Introduction
Ovarian cancer is the most lethal malignancy among
females and the prognosis is poor since ovarian cancer is often at
an advanced stage when it is detected (1–2). Early
diagnosis of ovarian cancer is likely to improved the cure rate
significantly. At present, the sensitivity and specificity of the
clinically used markers of ovarian cancer are low for early
diagnosis, so a number of studies have attempted to identify a more
effective diagnostic marker (3).
Human epididymis protein 4 (human epididymis gene product 4; HE4)
is a marker of ovarian tumors which has significant potential for
diagnosis (4). HE4 is a secreted
protein coded by the gene WFDC2 and belongs to the lactic acid
protein domain family (5–6). It has been demonstrated that HE4 mRNA
is highly expressed in ovarian cancer tissue and not expressed in
benign ovarian tissue (7). Moore
et al (8) observed that HE4
was a useful single marker for differentiating between benign
ovarian tumor and ovarian cancer patients. Köbel et al
(9) analyzed the expression of a
number of ovarian cancer markers in various pathological types of
malignant ovarian tumors and observed high expression of HE4 in
epithelial ovarian cancer. Since epithelial ovarian cancer accounts
for 85–90% of ovarian cancer among the various pathological types,
it is important to study the diagnostic value of HE4 for epithelial
ovarian cancer. Cytoreductive surgery combined with platinum-based
chemotherapy is the standard treatment for patients with ovarian
cancer (10). Accurate preoperative
assessments of the degree of malignancy and extent of metastasis
are critical for optimal debulking, which is the best available
approach for treating ovarian cancer at present (11). Previously, no tumor marker has been
established to predict whether optimal debulking is likely to be
achieved preoperatively. The aim of the present study was to
appraise the diagnostic and preoperative predictive value of serum
HE4 concentrations for optimal debulking in ovarian cancer.
Patients and methods
Source of specimens and clinical
data
Serum specimens were obtained from ovarian neoplasm
patients and diagnosed pathologically at the Department of
Gynecologic Oncology of the Affiliated Tumor Hospital of Guangxi
Medical University (Nanning, China). There were 180 malignant
ovarian epithelial carcinoma patients, including 93 with ovarian
serous adenocarcinoma, 38 with mucinous adenocarcinoma, 18 with
endometrial adenocarcinoma, 14 with clear cell carcinoma and 17
with undifferentiated carcinoma. The median age was 37.6 years
(range, 13–71 years). The surgical-pathological staging according
the to FIGO (2004) staging criteria was 57 cases of stages II–II
and 123 cases of stages III–IV. There were also 40 patients with
benign ovarian tumors, including 13 with ovarian serous adenoma, 4
with benign ovarian teratoma, 10 with ovarian cysts and 13 with
other types. The median age of the benign ovarian tumor patients
was 43.8 years (range, 14–62 years). Additionally, 40 healthy
female subjects were identified by physical examination, with a
median age of 42 years (range, 33–50 years). The study was approved
by the Ethics Committee of Guangxi Medical University. All patients
received an explanation of the aims of the study, provided written
informed consent and understood that they were able to withdraw
from the study at any time without influencing their oncological or
general medical treatment.
Methods
Sample collection
Venous blood (3 ml) was obtained from each patient
and placed in test tubes without anticoagulants. The blood samples
were allowed to stand for 1 h at room temperature after specimen
collection and the supernatant was collected after centrifuging at
3000 rpm. The samples were stored in a −80°C freezer until
tested.
Determination of serum HE4
The concentrations of serum HE4 were determined
using the double antibody sandwich enzyme-linked immunosorbent
assay (ELISA) method. ELISA kits for serum HE4 detection were
purchased from Fujirebio Diagnostics AB (Gothenburg, Sweden) and
used according to manufacturer’s instructions.
Determination of serum CA125
Serum CA125 was detected using the
electrochemiluminescent immunoassay (ECLIA) method. The ECLIA kit
was provided by Roche Diagnostics (Mannheim, Germany) and the
instrument used was a Roche El70 electrochemiluminescent analyzer
which was used according to the manufacturer’s instructions. Serum
CA125>35 U/ml was considered positive and serum CA125≤35 U/ml
was considered negative.
