.
Notably, the distribution of the fluorescence signal showed a
polarized pattern (Fig. 1A and B).
Subsequent to 12 h, the fluorescence signal had saturated the cells
(Fig. 1C). The cause of the
polarized pattern of entry remains to be investigated. Previously
published data have indicated that C6 transporters are involved in
the entry of C6 ceramide into the cells (16). However, in the present study, the
fluorescence signal was observed to occur between two dividing
cells and we hypothesized that entry is likely to occur at mitosis
initiation sites.
Effect of inhibitors of lipid
rafts/caveolae on C6 ceramide entry into ovarian cancer cells
Previous studies have indicated that the synergism
of C6 ceramide and taxol is mediated by the inhibition of the EGF
cell surface receptor and the ERK/AKT cell survival pathway, and
that the action occurs in the initial hours following treatment. C6
ceramide is a membrane-permeable molecule that is currently
hypothesized to enter the cells evenly through diffusion, although
an accumulating number of studies have also demonstrated the
existence of ceramide transporters (14–16).
Our data also demonstrated that C6 ceramide rapidly enters cells in
a polarized manner. Lipid rafts/caveolae have previously been
identified as important for the process of signal transduction
(19). To further investigate the
entry of C6 ceramide, in the present study, the cells were
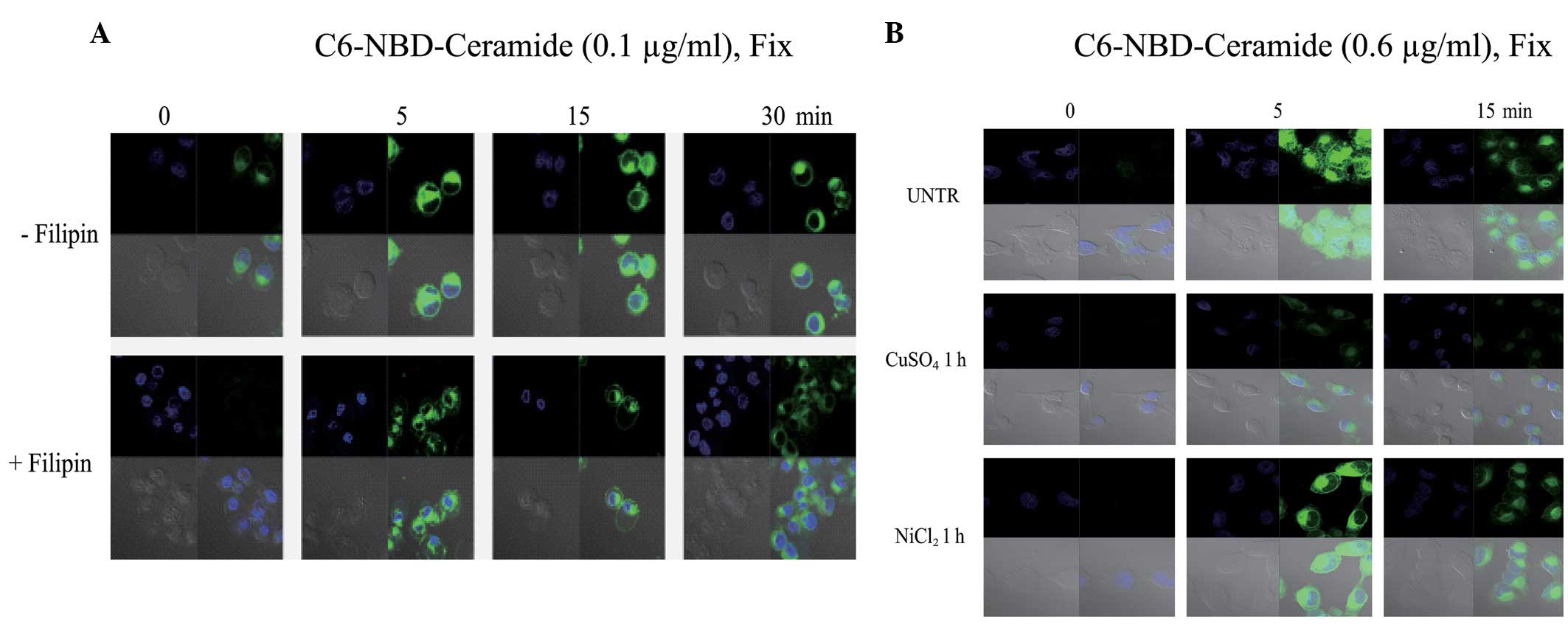
pretreated with filipin, an inhibitor of lipid rafts/caveolae.
