Introduction
NAD+ glycohydrolase (NADase; EC 3.2.2.5)
is an enzyme that catalyzes the hydrolysis of NAD+ to
nicotinamide and ADP-ribose. In eukaryotes, particularly mammals,
NAD+ glycohydrolase activity is largely attributed to
surface antigen CD38, a multifaceted protein detected primarily in
the activation stages of lymphocytes (1,2). CD38
is exposed as a transmembrane protein on the surface of
hematopoietic cells and several other cell types, and is involved
in heterotypic interactions with CD31 on endothelial cells, as well
as acting as a receptor that modulates signal transmission.
Finally, CD38 is an ectoenzyme with its catalytic site residing on
the outer surface of the cell membrane (3). As such, it exhibits three catalytic
activities, those of NAD+ glycohydrolase, ADP-ribosyl
cyclase and cyclic ADP-ribosyl hydrolase. Cyclic ADP-ribose, the
product of CD38 ADP-ribosyl cyclase activity, has received
considerable interest as an inositol 1,4,5-triphosphate
(IP-3)-independent Ca2+-mobilizer. Moreover, CD38
expression has gained importance as a prognostic marker in chronic
lymphocytic leukemia (CLL) (4,5), HIV
infection (6) and cancer (7,8).
A soluble form of CD38 with a molecular weight of 38
kDa also exists. The form was first identified in the supernatants
of a mixed lymphocyte culture and CD38+ leukemic cell
lines (9). The soluble form appears
to correspond to the extracellular portion of CD38 and exists
partially in dimeric form (10).
The presence of elevated levels of a soluble form of CD38 has also
been shown in serum samples from cancer patients (11). Soluble CD38 was reported to be
decreased in rheumatoid arthritis and systemic lupus erythematosus
patients (12), but increased in
carriers of viral hepatitis G markers (13). A decrease in the serum levels of the
dimeric form of soluble CD38 was observed in burned patients
(14). By contrast, CD38 has been
implicated to be involved in the prolongation of the lifespan of
memory B-cell responses (15). Such
data suggests a possible association between CD38 and various
physiological and pathological events. The levels of soluble CD38
appear to change depending on the underlying disorder. In view of
such variable data, the present study attempted to purify soluble
CD38, as the availability of purified protein would enable the
identification of its molecular and cellular interactions and
eventually the role(s) that CD38 may have in various pathogenic
networks.
Materials and methods
Materials
[Carbonyl-14C]NAD+, with a
specific activity of 53 mCi/mmol, was purchased from Amersham Life
Sciences (Piscataway, NJ, USA). Sephadex G-100, Affi-Gel blue
(Cibacron Blue F3GA), monoclonal anti-human CD38 clone HI157,
NAD+ glycohydrolase (from a Pig’s brain), Ampholine
carrier ampholytes and all chemicals of analytical grade were
obtained from Sigma-Aldrich (St. Louis, MO, USA). The AG1-X4 anion
exchange resin was a product of Bio-Rad Laboratories (Hercules, CA,
USA).
Serum samples were obtained from young and
apparently healthy individuals.
Purification of NAD+
glycohydrolase from serum
Steps
All procedures were performed at 4°C. The serum
samples were fractionated using successive steps of ammonium
sulfate precipitation, affinity chromatography (Affi-Gel blue), gel
filtration chromatography (Sephadex G-100) and isoelectric
focusing.
Ammonium sulfate fractionation. Well-powdered
solid ammonium sulfate was added slowly to the serum samples and
was continuously stirred until saturation levels of 35 and 65% were
attained. Once each level of saturation had been reached, the
stirring was continued for a further 30 min. Thereafter, the
samples were centrifuged for 20 min at 10,000 x g and the collected
precipitated proteins were dissolved in and dialyzed exhaustively
against 10 mM potassium phosphate (pH 7.4). The supernatant
obtained following the centrifugation of the precipitated proteins
at 65% saturation was also dialyzed against the same buffer.
Affinity (Cibacron Blue F3GA) chromatography.
Affinity chromatography was performed with an Affi-Gel blue column
as follows (16). The column
(1.5×30 cm) was equilibrated with buffer A [10 mM potassium
phosphate, pH 7.4, containing 0.5%
3-[(3-cholamidopropyl)dimethylammonio]-2-hydroxy-1-propane
sulphonate (CHAPS)]. Subsequent to adding the sample, the column
was washed with buffer A and enzymatic activity was eluted with the
same buffer containing 1.5 M NaCl.
Gel filtration chromatography. Fractionation
was performed at 4°C on a Sephadex G-100 Superfine gel. The column
(1×50 cm) was equilibrated with 50 mM Tris-HCl, pH 7.4 and 100 mM
KCl and calibrated using bovine serum albumin (Mr, 66
kDa), ovalbumin (Mr, 45 kDa) and carbonic anhydrase
(Mr, 29 kDa) as molecular weight markers. The column was
eluted with the same buffer at a flow rate of 0.05 ml/min. The
amount of protein in the 1-ml column fractions was monitored by
absorption measurements at 280 nm.
