Introduction
Giant sacral neurogenic tumors are rare lesions
which include schwannomas, neurofibromas and malignant peripheral
nerve sheath tumors (MPNSTs), and are estimated to represent ∼10%
of presacral tumors (1). The tumors
usually grow towards the presacral space and present with extremely
large dimensions (2). Previous
studies on the tumors are limited and consist of a relatively small
number of cases (2–5). Giant sacral neurogenic tumors are
characterized by an indolent growth pattern and non-specific
symptoms (6). Although the majority
of these tumors are benign, they may become locally aggressive,
cause catastrophic neurological impairment and weaken pelvic arch
stability. The initial treatment of a tumor by complete resection
may give a patient the best chance of an overall and disease-free
survival (7). However, surgical
resection is complex due to the anatomical characteristics of the
region, the extent of the tumor, the substantial blood loss during
surgery and the propensity for local recurrence (6,7). The
requirement for adequate tumor removal must be balanced against the
preservation of nerve function. Yang et al (8) identified that pre-operative arterial
embolization resulted in decreased intra-operative blood loss and
allowed a more aggressive approach to the complete resection of the
tumors. In the present study, the use of a posterior approach
following transcatheter arterial embolization was assessed in the
complete resection of giant sacral neurogenic tumors. Furthermore,
the oncological outcomes and Musculoskeletal Tumor Society (MSTS)
scores were evaluated.
Patients and methods
Patients
The records of 16 patients with giant sacral
neurogenic tumors who underwent surgical excision in The First
Affiliated Hospital of Soochow University (Suzhou, Jiangsu, China)
between January 2000 and June 2010 were identified and
retrospectively analyzed. The patients included 7 males and 9
females, with a mean age of 39.9 years (range, 17–62 years).
Previous surgical treatments had been provided to three patients at
other hospitals. The duration between the onset and presentation of
the symptoms was 1–252 months (average, 94 months). Pain in the
sacrococcygeal region was the most common presenting symptom,
followed by rectal dysfunction or urinary disturbance (Table I). The tumors were detected as firm
presacral masses by a clinical rectal examination in nine of the
patients. All patients received a histological diagnosis using a
computerized tomography-guided percutaneous needle biopsy. A total
of 12 patients (75%) had benign tumors, including 10 schwannomas
and 2 neurofibromas. MPNSTs were identified in four patients (25%).
Plain X-ray, computed tomography (CT) and magnetic resonance
imaging (MRI) scans were obtained pre-operatively. In the high
sacrum (above S3), 11 tumors were located. The remaining five were
situated in the low sacrum (S3 or below). The tumor size was
recorded as the maximum tumor dimension measured on the gross
specimen or on the pre-operative MRI. Approval for the present
study was obtained from the ethics committee of Soochow University
and all patients provided their informed consent.
 | Table I.Chief complaint at presentation. |
Table I.
Chief complaint at presentation.
| Complaint | Frequency, n (%) |
|---|
| Pain | 7 (43.75) |
| Urinary disturbance
or rectal dysfunction | 4 (25.00) |
| Palpable painless
mass | 3 (18.75) |
| Neurological
deficit | 1 (6.25) |
| None (health
examination) | 1 (6.25) |
The management of the giant sacral neurogenic tumors
was performed by a single approach involving a multidisciplinary
team that consisted of two or more surgical specialists, including
orthopedic surgeons, neurosurgeons, colorectal surgeons,
urologists, gynecologists and plastic surgeons. The team created an
appropriate surgical plan with the goal of obtaining adequate
margins.
Surgical procedure
All patients underwent pre-operative embolization of
the tumor-feeding arteries under digital subtraction angiography
(DSA; Siemens Angiostar Plus, Seimens, Munich, Germany). The
bilateral internal iliac, median sacral and other small
tumor-feeding arteries were embolized using gelatin sponges,
ranging in size from a strip to a particle. The angiogram was then
performed again across the abdominal aorta to ensure that all
tumor-supplying vessels were embolized.