Statistical analysis
Data were processed with SPSS 17.0 statistical
software and the mean ± standard deviation was used to denote the
measured data. The χ2 test was used to evaluate the
enumeration data (the frequency of the positive or negative
specimens). One-way analysis of variance was used to compare the
concentrations of serum samples and the least significant
difference two-sample t-test was used to compare pairwise mean
values between groups. The specificity and sensitivity of the
diagnosis of ovarian cancer using various HE4 concentrations were
calculated using ROC curves and the concentration of HE4 with the
greatest diagnostic value was selected as the best cut-off point.
Diagnosis consistency was used to calculate κ values. The life
table method was used to calculate survival rates and survival was
compared with Kaplan-Meier survival curves and the log-rank
test.
Results
Comparative analysis of the serum HE4
levels of each group
The concentration of serum HE4 was 355.2±221.29
pmol/l in ovarian cancer patients, 43.86±20.87 pmol/l in benign
ovarian tumors and 30.22±9.64 pmol/l in healthy individuals. The
difference between the HE4 serum levels of ovarian cancer patients
and the other two groups was statistically significant (P=0.000)
and the serum HE4 levels of ovarian cancer patients were
significantly higher. The difference between the HE4 serum levels
of the benign ovarian tumor lesion and healthy groups was not
statistically significant (P>0.05). The results are shown in
Table I.
 | Table I.Comparative analysis of the serum HE4
levels of each group (mean ± standard deviation). |
Table I.
Comparative analysis of the serum HE4
levels of each group (mean ± standard deviation).
| Group | No. of cases | Content of HE4
(pmol/l) | P-value |
|---|
| Ovarian cancer | 180 | 355.2±221.29 | 0.000a |
| Benign tumor | 40 | 43.86±20.87 | 0.002b |
| Healthy control | 40 | 30.22±9.64 | 0.453c |
Analysis of the associations between
serum HE4 levels, pathological types and clinical stages of ovarian
cancer
The levels of serum HE4 were highest in the serous
adenocarcinoma and clear cell carcinoma groups and the difference
was statistically significant (P=0.019) compared with the other
types of ovarian cancer. No statistically significant difference
was observed between the mucinous adenocarcinoma, endometrial
adenocarcinoma and undifferentiated carcinoma groups (P>0.05).
In the comparison of the HE4 content between ovarian cancer stages
I–II and III–IV, the difference was statistically significant
(P=0.001). The results are shown in Table II.
 | Table II.Associations between serum HE4 levels,
pathological types and clinical stages of ovarian cancer. |
Table II.
Associations between serum HE4 levels,
pathological types and clinical stages of ovarian cancer.
| Clinicopathological
factors | No. of cases | Content of HE4
(pmol/l) |
|---|
| Clinical stage | | |