Isoelectric focusing. Following the gel
filtration chromatography, the fractions with molecular weights of
35-40 kDa and the NAD+ glycohydrolase activity were
collected, dialyzed against 10 mM Tris-HCl (pH 7.4) and subjected
to isoelectric focusing with an LKB 8100-1 column, according to the
manufacturer’s instructions. Briefly, half of the dialyzed protein
sample was mixed with 1.8 ml carrier ampholytes (pH range,
3.5–10.0) in a total volume of 60 ml. This solution also contained
28 g sucrose (dense solution). The other half of the protein sample
was mixed with 0.6 ml carrier ampholytes (pH range, 3.5–10.0),
again in a total volume of 60 ml (light solution). The two
solutions were mixed in a series of reaction tubes so that a
density gradient was formed and, thereafter, layered in a stepwise
manner in the column over the anode solution (0.2 ml phosphoric
acid, 12 g sucrose and 14 ml dH2O). Finally, the cathode
solution (0.2 ml ethylenediamine and 10 ml dH2O) was
added to the top of the gradient. The isoelectric focusing was
performed for 24 h at 4°C and the operational power level was
maintained at <W. Upon completion, the column content was
harvested in 1-ml fractions and their A280, pH-values
and enzymatic activities were determined.
Gel filtration chromatography. Following
isoelectric focusing, the fractions with NAD+
glycohydrolase activity were once again subjected to Sephadex G-100
chromatography, as described previously, in order to remove the
ampholytes.
NAD+ glycohydrolase
activity assay
NAD+ glycohydrolase activity was
determined by separating [carbonyl-14C]nicotinamide from
[carbonyl-14C]NAD+ with a BioRad AG-1X4 anion
exchange resin (17). The reaction
mixture (20 μl), containing 12 μl serum fractions, 7
μl mix (10 mM NaCl, 500 μM ZnCl2, 50
μM CaCl2 and 20 mM Tris-HCl, pH 9.0) and 10
μM [carbonyl-14C]NAD+, was incubated
for 30 min at 37°C. The samples were then added to the BioRad
AG-1X4 column and the [carbonyl-14C]nicotinamide that
was released due to NAD+ glycohydrolase action was
eluted with dH2O. The [carbonyl-14C]NAD+ that
was retained on the column was subsequently eluted with 0.5 M NaCl.
The radioactivity that was eluted with dH2O and 0.5 M
NaCl was determined in Bray’s solution using a liquid scintillation
counter (Packard Tri-Carb 1000TR, Meriden, CT, USA). The specific
activity of the NAD+ glycohydrolase activity was defined
as the nmol of [carbonyl-14C]nicotinamide released under
the aforementioned reaction conditions per mg of protein.