The surgical excision of the tumor was performed
within 48 h following the embolization. A pre-operative bowel
preparation and urethral catheterization were performed in all
patients. With the patient in the prone position, and once the
rectum had been identified by palpating the anal tube, the
posterior ‘I’ incision was performed. The approach offered a good
exposure of the sacrum, the dorsal sections of the iliac wings and
the surrounding soft tissues, while allowing exposure of the upper
lumbar spine where necessary. The posterior, caudal and bilateral
aspects of the tumor were excised using wide or marginal
approaches, while the anterior aspect was separated by blunt
dissection through the retroperitoneal interstitial space, with
care being taken to ensure that the integrity of the wall was not
violated. Palpating the anal tube was an efficient way to certify
the location of the rectum. The anterior aspect of the sacrum was
exposed by resecting parts of the iliac bone where necessary. The
bilateral S1–S2 nerve roots, or at least one S3 nerve root, were
preserved in the patients with benign tumors. In the instances
where a nerve root penetrated the tumor, the membrane of this nerve
was dissected. For the patients with malignant neurogenic tumors,
the affected sacral nerves were sacrificed in order to achieve
adequate margins. The dura was ligated twice using a silk suture to
avoid a dural leak. Where the S1 segment, total sacrum and
substantial parts of the iliac wings were excised, the stability of
the pelvic ring was reconstructed using pedicle screws, iliac
screws and lumbopelvic rods.
The minimum duration of patient follow-up was two
years subsequent to the surgery. All data was obtained from
available records or a telephone interview. The follow-up procedure
included plain radiograph, CT and MRI evaluations at six-month
intervals. The ability of the patient to walk with or without an
assistance device was recorded in the last follow-up. The
evaluation of function was graded according to the MSTS score
(9). The oncological outcomes were
assessed clinically using clinical examinations and imaging
studies.
Statistical analysis