| I–II | 57 | 226.43±196.87 |
| III–IV | 123 | 366.13±192.16 |
| Pathological
type | | |
| Serous
adenocarcinoma | 93 | 448.11±159.59 |
| Mucinous
adenocarcinoma | 38 | 299.90±206.27 |
| Endometrial
adenocarcinoma | 18 | 309.90±206.27 |
| Clear cell
carcinoma | 14 | 418.11±159.77 |
Diagnostic value of serum HE4 for ovarian
cancer
ROC curve analysis
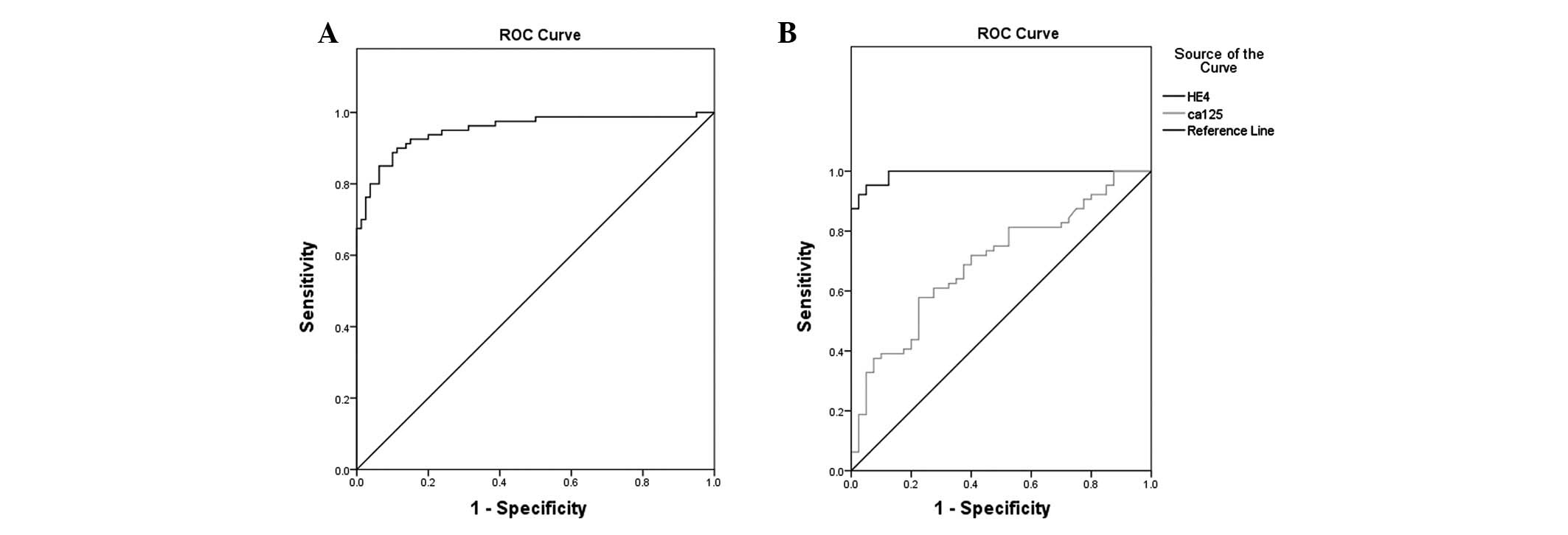
An ROC curve was created which showed that the area
under the curve was 0.984 (95% CI, 0.970–0.998, P<0.001) and the
κ value was 0.814 (P=0.000). The maximum diagnostic value occurred
when the cut-off for the diagnosis of serum HE4 for ovarian cancer
was 65.52 pmol/l. The specificity and sensitivity were 96.2% and
83.8%%, respectively, and the positive predictive and negative
predictive values were 95.7 and 85.6%, respectively. The results
are shown in Fig. 1A.
Comparative analysis of diagnostic
value between serum HE4 and CA125 for ovarian cancer
The specificity, sensitivity, positive predictive
value and negative predictive value were all higher for HE4
diagnosis of ovarian cancer compared with CA125. The difference
between the sensitivities was statistically significant (P=0.004).
The difference in specificities was also statistically significant
(P=0.003). The diagnostic performance of serum HE4 is superior to
that of CA125, particularly for stage I–II patients. The difference
between the sensitivities of the HE4 and CA125 of stage I–II
patient groups was statistically significant (P=0.046). The results
are shown in Table III.
 | Table III.Comparative analysis of diagnostic
value of serum HE4 and CA125. |
Table III.
Comparative analysis of diagnostic
value of serum HE4 and CA125.
| Group | HE4
| CA125
|
|---|
| Sensitivity (%) | Specificity (%) | Positive predictive
value (%) | Negative predictive
value (%) | Sensitivity (%) | Specificity (%) | Positive predictive
value (%) | Negative predictive
value (%) |
|---|
| Ovarian cancer | 83.8 | 96.2 | 95.7 | 85.6 | 62.5 | 80.0 | 75.8 | 68.1 |
| Stage I–II | 70.4 | 96.2 | 86.4 | 90.6 | 44.4 | 80.0 | 60.0 | 68.1 |
Value of the combination of serum HE4
and CA125 in the diagnosis of ovarian cancer
Fig. 1B shows the
ROC curves of serum HE4 and CA125 used alone or combination in the
diagnosis of ovarian cancer. Table
IV shows a comparison of the areas under the ROC curves. The
diagnostic performance was compared between serum HE4, CA125 and
HE4 + CA125, if HE4 and CA125 were positive. The specificity of HE4
+ CA125 was significantly higher than HE4 or CA125 alone, while the
sensitivity was lower compared with HE4 alone, but higher compared
with CA125 alone. Table V shows the
comparisons of sensitivity, specificity, positive predictive value,
negative predictive value, positive likelihood ratio and negative
likelihood ratio.
 | Table IV.Comparison of the area under the ROC
curves of serum HE4 and CA125. |
Table IV.