Filipin inhibited the C6 ceramide entry into the CaOV3 cells
(Fig. 2), indicating that lipid
rafts/caveolae may be involved in the entry of C6 ceramide into the
cells. Maintaining membrane structure or topology may potentiate
the synergistic effect of taxol and ceramide on the apoptosis of
cancer cells.
Effect of water channel inhibitors on
entry of C6 ceramide into ovarian cancer cells
Previous studies have demonstrated that molecules
other than water may also enter the cells via water channels or
aquaporins (20,21). Aquaporins also play critical roles
in processes other than the transport of water (21,22).
Our previous studies revealed that the EGFR-mediated expression of
aquaporins is associated with cell migration in normal and cancer
cells (23,24). To investigate whether C6 ceramide
enters the cells via aquaporins or whether the entry is only
partially associated with aquaporins, the inhibitors of aquaporins,
CuSO4 and NiCl2, were utilized. The results
revealed that CuSO4 inhibits C6 ceramide entry into the
cells, but that NiCl2 does not (Fig. 2B), indicating that C6 ceramide may
partially enter the cells via aquaporins.
Effect of taxol and doxorubicin on the
polarized entry of C6 ceramide into ovarian cancer cells
Paclitexal and doxorubicin have been successfully
administered clinically in numerous cancer types (25). Our previous study demonstrated that
together with C6 ceramide, taxol synergistically inhibits cell
proliferation and cell migration (18). To further determine whether taxol
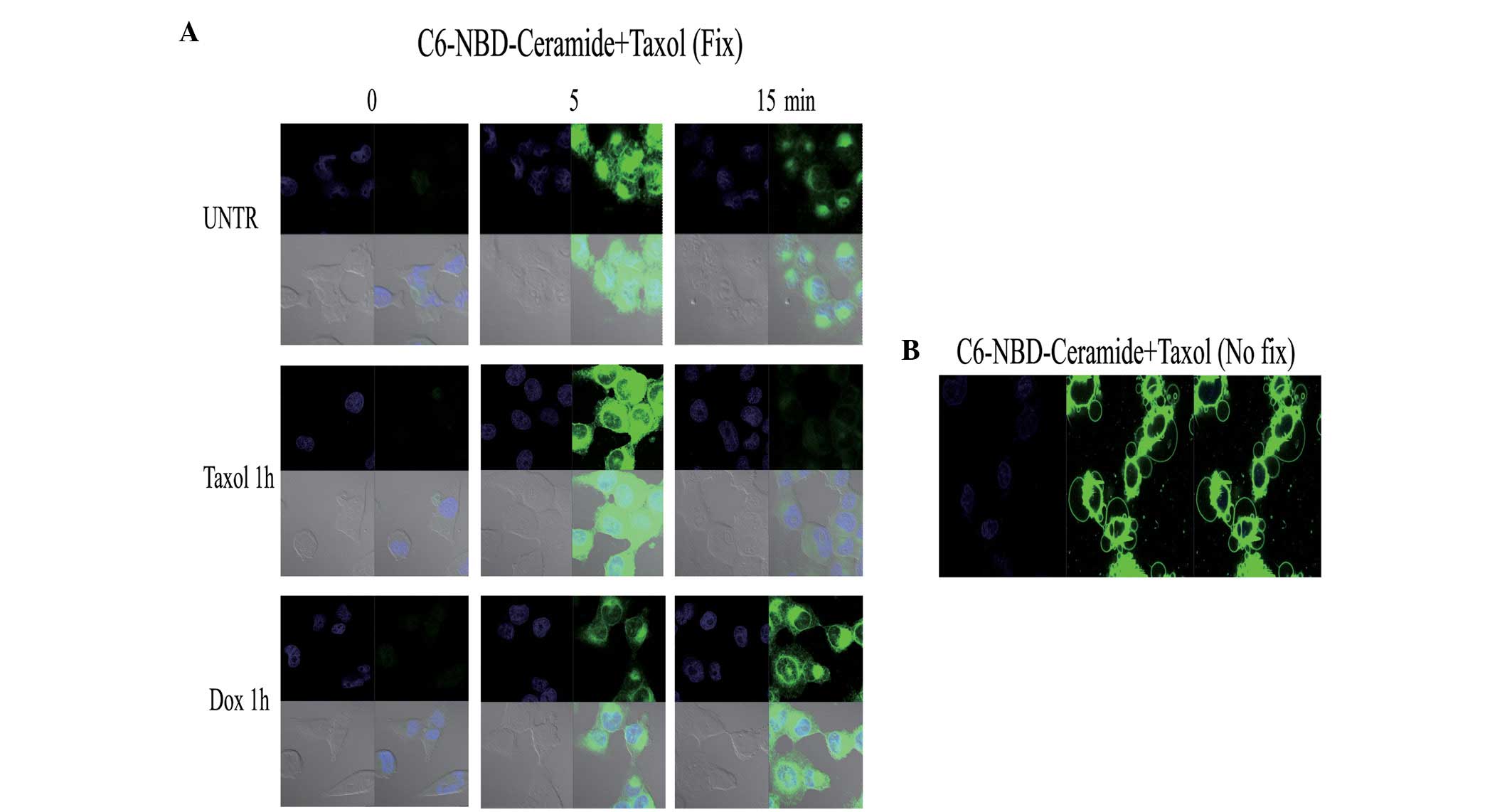
affects C6 ceramide entry into ovarian cancer cells, in the present
study, the cells were pretreated with taxol for 1 h and then
treated with C6-NBD. The results indicated that taxol disrupted the
polarized pattern of C6 entry. Notably, doxorubicin, a commonly
utilized therapeutic agent, had no such effect (Fig. 3A). A previous study has shown that
exogenous C6-ceramide induces endocytic vesicle formation and
causes enlarged late endosomes and lysosomes in mouse fibroblasts
(2). In the present study, the
combination of C6-NBD and taxol was also observed to induce vesicle
formation (Fig. 3B). The cause and
effect of the formation of these vesicles requires further
investigation, however, we hypothesize that vesicle formation may
enhance apoptotic activity when using taxol and C6 ceramide in
combination, as observed in our previous study (18).
Acknowledgements
The present study was supported, in
part, by grants from the National Natural Science Foundation of