A statistical analysis was implemented using SPSS
16.0 software (SPSS, Chicago, IL, USA). The comparisons of the
variables comprised of continuous data were performed using
two-sample t-tests. The comparisons of the discrete variables were
performed using χ2 tests. P<0.05 was considered to
indicate a statistically significant difference.
Results
All tumor masses were removed completely without
intraoperative shock or patient fatalities. The mean tumor size was
17.5 cm (range, 11.5–28 cm) at the greatest diameter. The average
duration of the embolization procedure was 35 min (range, 20–70
min). The mean number of embolized vessels was 13.5 branches
(range, 7–26 branches). The duration of the surgery was 2.5–8
hours, with an average time of 4.2 h. The average level of
intraoperative blood loss was 1,293 ml (range, 400–4,500 ml). The
average level of blood loss in the patients with malignant
schwannomas (1,850 ml) was greater than in patients with benign
neurogenic tumors (1,041.7 ml; P<0.05).
The sacral nerve roots were preserved as much as
possible in the patients with benign neurogenic tumors in order to
maintain a greater neurological function. A marginal resection was
performed in seven patients (58.3%) and the remaining five (41.7%)
underwent intralesional resections due to extensive involvement of
the sacral nerve roots. During surgery, the bilateral S1–S2 nerve
roots, or at least the unilateral S3 nerve roots, were preserved in
11 patients. For the patients with MPNSTs, the affected sacral
nerves were sacrificed in order to achieve adequate margins. As a
result, one patient (25%) underwent a wide resection and three
(75%) underwent marginal resections. The bilateral S1–S2 nerve
roots, or at least the unilateral S3 nerve roots, were preserved in
two patients; the unilateral S1–S2 nerve roots were preserved in
one patient and the bilateral S1 nerve roots were preserved in the
remaining patient. One of the patients with an MPNST had the
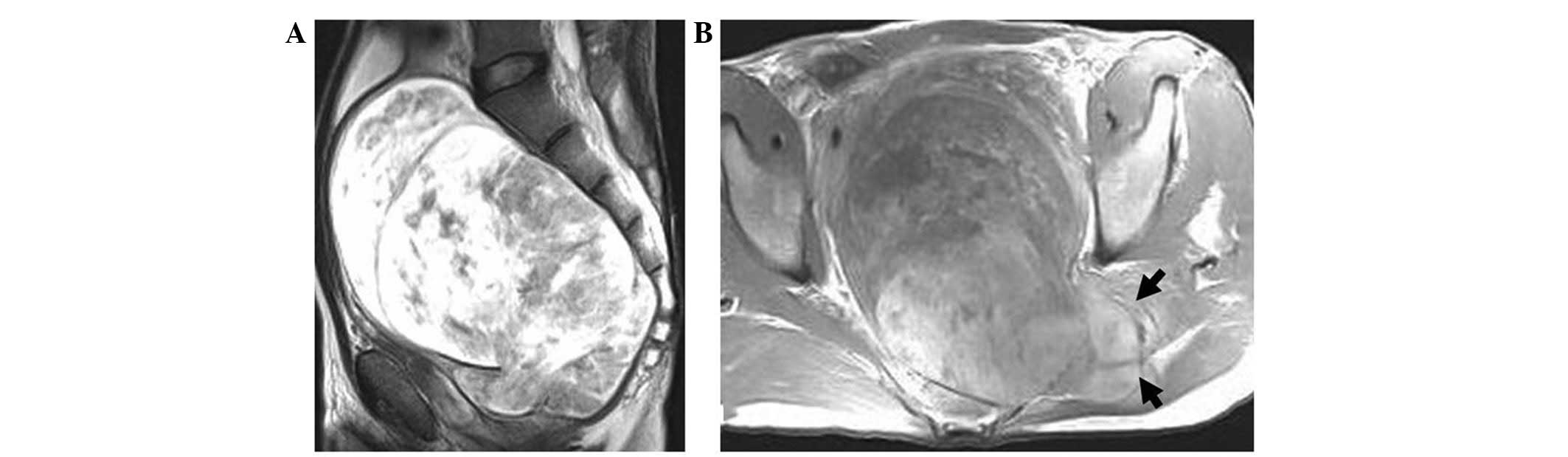
largest tumor volume of ∼13×16×28 cm3 (Fig. 1). All the tumor-supplying vessels
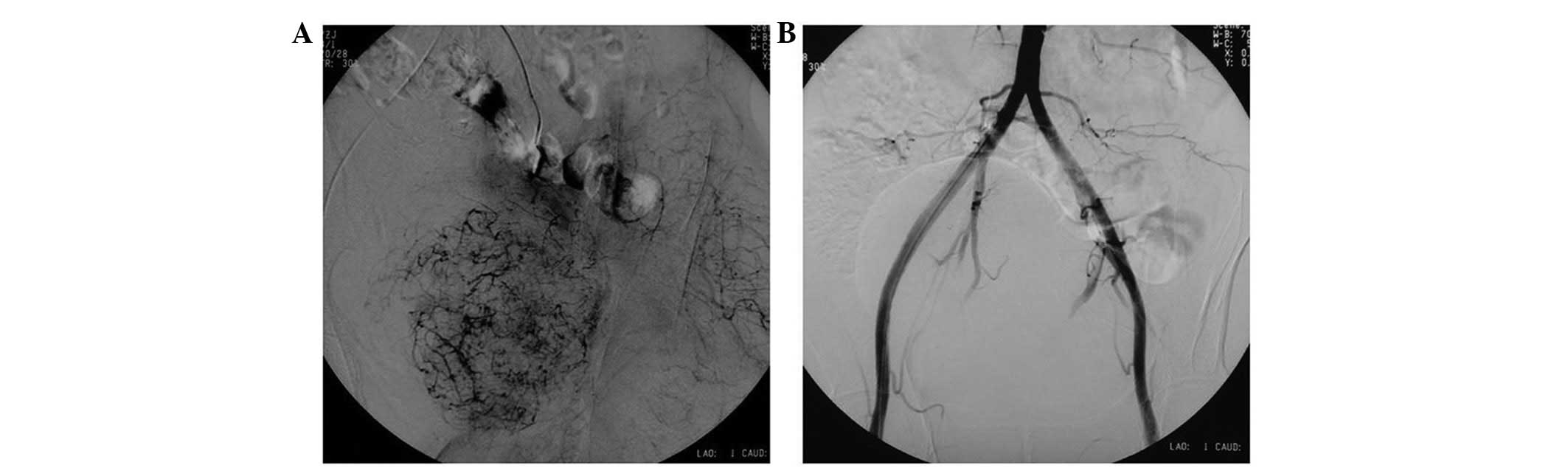
were embolized pre-operatively (Fig.