Comparison of the area under the ROC
curves of serum HE4 and CA125.
| Detected marker | Area | Standard error | P-value | 95% confidence
interval |
|---|
| HE4 | 0.988 | 0.007 | 0.000 | 0.971–1.000 |
| CA125 | 0.715 | 0.048 | 0.000 | 0.622–0.809 |
 | Table V.Comparison of the diagnostic
performance of serum HE4, CA125 and HE4 + CA125. |
Table V.
Comparison of the diagnostic
performance of serum HE4, CA125 and HE4 + CA125.
| Marker | Sensitivity (%) | Specificity (%) | Positive predictive
value (%) | Negative predictive
value (%) | Positive likelihood
ratio | Negative likelihood
ratio |
|---|
| HE4 | 83.8 | 96.2 | 95.7 | 85.6 | 22.3 | 0.17 |
| CA125 | 62.5 | 80.0 | 75.8 | 68.1 | 3.13 | 0.47 |
| HE4 + CA125 | 65.0 | 98.7 | 98.1 | 73.8 | 52.0 | 0.35 |
Association of serum HE4 levels with the
prognosis of ovarian cancer patients
All ovarian cancer patients were followed up until
December 2011. The one-, two-, three- and four-year cumulative
survival rates were 90, 62, 36 and 26%, respectively, and the
median survival time was 28 months.
Kaplan-Meier survival curve
analysis
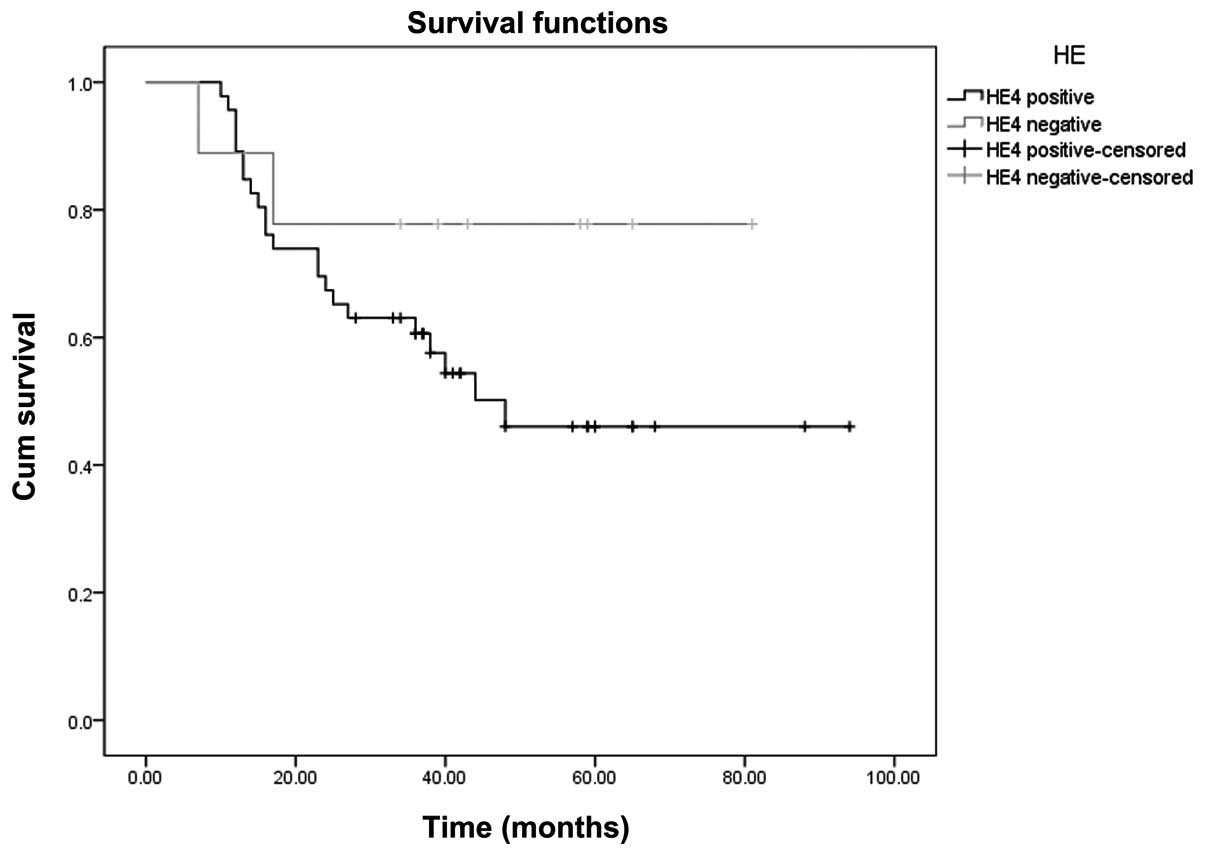
The Youden index of the ROC curve (Fig. 1A) is at the maximum, the
concentration of the serum HE4 is 148.8 pmol/l. With 148.8 pmol/l
as a positive threshold value, the Kaplan-Meier survival curves of
serum HE4-positive and negative patients were compared. The
log-rank test showed that the curves were significantly different
(P=0.036). The results are shown in Fig. 2.