Sodium dodecyl sulfate-polyacrylamide
gel electrophoresis (SDS-PAGE)
SDS-PAGE was performed as described previously
(18). Bovine serum albumin
(Mr, 66 kDa), ovalbumin (Mr, 45 kDa),
carbonic anhydrase (Mr, 29 kDa) and trypsinogen
(Mr, 24 kDa) were used as molecular weight standards.
Aliquots (∼10 μl) of enzyme preparations in sample buffer
were heated for 3 min in a boiling water bath prior to being added
to the gel. The protein bands in the gel slabs were visualized with
silver staining (19).
Results and Discussion
Purification of NAD+
glycohydrolase
Ammonium sulfate precipitation
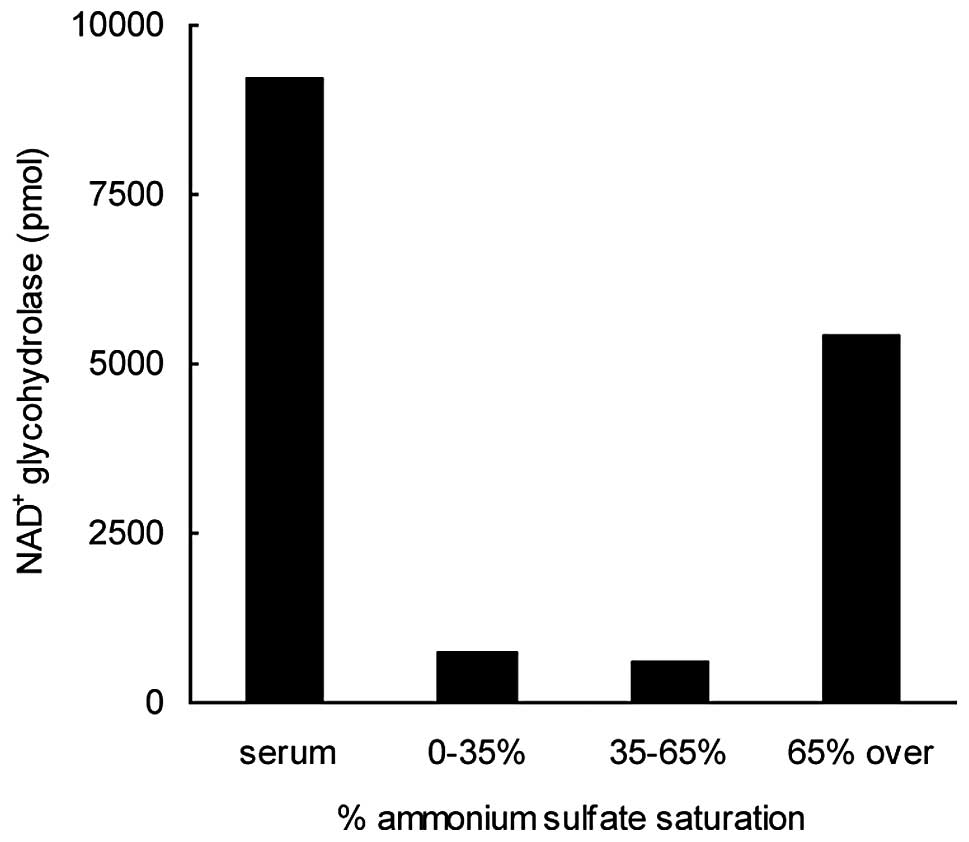
Serum (60 ml) was subjected to fractionation with
ammonium sulfate at concentrations that were increased in a
stepwise manner, first to 35 and then to 65% saturation. Following
each saturation step, the precipitated proteins were collected by
centrifugation, dissolved in and dialyzed against 10 mM potassium
phosphate, pH 7.4, and assayed for NAD+ glycohydrolase
activity (Fig. 1). The supernatant
fraction obtained following the centrifugation of the proteins
precipitated at 65% ammonium sulfate saturation was also subjected
to dialysis and a subsequent activity test. NAD+
glycohydrolase activity was observed almost exclusively in the 65%
supernatant. This step resulted in the removal of a significant
portion of the serum proteins and in an ∼20-fold enrichment of the
enzymatic activity, with a yield of 32% (Table I).
 | Table I.Purification of NAD+
glycohydrolase from human serum. |
Table I.
Purification of NAD+
glycohydrolase from human serum.
| Fraction | Total protein
(mg) | Volume (ml) | Total activity
(nmol) | Specific activity
(nmol/mg) | Purification
(fold) | Yield (%) |
|---|
| Serum | 4920 | 60 | 285 | 0.058 | 1 | 100 |
| Ammonium
sulfate | 80 | 34 | 92 | 1.15 | 19.8 | 32.3 |
| Affi-Gel Blue | 17 | 10 | 21 | 1.24 | 21.4 | 7.4 |
| Sephadex G-100
(1) | 0.94 | 4 | 3.3 | 3.5 | 60.3 | 1.15 |
| Isoelectric
focusing | 0.49 | 3 | 3.5 | 7.1 | 122.4 | 1.23 |
| Sephadex G-100
(2) | 0.1 | 3 | 2.8 | 28 | 483 | 0.97 |
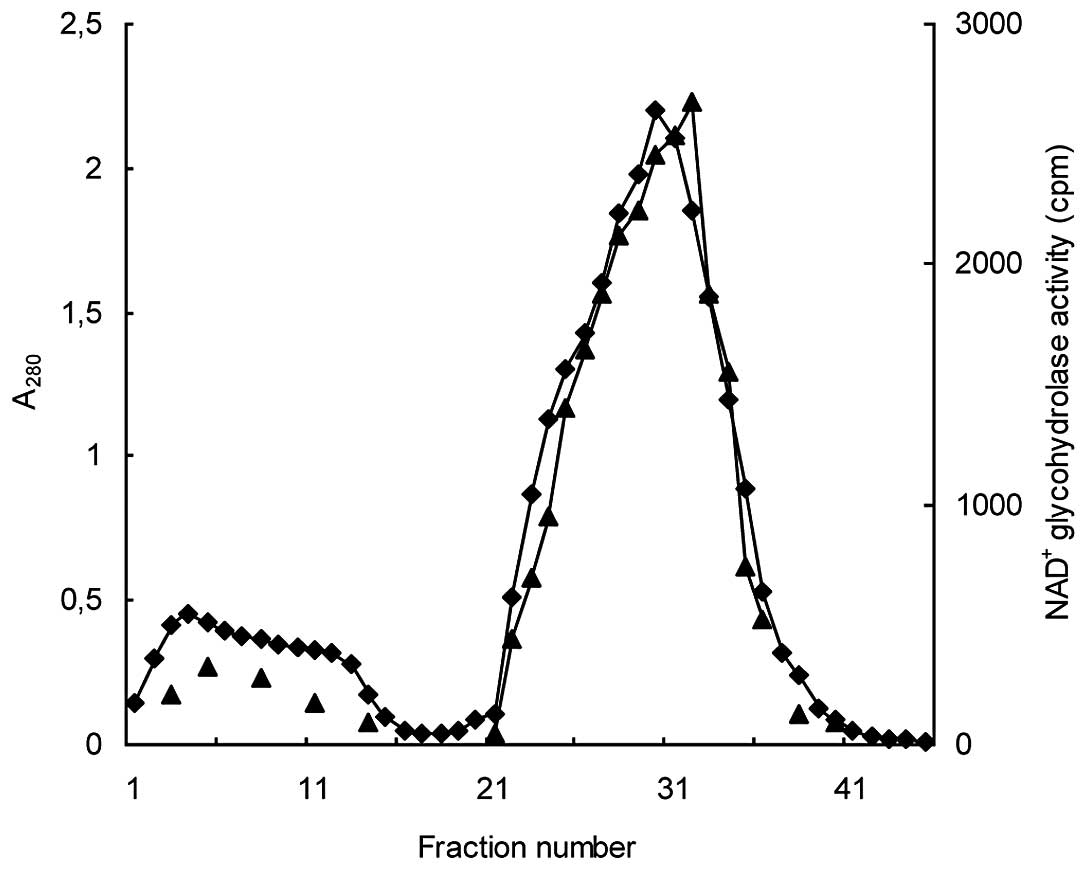
Affi-Gel blue affinity chromatography. The
65% supernatant fraction was then injected into the Affi-Gel blue
column from which proteins were successively eluted with buffer A
and buffer A plus 1.5 M NaCl. A stepwise elution approach at lower
NaCl concentrations failed to isolate the activity in a
well-defined concentration range. Instead, the activity was
observed to be spread over all the attempted concentrations.