2) and the tumor was marginally resected while preserving the
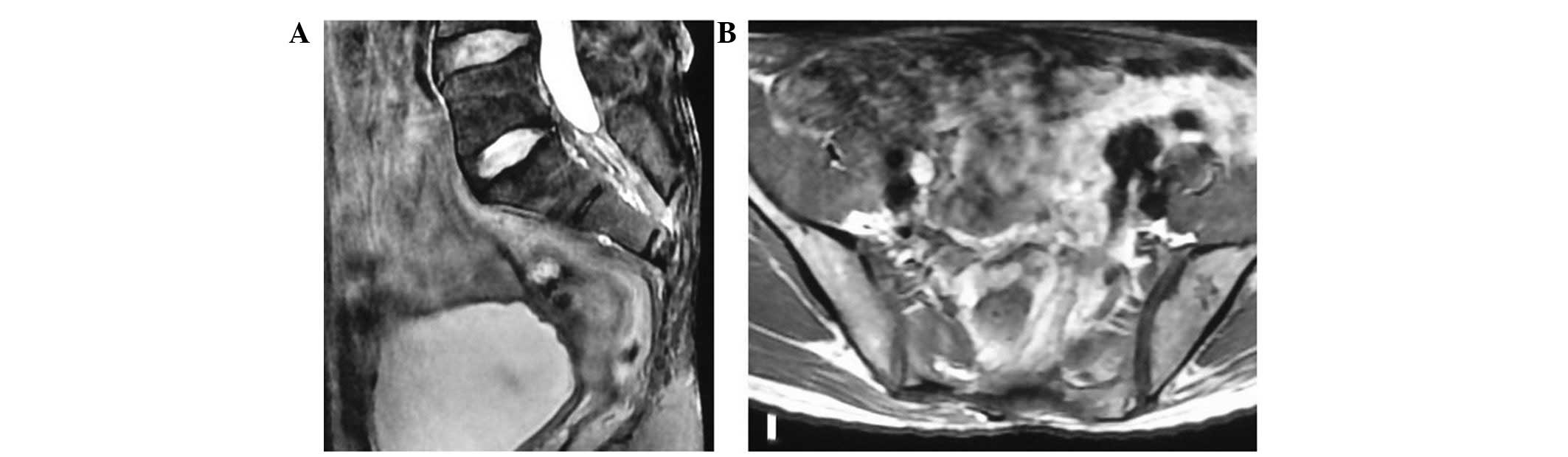
bilateral S2 nerve roots. The patient was disease-free at the
four-year follow-up (Fig. 3). A
pelvic reconstruction was performed in three of the 16 patients. No
patients were treated pre- or post-operatively with adjuvant
radiation therapy or chemotherapy. The mean hospital stay was 27
days (range, 15–48 days). Wound complications occurred in four
patients (25%); three experienced cutaneous necrosis and one
suffered a superficial wound infection. The surgical wounds of two
of the patients healed with local wound care and debridement, and
the wounds of the remaining two patients healed with flap closure
and debridement. No patients experienced a clinical pulmonary
embolism.
Overall, the average MSTS score of the 16 patients
was 82% at the final follow-up. The score was 92.8% (range,
68–100%) in the benign cases, and 60.3% (range, 38.2–87.6%) for the
patients with malignant disease. This difference was statistically
significant (P<0.05). The MSTS scores of the patients with
preservation of the bilateral S1/S2 nerve roots, or at least the
unilateral S3 nerve roots (89%) were better than the scores of the
patients in whom the bilateral S3 nerve was sacrificed (46%;
P<0.05). The patients >40 years of age had a poor functional
score compared with the patients <40 years, but the difference
was not statistically significant (P>0.05).
Of the 12 patients with benign neurogenic tumors, 10
(83.3%) had normal bladder function and 11 (91.6%) had normal bowel
control. For the patients with MPNSTs, two (50%) experienced
impaired bladder control and two (50%) had impaired bowel control.
Finally, two patients (12.5%) were managed by colostomy and three
patients (18.8%) received intermittent urethral catheterization.
All patients were able to ambulate independently and showed no
signs of significant lower extremity weakness.
Follow-up information was obtained for all patients.
The mean follow-up time was 59 months (range, 24–110 months). The
oncological outcomes are presented in Table II. Recurrence or mortality as a
result of the disease did not occur in the patients with benign
tumors. The survival rate of the patients who presented with
malignant lesions was 75% (3/4 patients), since 1 patient (25%) had
multiple local recurrences and succumbed to the disease. However,
no pulmonary or other metastases were identified in this
patient.
 | Table II.Surgical type and outcome of 16 cases
of sacral neurogenic tumors. |
Table II.