Cox model analysis of serum HE4 as an
independent factor affecting the prognosis of ovarian cancer
patients
The Cox proportional hazards regression model was
analyzed in accordance with the following factors: serum HE4
>67.52 pmol/l, age, pathological type, clinical stage (I–II and
III–IV), retroperitoneal lymph node metastasis, omentum metastasis,
distant organ transfer and of postoperative residual focal factors.
The results showed that the independent factors affecting the
prognoses of ovarian cancer patients were the clinical stage,
distant organ metastasis and postoperative residual tumors >2
cm. However, HE4, age, pathological type, retroperitoneal lymph
node metastasis and omentum majus metastasis were not independent
factors. The results are shown in Table
VI.
 | Table VI.Status of each factor in affecting the
prognoses of ovarian cancer patients by Cox proportional hazards
model analysis. |
Table VI.
Status of each factor in affecting the
prognoses of ovarian cancer patients by Cox proportional hazards
model analysis.
| Factor | Regression
coefficient | Standard error | Statistic | Degree of
freedom | P-value | 95.0% confidence
interval
|
|---|
| Lower limit | Upper limit |
|---|
| HE4 | 1.090 | 1.608 | 0.460 | 1 | 0.498 | 0.127 | 69.598 |
| Age | 0.028 | 0.019 | 2.146 | 1 | 0.143 | 0.991 | 1.067 |
| Pathological
type | −0.138 | 1.476 | 0.009 | 1 | 0.926 | 0.048 | 15.726 |
| Stage | 3.526 | 1.415 | 6.211 | 1 | 0.013 | 2.123 | 543.591 |
| Lymph node | −0.328 | 0.544 | 0.365 | 1 | 0.546 | 0.248 | 2.090 |
| Omentum majus | 0.965 | 0.713 | 1.830 | 1 | 0.176 | 0.648 | 10.623 |
| Ascites | 0.491 | 0.544 | 0.815 | 1 | 0.367 | 0.562 | 4.748 |
| Metastasis | −1.375 | 0.651 | 4.463 | 1 | 0.035 | 0.071 | 0.905 |
| Postoperative
residual foci | −2.758 | 1.064 | 6.721 | 1 | 0.010 | 0.008 | 0.510 |
Association between the serum
concentration of HE4 and CA125 and the possibility of optimal
debulking in epithelial ovarian cancer
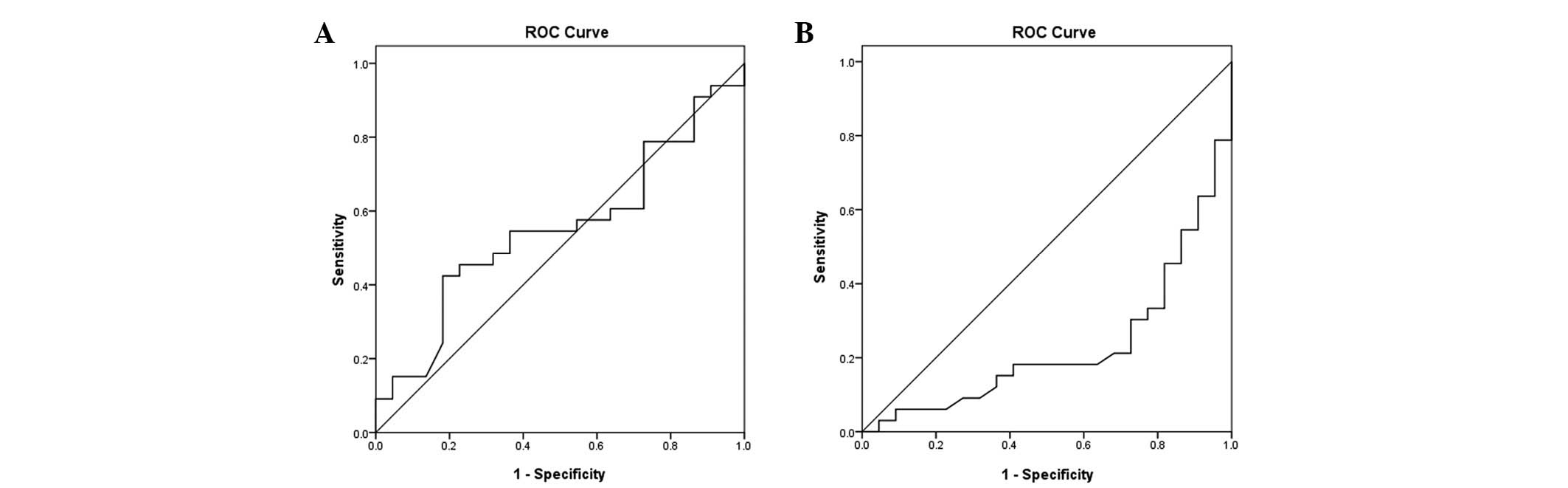
Debulking was performed in all patients. The results
of debulking were compared with the preoperative serum
concentrations of HE4 and CA125. With 500 U/ml as the demarcation
criterion (CA125), the larger the numbers were, the lower the
possibility of optimal cytoreduction surgery. CA125 predicted
incomplete cytoreductive surgery with 72 and 30% sensitivity and
specificity, respectively (Fig.