Moreover, the 1.5 M NaCl-eluate appeared to contain the bulk of the
protein that was applied to the column, aside from the
NAD+ glycohydrolase activity. Thus, this step did not
result in a meaningful enrichment of the enzymatic activity
(Fig. 2).
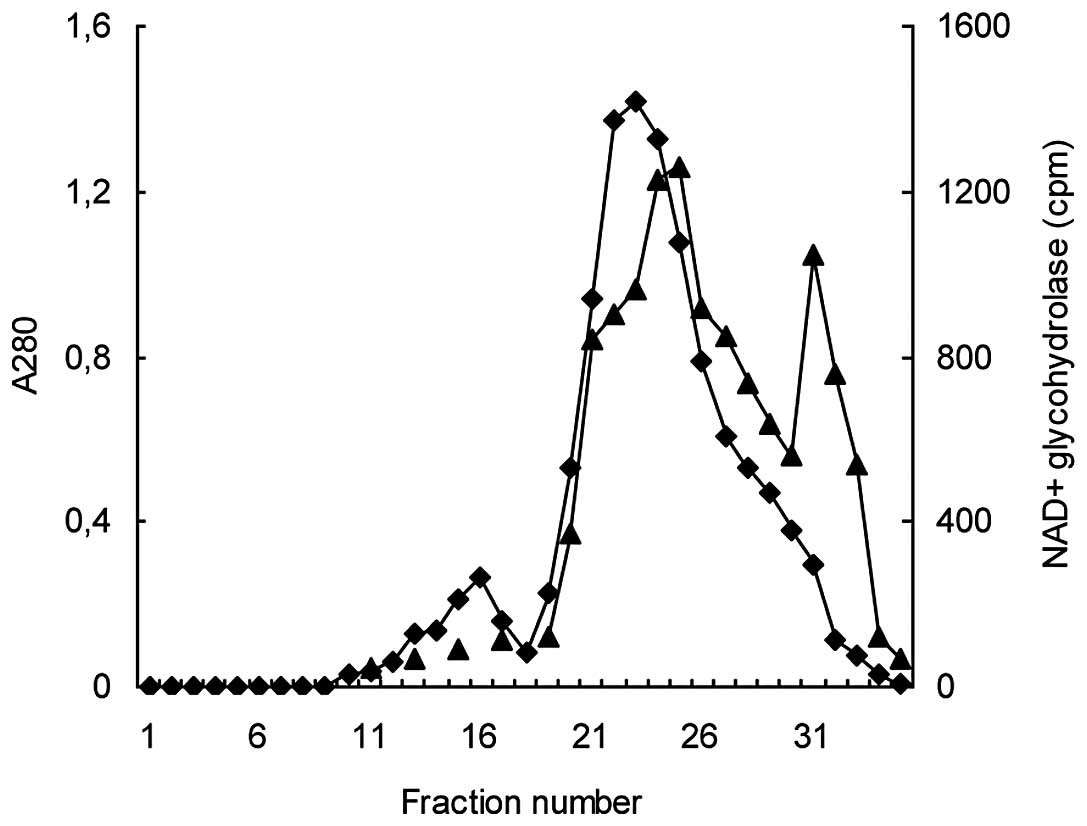
Gel filtration chromatography (Sephadex
G-100). Following Affi-Gel blue affinity chromatography, the
1.5 M NaCl-eluate was subjected to gel filtration using a Sephadex
G-100 column. This step resulted in the separation of the
NAD+ glycohydrolase activity into peaks, with molecular
weight ranges of 70–75 kDa and 35–40 kDa. The major peak with the
higher molecular weight, which comprised more than two-thirds of
the total NAD+ glycohydrolase activity, also overlapped
with the main A280 peak. The minor, sharp activity peak
directly followed the descending section of the A280
peak. Having been separated from the bulk of the protein, this peak
(35–40 kDa) revealed a relatively high specific activity and
appeared to be the most suitable for the further purification steps
(Fig. 3).