Surgical type and outcome of 16 cases
of sacral neurogenic tumors.
| Tumor type | Type of surgery
| Local recurrence | Metastasis | NED | AWD | Mortality |
|---|
| Wide | Marginal | Intralesional |
|---|
| Benign, n | 0 | 7 | 5 | 0 | 0 | 10 | 2 | 0 |
| Malignant, n | 1 | 3 | 0 | 1 | 0 | 3 | 0 | 1 |
Discussion
Giant sacral neurogenic tumors are uncommon and
their true incidence is unknown. However, they are estimated to
represent 10% of the total presacral tumors (1) The majority of giant sacral neurogenic
tumors are benign, with malignant schwannomas being rare. The
literature on the surgical management of these tumors is limited to
case reports and small studies (2–4). The
growth of these tumors is frequently indolent and the lack of
specific symptoms and signs make an early diagnosis difficult. The
tumors often reach considerable sizes and may involve surrounding
structures, making their management a surgical challenge.
The treatment of giant sacral neurogenic tumors
remains controversial, since the main objective of surgical
treatment is to achieve a complete surgical resection (4,6,10).
However, the extent of the surgical resection is frequently limited
by extensive intraoperative blood loss, particularly in giant
MPNSTs (7,11). Methods to control the blood loss
include occluding the abdominal aorta (6), ligation of the internal vein and
artery (12), autologous blood
transfusion (13) and manipulative
hypotensive anesthesia. Nonetheless, studies have documented the
mean level of blood loss during surgery as 1,600–4,300 ml (3,4,6). In
the present study, a gelatin sponge was used as an embolic agent.
The intratumor blood supply vessels and the main stem of the
extratumor artery, including the bilateral internal iliac and
median sacral arteries, were embolized completely. The level of
blood transfusion averaged 1,293 ml (range, 0–4,000 ml) during the
surgery. The level of blood loss was significantly decreased
compared with previous data (3,4,6).
Several surgical approaches, including anterior,
posterior and combined approaches, are used to resect sacral
neurogenic tumors (4,6,14). The
choice of approach is dictated by the location and size of the mass
and its association with adjacent structures. For malignant pelvic
tumors, Yokoyama et al (15)
suggested that a patient's chances of definitive surgery may be
enhanced by a multidisciplinary approach. Generally, the posterior
approach is recommended for tumors that are situated at the third
sacral (S3) segment or below. A combined anterior and posterior
approach is advised for lesions that extend to the S1 or S2
segments. The decision to use only a posterior approach on patients
in the present study is controversial. In our experience, the tumor
rarely invades the rectal wall due to the presence of the presacral
fascia, which resists tumor transgression. It is relatively easy to
separate the anterior tissues using blunt dissection through the
retroperitoneal interstitial spaces. In the present study, all
tumor masses were removed completely without intraoperative shock
or fatalities. Wide or marginal resection was performed on the four
patients with MPNSTs. A posterior approach may be considered
satisfactory for the resection of sacral neurogenic tumors,
including those above S3, when combined with embolization.
Moreover, pre-operative arterial embolization may have the
potential to assist surgeons in completing resections using only a
posterior approach.
Previous studies on sacral neurogenic tumors have
focused on the oncological results without addressing the
functional outcomes (4,6). Alderete et al (7) studied 38 patients with pelvic
neurogenic tumors. Of these patients, twelve presented with
malignant tumors. The patients with benign tumors had a mean MSTS
score of 94%, while the survivors of malignant disease had a mean
score of 57%. The majority of patients with benign tumors in the
present study had higher MSTS scores and a better functional
outcome when compared with the patients with malignant tumors,
which is consistent with the results from the previous studies.
Moreover, the results from the present study demonstrated that the
preservation of the bilateral S1–S2, or at least the unilateral S3,
nerve roots is significant in maintaining normal function.
The oncological results of sacral neurogenic tumors,
particularly MPNSTs (7), are not as
promising as those observed for the limbs. Due to the aggressive
nature of MPNSTs, the overall survival rate is worse than for
benign tumors. In the present study, the recurrence rate of MPNSTs
was 25% (1/4 patients), which is comparable to the recurrence rates
(40–71.4%) in the literature (6,7). No
tumor recurrence or patient fatalities occurred in any of the
benign cases. The prognosis for a sacral neurogenic tumor has been
reported to be associated with tumor size, surgical margins or
having had a prior surgical procedure (11). Sacrectomy has been suggested for
treating giant sacral schwannomas (3,5).