China (no. 30772306) and the NIH (P20 RR016457 from INBRE Program
of the National Center for Research Resources).
References
|
1
|
Bourteele S, Hausser A, Döppler H,
Horn-Müller J, Röpke C, Schwarzmann G, Pfizenmaier K and Müller G:
Tumor necrosis factor induces ceramide oscillations and negatively
controls sphingolipid synthases by caspases in apoptotic Kym-1
cells. J Biol Chem. 273:31245–31251. 1998. View Article : Google Scholar
|
|
2
|
Zhan D, Santin AD, Liu Y, Parham GP, Li C,
Meyers C and Hermonat PL: Binding of the human papillomavirus type
16 p97 promoter by the adeno-associated virus Rep78 major
regulatory protein correlates with inhibition. J Biol Chem.
274:31619–31624. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Chapman JV, Gouazé-Andersson V, Messner
MC, Flowers M, Karimi R, Kester M, Barth BM, Liu X, Liu YY,
Giuliano AE and Cabot MC: Metabolism of short-chain ceramide by
human cancer cells - implications for therapeutic approaches.
Biochem Pharmacol. 80:308–315. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lavie Y, Cao HT, Volner A, Lucci A, Han
TY, Geffen V, Giuliano AE and Cabot MC: Agents that reverse
multidrug resistance, tamoxifen, verapamil and cyclosporin A, block
glycosphingolipid metabolism by inhibiting ceramide glycosylation
in human cancer cells. J Biol Chem. 272:1682–1687. 1997. View Article : Google Scholar
|
|
5
|
Chang MS, Sasaki H, Campbell MS, Kraeft
SK, Sutherland R, Yang CY, Liu Y, Auclair D, Hao L, Sonoda H,
Ferland LH and Chen LB: HRad17 colocalizes with NHP2L1 in the
nucleolus and redistributes after UV irradiation. J Biol Chem.
274:36544–36549. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lucci A, Giuliano AE, Han TY, Dinur T, Liu
YY, Senchenkov A and Cabot MC: Ceramide toxicity and metabolism
differ in wild-type and multidrug-resistant cancer cells. Int J
Oncol. 15:535–540. 1999.PubMed/NCBI
|
|
7
|
Giussani P, Bassi R, Anelli V, Brioschi L,
De Zen F, Riccitelli E, Caroli M, Campanella R, Gaini SM, Viani P
and Riboni L: Glucosylceramide synthase protects glioblastoma cells
against autophagic and apoptotic death induced by temozolomide and
Paclitaxel. Cancer Invest. 30:27–37. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lucci A, Han TY, Liu YY, Giuliano AE and
Cabot MC: Modification of ceramide metabolism increases cancer cell
sensitivity to cytotoxics. Int J Oncol. 15:541–546. 1999.PubMed/NCBI
|
|
9
|
Carlier MF and Pantaloni D: Taxol effect
on tubulin polymerization and associated guanosine 5′-triphosphate
hydrolysis. Biochemistry. 22:4814–4822. 1983.