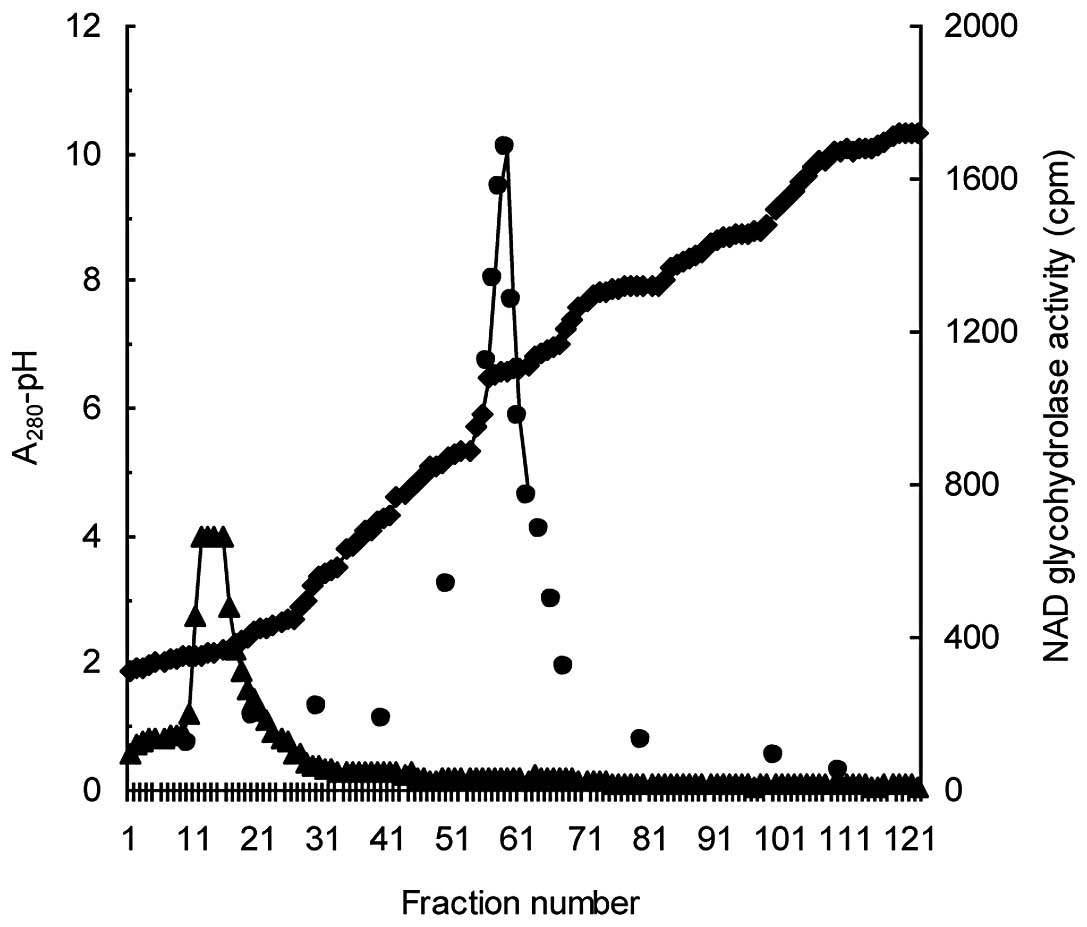
Isoelectric focusing. The fractions from the
Sephadex G-100 column chromatography that corresponded to the minor
activity peak within the molecular weight range of 35–40 kDa were
pooled and subjected to isoelectric focusing. At the end of the
isoelectric focusing, NAD+ glycohydrolase activity was
observed in the pH range of 6.4–6.6 (Fig. 4). The active fractions had low
A280 values, whereas the main A280 peak was
located at the bottom of the column and pH-gradient. The active
fractions were collected and applied once more to the Sephadex
G-100 column in order to remove the ampholytes.
Assessment of purification
A SDS-PAGE analysis of the active fraction obtained
at the end of the purification procedure revealed the presence of a
single protein band that corresponded to a molecular weight of ∼39
kDa (Fig. 5). This value appears to
be in line with the molecular weight of the monomeric form of
soluble CD38 that has been reported previously (9,10).