However, achieving a wide margin may sacrifice the normal adjacent
neurovascular structures and associated function. Conversely,
Pongsthorn et al (4)
observed a good outcome with the use of a piecemeal subtotal
excision in six giant sacral schwannoma cases. For benign sacral
neurogenic tumors, local recurrences and malignant transformations
are rare. Therefore, marginal or intralesional resections may be
considered in order to preserve the sacral nerve roots and
function. In the present study, adequate surgical treatment was
performed for the MPNSTs. At the final follow-up, three patients
were alive with no evidence of disease (NED) and one patient
succumbed to repeated local recurrences.
The limitations of the present study include its
retrospective nature, the relatively small numbers of patients and
the use of a heterogenous group of patients with malignant and
benign sacral neurogenic tumors. Despite these limitations, the
present study may assist surgeons in recognizing the behavior of
sacral neurogenic tumors. The results demonstrate that a surgical
resection utilizing a posterior approach with pre-operative
embolization is a viable treatment option for giant sacral
neurogenic tumors.
References
|
1.
|
Jao SW, Beart RW Jr, Spencer RJ, Reiman HM
and Ilstrup DM: Retrorectal tumors. Mayo Clinic experience,
1960–1979. Dis Colon Rectum. 28:644–652. 1985.PubMed/NCBI
|
|
2.
|
Ortolan EG, Sola CA, Gruenberg MF and
Carballo Vazquez F: Giant sacral schwannoma. A case report. Spine
(Phila Pa 1976). 21:522–526. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
Min K, Espinosa N, Bode B and Exner GU:
Total sacrectomy and reconstruction with structural allografts for
neurofibrosarcoma of the sacrum. A case report. J Bone Joint Surg
Am. 87:864–869. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Pongsthorn C, Ozawa H, Aizawa T, Kusakabe
T, Nakamura T and Itoi E: Giant sacral schwannoma: a report of six
cases. Ups J Med Sci. 115:146–152. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Santi MD, Mitsunaga MM and Lockett JL:
Total sacrectomy for a giant sacral schwannoma. A case report. Clin
Orthop Relat Res. 294:285–289. 1993.PubMed/NCBI
|
|
6.
|
Wei G, Xiaodong T, Yi Y and Ji T: Strategy
of surgical treatment of sacral neurogenic tumors. Spine (Phila Pa
1976). 34:2587–2592. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Alderete J, Novais EN, Dozois EJ, Rose PS
and Sim FF: Morbidity and functional status of patients with pelvic
neurogenic tumors after wide excision. Clin Orthop Relat Res.
468:2948–2953. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Yang H, Zhu L, Ebraheim NA, et al:
Surgical treatment of sacral chordomas combined with transcatheter
arterial embolization. J Spinal Disord Tech. 23:47–52. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Enneking WF, Dunham W, Gebhardt MC,
Malawar M and Pritchard DJ: A system for the functional evaluation
of reconstructive procedures after surgical treatment of tumors of
the musculoskeletal system. Clin Orthop Relat Res. 286:241–246.
1993.PubMed/NCBI
|
|
10.
|
Gachiani J, Kim D, Nelson A and Kline D:
Surgical management of malignant peripheral nerve sheath tumors.
Neurosurg Focus. 22:E132007. View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Dozois EJ, Wall JC, Spinner RJ, et al:
Neurogenic tumors of the pelvis: clinicopathologic features and
surgical outcomes using a multidisciplinary team. Ann Surg Oncol.
16:1010–1016. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Sahakitrungruang C, Chantra K, Dusitanond
N, Atittharnsakul P and Rojanasakul A: Sacrectomy for primary
sacral tumors. Dis Colon Rectum. 52:913–918. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Nakai S, Yoshizawa H, Kobayashi S, Naga K
and Ichinose H: Role of autologous blood transfusion in sacral
tumor resection: patient selection and recovery after surgery and
blood donation. J Orthop Sci. 5:321–327. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Acciarri N, Staffa G and Poppi M: Giant
sacral schwannoma: removal by an anterior, transabdominal approach.
Br J Neurosurg. 10:489–492. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Yokoyama R, Beppu Y, Tobisu Ki K, et al: A
multidisciplinary approach to the treatment of malignant pelvic
bone tumors: results with eight consecutive patients. J Orthop Sci.
5:449–456. 2000. View Article : Google Scholar : PubMed/NCBI
|