|
|
10
|
Myrick D, Blackinton D, Klostergaard J,
Kouttab N, Maizel A, Wanebo H and Mehta S: Paclitaxel-induced
apoptosis in Jurkat, a leukemic T cell line, is enhanced by
ceramide. Leuk Res. 23:569–578. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Gatei M, Young D, Cerosaletti KM,
Desai-Mehta A, Spring K, Kozlov S, Lavin MF, Gatti RA, Concannon P
and Khanna K: ATM-dependent phosphorylation of nibrin in response
to radiation exposure. Nat Genet. 25:115–119. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Charles AG, Han TY, Liu YY, Hansen N,
Giuliano AE and Cabot MC: Taxol-induced ceramide generation and
apoptosis in human breast cancer cells. Cancer Chemother Pharmacol.
47:444–450. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Liu Y, Qiu L and Li C: Effect of radiation
on nasal mucosa and the microvascular casting of guinea pig. Lin
Chuang Er Bi Yan Hou Ke Za Zhi. 20:793–796. 2006.(In Chinese).
|
|
14
|
Devalapally H, Duan Z, Seiden MV and Amiji
MM: Paclitaxel and ceramide co-administration in biodegradable
polymeric nanoparticulate delivery system to overcome drug
resistance in ovarian cancer. Int J Cancer. 121:1830–1838. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kolesnick R, Altieri D and Fuks Z: A
CERTain role for ceramide in taxane-induced cell death. Cancer
Cell. 11:473–475. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Swanton C, Marani M, Pardo O, Warne PH,
Kelly G, Sahai E, Elustondo F, Chang J, Temple J, Ahmed AA, Brenton
JD, Downward J and Nicke B: Regulators of mitotic arrest and
ceramide metabolism are determinants of sensitivity to paclitaxel
and other chemotherapeutic drugs. Cancer Cell. 11:498–512. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lee AJ, Roylance R, Sander J, Gorman P,
Endesfelder D, Kschischo M, Jones NP, East P, Nicke B, Spassieva S,
Obeid LM, Birkbak NJ, Szallasi Z, McKnight NC, Rowan AJ, Speirs V,
Hanby AM, Downward J, Tooze SA and Swanton C: CERT depletion
predicts chemotherapy benefit and mediates cytotoxic and
polyploid-specific cancer cell death through autophagy induction. J
Pathol. 226:482–494. 2012. View Article : Google Scholar
|
|
18
|
Qiu L, Zhou C, Sun Y, Di W, Scheffler E,
Healey S, Wanebo H, Kouttab N, Chu W and Wan Y: Paclitaxel and
ceramide synergistically induce cell death with transient
activation of EGFR and ERK pathway in pancreatic cancer cells.
Oncol Rep. 16:907–913. 2006.PubMed/NCBI
|
|
19
|
Kuebler WM, Yang Y, Samapati R and Uhlig
S: Vascular barrier regulation by PAF, ceramide, caveolae and NO -
an intricate signaling network with discrepant effects in the
pulmonary and systemic vasculature. Cell Physiol Biochem. 26:29–40.
2010. View Article : Google Scholar
|
|
20
|
Hara-Chikuma M, Chikuma S, Sugiyama Y,
Kabashima K, Verkman AS, Inoue S and Miyachi Y: Chemokine-dependent
T cell migration requires aquaporin-3-mediated hydrogen peroxide
uptake. J Exp Med. 209:1743–1752. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Virreira M, Perret J and Delporte C:
Pancreatic beta-cells: Role of glycerol and aquaglyceroporin 7. Int
J Biochem Cell Biol. 43:10–13. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zheng X and Bollinger Bollag W: Aquaporin
3 colocates with phospholipase d2 in caveolin-rich membrane
microdomains and is downregulated upon keratinocyte
differentiation. J Invest Dermatol. 121:1487–1495. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Cao C, Sun Y, Healey S, Bi Z, Hu G, Wan S,
Kouttab N, Chu W and Wan Y: EGFR-mediated expression of aquaporin-3
is involved in human skin fibroblast migration. Biochem J.
400:225–234. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ji C, Cao C, Lu S, Kivlin R, Amaral A,
Kouttab N, Yang H, Chu W, Bi Z, Di W and Wan Y: Curcumin attenuates
EGF-induced AQP3 up-regulation and cell migration in human ovarian
cancer cells. Cancer Chemother Pharmacol. 62:857–865. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kelland LR: Emerging drugs for ovarian
cancer. Expert Opin Emerg Drugs. 10:413–424. 2005. View Article : Google Scholar : PubMed/NCBI
|