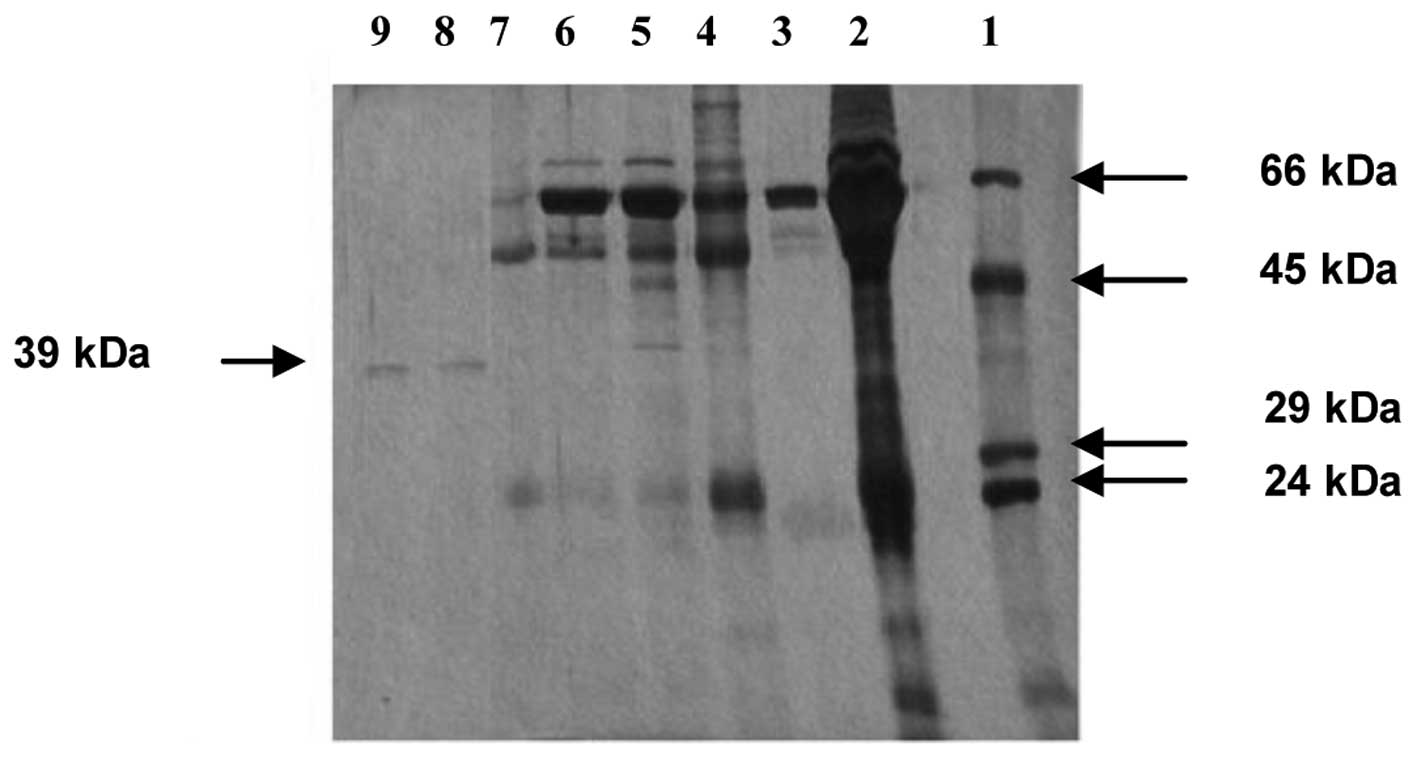 | Figure 5.SDS-PAGE analysis of serum
NAD+ glycohydrolase fractions following successive
purification steps. Bovine serum albumin (Mr, 66 kDa),
ovalbumin (Mr, 45 kDa), carbonic anhydrase
(Mr, 29 kDa) and trypsinogen (Mr, 24 kDa)
were used as molecular weight standards. The gel lanes, in order
from 1–9, correspond to protein molecular weight standards,
unfractionated serum proteins, supernatant of 65% ammonium sulfate
saturation, eluate from Affi-Gel blue chromatography (fractions
24–34), eluate from Sephadex G-100 chromatography (fractions
20–23), eluate from Sephadex G-100 chromatography (fractions
30–33), eluate from isoelectric focusing (fractions 58–60), eluate
from second Sephadex G-100 chromatography (fraction 31), eluate
from second Sephadex G-100 chromatography (fraction 32). SDS-PAGE,
sodium dodecyl sulfate-polyacrylamide gel electrophoresis. |
We suggest that the soluble monomer of CD38 was
purified from the human serum in the present study since the
molecular weight of the purified protein matched that reported for
soluble monomeric CD38 (9,10). It is notable that the second gel
filtration step performed on the Sephadex G-100 column with the aim
of depleting the ampholytes, actually led to a final, clear
enrichment of this monomeric form of soluble NAD+
glycohydrolase. The findings of the SDS-PAGE analysis are also in
line with the considerable increase in specific activity obtained
subsequent to the second gel filtration step (Table I). It appears that a more efficient
fractionation by gel filtration was possible only following the
removal of the serum albumin load by ammonium sulfate fractionation
and isoelectric focusing.
