|
1.
|
Deaglio S, Aydin S, Vaisitti T, Bergui L
and Malavasi F: CD38 at the junction between prognostic marker and
therapeutic target. Trends Mol Med. 14:210–218. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
2.
|
Malavasi F, Deaglio S, Funaro A, Ferrero
E, Horenstein AL, Ortolan E, Vaisitti T and Aydin A: Evolution and
function of ADP-ribosyl cyclase/CD38 gene family in physiology and
pathology. Physiol Rev. 88:841–886. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
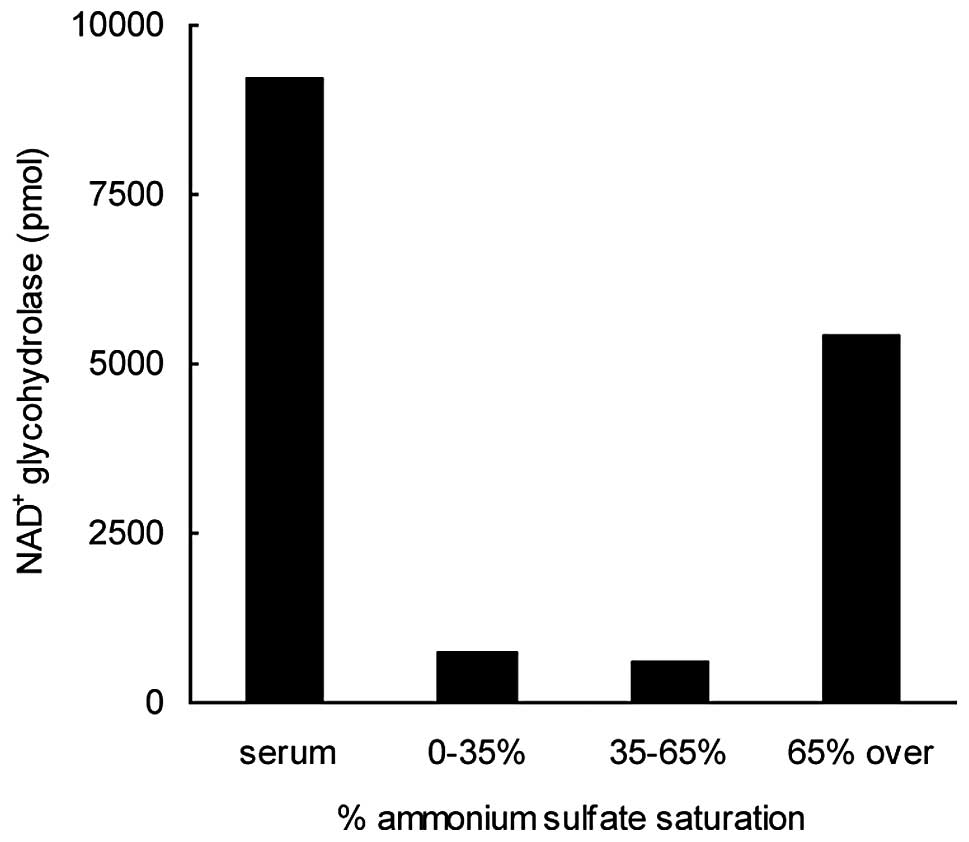
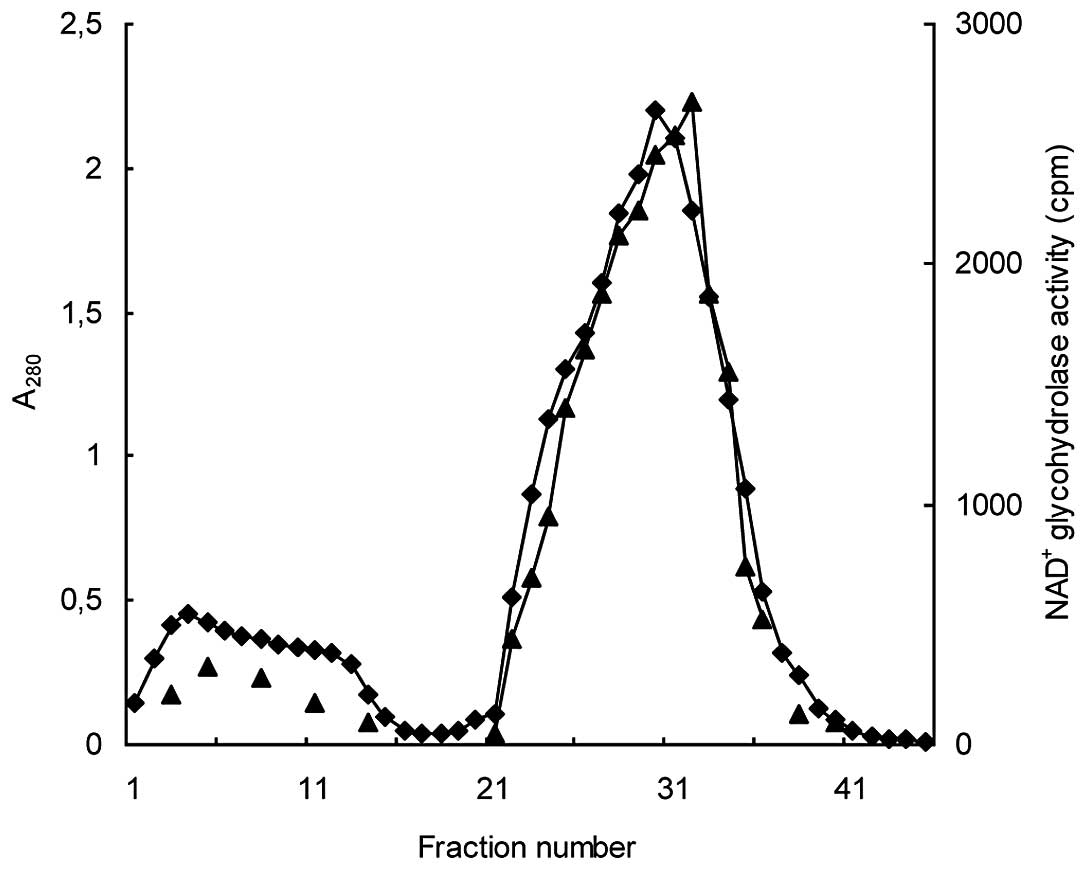
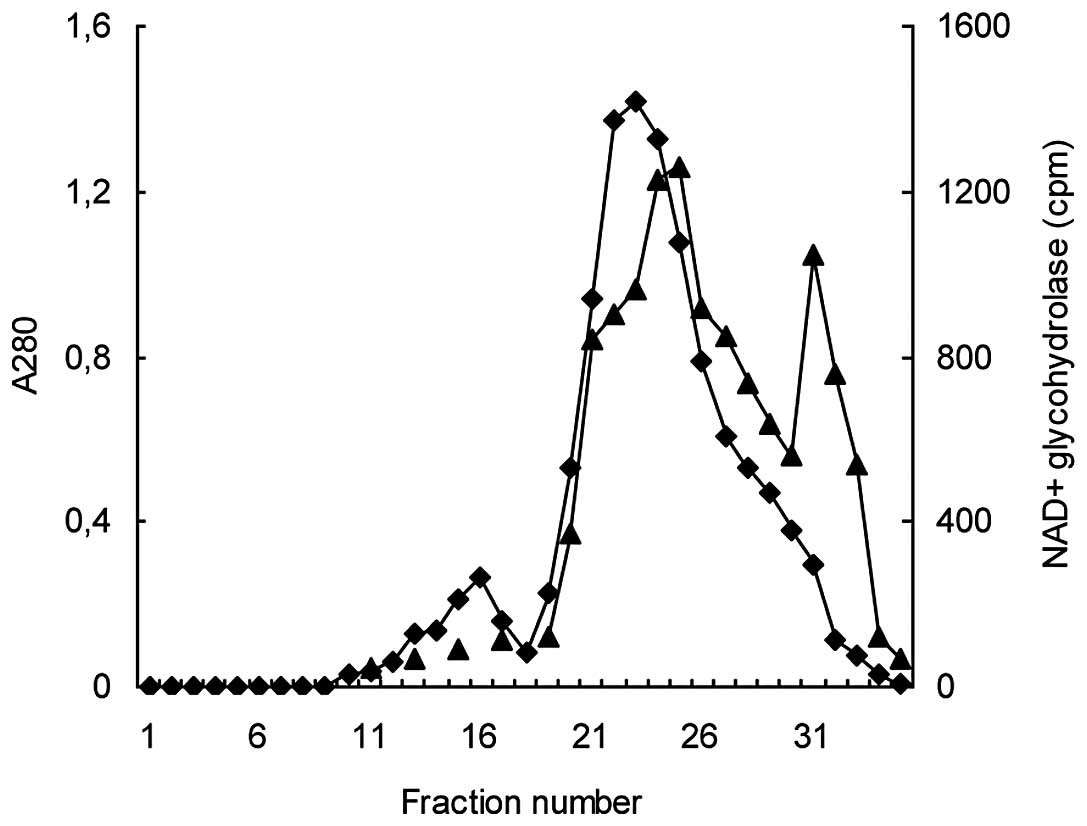
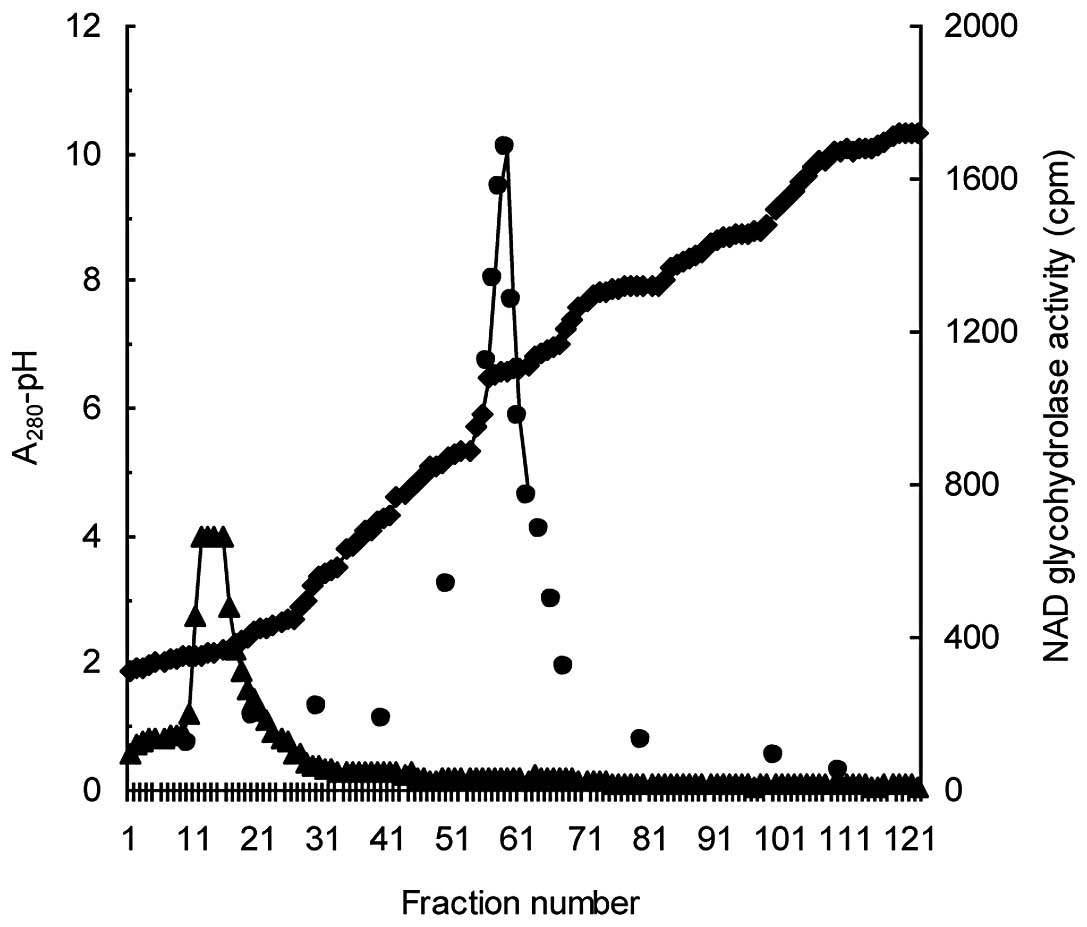
Coşkun O and Nurten R: Purification of
NAD+glycohydrolase enzyme from erythrocyte membrane.