As indicated in Table
I, the NAD+ glycohydrolase was purified by 483-fold
compared with the starting serum fraction, although the yield of
the purification was only 1%. We hypothesize that there are two
main reasons for this modest yield. Firstly, the Affi-Gel blue step
resulted in the loss of a significant part of the activity with
practically no gain in specific activity. Accordingly, a
‘cost-benefit analysis’ does not justify the inclusion of this step
in the present purification procedure, at least not at such an
early stage. Affi-Gel blue chromatography has previously been used
successfully in the purification of proteins with dinucleotide fold
(20) and NAD+
glycohydrolase from calf spleens and thyroid glands (16,21).
However, the present study demonstrated that Affi-Gel blue
chromatography was of little use in the purification of serum
NAD+ glycohydrolase, since serum albumin also exhibits a
marked affinity for Cibacron Blue F3GA. The affinity of serum
albumin for Cibacron Blue F3GA has resulted in an expansion in the
development of chromatographic methods for albumin depletion in the
preparation of low abundance proteins for proteomic analysis
(22–26). In the present study, a stepwise
development of Affi-Gel blue affinity chromatography, with the aim
of differentially separating NAD+ glycohydrolase from
serum albumin, was not successful. Moreover, the co-elution of the
major, high molecular weight (possibly dimeric) form of this
protein together with serum albumin in gel filtration
chromatography dissuaded us from concentrating on its purification.
That the purification of this dimeric form was not attempted is
thus the second reason accounting for the low yield of the present
procedure.
However, a change in the purification protocol, with
the isoelectric focusing step directly following ammonium sulfate
fractionation, may enable the efficient separation of the two forms
of NAD+ glycohydrolase from serum albumin. The fairly
low isoelectric point (pI) of serum albumin (4.4) (27) provides an opportunity which may be
exploited.
In the present attempt to purify NAD+
glycohydrolase (soluble CD38) from human serum, the isolation of
its monomeric form in an apparently homogeneous state was achieved.
However, the abundance of serum albumin and its co-elution in
Affi-Gel blue affinity chromatography, as well as gel filtration
with Sephadex G-100, prevented the similar purification of the
putative dimer of NAD+ glycohydrolase. This result
demonstrates the difficulties that are likely to be encountered in
the purification of low abundance proteins from serum when a
differential depletion of serum albumin is not possible.
The information gained in this first attempt
provides valuable information for designing future approaches that
should take into account the similar chromatographic behavior of
the NAD+ glycohydrolase dimer and serum albumin.
Therefore, we propose that purification protocols that initially
deplete serum albumin, particularly by making use of its low
pI-value, are likely to have an improved prospect for the
purification of the monomeric and dimeric forms of NAD+
glycohydrolase. At present, research is in progress to aid in the
implementation of this strategy.
Acknowledgements
The present study was supported by the
Research Fund of the University, Fatih, Istanbul (project
T-1157/18062001).
References
|
1.
|
Deaglio S, Aydin S, Vaisitti T, Bergui L
and Malavasi F: CD38 at the junction between prognostic marker and
therapeutic target. Trends Mol Med. 14:210–218. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
2.
|
Malavasi F, Deaglio S, Funaro A, Ferrero
E, Horenstein AL, Ortolan E, Vaisitti T and Aydin A: Evolution and
function of ADP-ribosyl cyclase/CD38 gene family in physiology and
pathology. Physiol Rev. 88:841–886. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
Coşkun O and Nurten R: Purification of
NAD+glycohydrolase enzyme from erythrocyte membrane.
Turkiye Klinikleri J Cardiovasc Sci. 22:318–323. 2010.(In
Turkish).
|
|
4.
|
Damle RN, Wasil T, Fais F, Ghiotto F,
Valetto A, Allen SL, Buchbinder A, Budman D, Dittmar K, Kolitz J,
Lichtman SM, Schulman P, Vinciguerra VP, Rai KR, Ferrarini M and
Chiorazzi N: Ig V gene mutation status and CD38 expression as novel
prognostic indicators in chronic lymphocytic leukemia. Blood.
94:1840–1847. 1999.PubMed/NCBI
|
|
5.
|
Matrai Z: CD38 as a prognostic marker in
CLL. Hematology. 10:39–46. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Liu Z, Cumberland WG, Hultin LE, Prince
HE, Detels R and Giorgi JV: Elevated CD38 expression on
CD8+ T cells is a stronger marker for the risk of
chronic HIV disease progression to AIDS and death in the
Multicenter AIDS Cohort Study than CD4+ cell count,
soluble immune activation markers, or combination of HLA-DR and
CD38 expression. J Acquir Immune Defic Syndr Hum Retrovirol.
16:83–92. 1997.PubMed/NCBI
|
|
7.
|
Albeniz I, Demir O, Nurten R and Bermek E:
NAD glycohydrolase activities and ADP-ribose uptake in erythrocytes
from normal subjects and cancer patients. Biosci Rep. 24:41–53.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Albeniz I, Demir O, Türker-Şener L,
Yalçintepe L, Nurten R and Bermek E: Erythrocyte CD38 as a
prognostic marker in cancer. Hematology. 12:409–414. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Funaro A, Horenstein AL, Calosso L, Morra
M, Tarocco RP, Franco L, De Flora A and Malavasi F: Identification
and characterization of an active soluble form of human CD38 in
normal and pathological fluids. Int Immunol. 8:1643–1650. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Mallone R, Ferrua S, Morra M, Zocchi E,
Mehta K, Notarangelo LD and Malavasi F: Characterization of a
CD38-like 78-kilodalton soluble protein released from B cell lines
derived from patients with X-linked agammaglobulinemia. J Clin
Invest. 101:2821–2830. 1998. View
Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Korkut C, Yalçintepe L, Kiremit-Korkut N,
Uzun-Altınöz S, Işsever S, Gümüşel F, Tiryaki D and Bermek E: Serum
proteins with NAD+ glycohydrolase activity and anti-CD38
reactivity - elevated levels in serum of tumour patients. Cancer
Lett. 126:105–109. 1998.PubMed/NCBI
|
|
12.
|
Kong KO, Leung BP, Chng HH, Thong BY, Koh
ET, Leong KP, Badsha H, Lian TY, Khoo KM and Howe HS: Usefulness of
serum soluble CD38 and CD157 levels in differentiating SLE, RA and
healthy adults and their relationship with disease activity. Ann
Acad Med Singapore. 32(5 Suppl): S16–S17. 2003.PubMed/NCBI
|
|
13.
|
Kravchenko GA, Novikov DV, Ptitsyna IuS
and Novikov VV: Serum levels of soluble forms of membranous
antigens of immune system cells in carriers of virus hepatitis G
markers. Vopr Virusol. 50:19–22. 2005.(In Russian).
|
|
14.
|
Lebedev MJ, Egorova NI, Sholkina MN,
Vilkov SA, Baryshnikov AJ and Novikov VV: Serum levels of different
forms of soluble CD38 antigen in burned patients. Burns.
30:552–556. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Liu XQ, Hart DN, MacPherson GG, Good MF
and Wykes MN: Soluble CD38 significantly prolongs the lifespan of
memory B-cell responses. Immunology. 125:14–20. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16.
|
Muller-Steffner H, Schenherr-Gusse I,
Tarnus C and Schuber F: Calf spleen NAD+ glycohydrolase:
solubilization, purification, and properties of the intact form of
the enzyme. Arch Biochem Biophys. 304:154–162. 1993.
|
|
17.
|
Kim H, Jacobson EL and Jacobson MK:
Synthesis and degradation of cyclic ADP-ribose by NAD
glycohydrolases. Science. 261:1330–1333. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
18.
|
Laemmli UK: Cleavage of structural
proteins during the assembly of the head of bacteriophage T4.
Nature. 227:680–685. 1970. View
Article : Google Scholar : PubMed/NCBI
|
|
19.
|
Morrissey JH: Silver stain for proteins in
polyacrylamide gels: a modified procedure with enhanced uniform
sensitivity. Anal Biochem. 117:307–310. 1981. View Article : Google Scholar : PubMed/NCBI
|
|
20.
|
Thompson ST and Stellwagen E: Binding of
Cibacron blue F3GA to proteins containing the dinucleotide fold.
Proc Natl Acad Sci USA. 73:361–365. 1976. View Article : Google Scholar : PubMed/NCBI
|
|
21.
|
De Wolf MJ, Van Dessel GA, Lagrou AR,
Hilderson HJ and Dierick WS: Topology, purification and
characterization of thyroidal NAD+ glycohydrolase.
Biochem J. 226:415–427. 1985.PubMed/NCBI
|
|
22.
|
Ahmed N, Barker G, Oliva K, Garfin D,
Talmadge K, Georgiu H, Quinn M and Rice G: An approach to remove
albumin for the proteomic analysis of low abundance biomarkers in
human serum. Proteomics. 3:1980–1987. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
23.
|
Colantonio DA, Dunkinson C, Bovenkamp DE
and Van Eyke JE: Effective removal of albumin from serum.
Proteomics. 5:3831–3835. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
24.
|
Ma ZY, Guan YP and Liu HZ: Affinity
adsorption of albumin on Cibacron Blue F3GA-coupled non-porous
micrometer-sized magnetic polymer microspheres. React Funct Polym.
66:618–624. 2006. View Article : Google Scholar
|
|
25.
|
Altintaş EB and Denizli A: Efficient
removal of albumin from human serum by monosize dye-affinity beads.
J Chromatogr B Analyt Technol Biomed Life Sci. 832:216–223.
2006.
|
|
26.
|
Sahab ZJ, Iczkowski KA and Sang QX: Anion
exchange fractionation of serum proteins versus albumin
elimination. Anal Biochem. 368:24–32. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
27.
|
Liu R, Pidikiti R, Ha CE, Petersen CE,
Bhagavan NV and Eckenhoff RG: The role of electrostatic
interactions in human serum albumin binding and stabilization by
halothane. J Biol Chem. 277:36373–36379. 2002. View Article : Google Scholar : PubMed/NCBI
|