Turkiye Klinikleri J Cardiovasc Sci. 22:318–323. 2010.(In
Turkish).
|
|
4.
|
Damle RN, Wasil T, Fais F, Ghiotto F,
Valetto A, Allen SL, Buchbinder A, Budman D, Dittmar K, Kolitz J,
Lichtman SM, Schulman P, Vinciguerra VP, Rai KR, Ferrarini M and
Chiorazzi N: Ig V gene mutation status and CD38 expression as novel
prognostic indicators in chronic lymphocytic leukemia. Blood.
94:1840–1847. 1999.PubMed/NCBI
|
|
5.
|
Matrai Z: CD38 as a prognostic marker in
CLL. Hematology. 10:39–46. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Liu Z, Cumberland WG, Hultin LE, Prince
HE, Detels R and Giorgi JV: Elevated CD38 expression on
CD8+ T cells is a stronger marker for the risk of
chronic HIV disease progression to AIDS and death in the
Multicenter AIDS Cohort Study than CD4+ cell count,
soluble immune activation markers, or combination of HLA-DR and
CD38 expression. J Acquir Immune Defic Syndr Hum Retrovirol.
16:83–92. 1997.PubMed/NCBI
|
|
7.
|
Albeniz I, Demir O, Nurten R and Bermek E:
NAD glycohydrolase activities and ADP-ribose uptake in erythrocytes
from normal subjects and cancer patients. Biosci Rep. 24:41–53.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Albeniz I, Demir O, Türker-Şener L,
Yalçintepe L, Nurten R and Bermek E: Erythrocyte CD38 as a
prognostic marker in cancer. Hematology. 12:409–414. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Funaro A, Horenstein AL, Calosso L, Morra
M, Tarocco RP, Franco L, De Flora A and Malavasi F: Identification
and characterization of an active soluble form of human CD38 in
normal and pathological fluids. Int Immunol. 8:1643–1650. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Mallone R, Ferrua S, Morra M, Zocchi E,
Mehta K, Notarangelo LD and Malavasi F: Characterization of a
CD38-like 78-kilodalton soluble protein released from B cell lines
derived from patients with X-linked agammaglobulinemia. J Clin
Invest. 101:2821–2830. 1998. View
Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Korkut C, Yalçintepe L, Kiremit-Korkut N,
Uzun-Altınöz S, Işsever S, Gümüşel F, Tiryaki D and Bermek E: Serum
proteins with NAD+ glycohydrolase activity and anti-CD38
reactivity - elevated levels in serum of tumour patients. Cancer
Lett. 126:105–109. 1998.PubMed/NCBI
|
|
12.
|
Kong KO, Leung BP, Chng HH, Thong BY, Koh
ET, Leong KP, Badsha H, Lian TY, Khoo KM and Howe HS: Usefulness of
serum soluble CD38 and CD157 levels in differentiating SLE, RA and
healthy adults and their relationship with disease activity. Ann
Acad Med Singapore. 32(5 Suppl): S16–S17. 2003.PubMed/NCBI
|
|
13.
|
Kravchenko GA, Novikov DV, Ptitsyna IuS
and Novikov VV: Serum levels of soluble forms of membranous
antigens of immune system cells in carriers of virus hepatitis G
markers. Vopr Virusol. 50:19–22. 2005.(In Russian).
|
|
14.
|
Lebedev MJ, Egorova NI, Sholkina MN,
Vilkov SA, Baryshnikov AJ and Novikov VV: Serum levels of different
forms of soluble CD38 antigen in burned patients. Burns.
30:552–556. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Liu XQ, Hart DN, MacPherson GG, Good MF
and Wykes MN: Soluble CD38 significantly prolongs the lifespan of
memory B-cell responses. Immunology. 125:14–20. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16.
|
Muller-Steffner H, Schenherr-Gusse I,
Tarnus C and Schuber F: Calf spleen NAD+ glycohydrolase:
solubilization, purification, and properties of the intact form of
the enzyme. Arch Biochem Biophys. 304:154–162. 1993.
|
|
17.
|
Kim H, Jacobson EL and Jacobson MK:
Synthesis and degradation of cyclic ADP-ribose by NAD
glycohydrolases. Science. 261:1330–1333. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
18.
|
Laemmli UK: Cleavage of structural
proteins during the assembly of the head of bacteriophage T4.
Nature. 227:680–685. 1970. View
Article : Google Scholar : PubMed/NCBI
|
|
19.
|
Morrissey JH: Silver stain for proteins in
polyacrylamide gels: a modified procedure with enhanced uniform
sensitivity. Anal Biochem. 117:307–310. 1981. View Article : Google Scholar : PubMed/NCBI
|
|
20.
|
Thompson ST and Stellwagen E: Binding of
Cibacron blue F3GA to proteins containing the dinucleotide fold.
Proc Natl Acad Sci USA. 73:361–365. 1976. View Article : Google Scholar : PubMed/NCBI
|
|
21.
|
De Wolf MJ, Van Dessel GA, Lagrou AR,
Hilderson HJ and Dierick WS: Topology, purification and
characterization of thyroidal NAD+ glycohydrolase.
Biochem J. 226:415–427. 1985.PubMed/NCBI
|
|
22.
|
Ahmed N, Barker G, Oliva K, Garfin D,
Talmadge K, Georgiu H, Quinn M and Rice G: An approach to remove
albumin for the proteomic analysis of low abundance biomarkers in
human serum. Proteomics. 3:1980–1987. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
23.
|
Colantonio DA, Dunkinson C, Bovenkamp DE
and Van Eyke JE: Effective removal of albumin from serum.
Proteomics. 5:3831–3835. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
24.
|
Ma ZY, Guan YP and Liu HZ: Affinity
adsorption of albumin on Cibacron Blue F3GA-coupled non-porous
micrometer-sized magnetic polymer microspheres. React Funct Polym.
66:618–624. 2006. View Article : Google Scholar
|
|
25.
|
Altintaş EB and Denizli A: Efficient
removal of albumin from human serum by monosize dye-affinity beads.
J Chromatogr B Analyt Technol Biomed Life Sci. 832:216–223.
2006.
|
|
26.
|
Sahab ZJ, Iczkowski KA and Sang QX: Anion
exchange fractionation of serum proteins versus albumin
elimination. Anal Biochem. 368:24–32. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
27.
|
Liu R, Pidikiti R, Ha CE, Petersen CE,
Bhagavan NV and Eckenhoff RG: The role of electrostatic
interactions in human serum albumin binding and stabilization by
halothane. J Biol Chem. 277:36373–36379. 2002. View Article : Google Scholar : PubMed/NCBI
|