3A). The demarcation criterion of HE4 was 600 pmol/l, where a
value >600 pmol/l indicates a lower possibility of the optimal
cytoreduction surgery. HE4 predicted incomplete cytoreductive
surgery with a sensitivity and specificity of 77 and 32%,
respectively (Fig. 3B).
Discussion
The present study showed that the concentration of
HE4 in ovarian cancer patients was significantly higher than that
in benign ovarian tumor and normal control patients (P<0.01),
and no statistically significant differences were observed
(P>0.05) between the benign ovarian tumor lesion and normal
control groups. The mechanism of HE4 overexpression in ovarian
cancer is not clear. However, the results of Berry et al
(12) showed that the chromosomal
region where HE4 is located is frequently amplified in breast
cancer and ovarian cancer. However, few HE4 promoters are active in
ovarian surface epithelial (OSE) cells, indicating that the
increase in HE4 levels observed in ovarian cancer does not appear
in normal ovarian epithelia culture. Moreover, HE4 is not expressed
in the normal ovaries, early and late corpus luteum or fallopian
tubes. Consequently, the level of serum HE4 may be used as marker
for the diagnosis of ovarian cancer. The results of the present
study are consistent with those of Moore et al (13) who detected the levels of serum HE4
in epithelial ovarian cancer (129 cases) and benign ovarian tumor
patients (352 cases) and observed that HE4 was significantly
increased in the epithelial ovarian cancer patients. The present
study also was consistent with Köbel et al’s results
(9). Kirchoff et al
(6) observed that HE4 was expressed
mainly in the distal epithelial cells of the epididymis and
epithelial cells of the vas deferens. To further study the
correlation between HE4 and ovarian cancer, Wang (14)et al studied the expression of
HE4 in various ovarian tissues and revealed that HE4 was highly
expressed in cancer tissue but not in normal ovarian tissue and
pericancerous tissues. Another study showed that the HE4 secreted
by ovarian cancer is a secreted protein resulting from
N-glycosylation. Its molecular weight is less than CA125, so HE4 is
more likely to be secreted into the blood than CA125 (15) and HE4 may be more effective than
CA125 in early diagnosis. The present data also showed that the
diagnostic value of HE4 was superior to that of CA125 in stage I–II
patients. Montagnana et al (16) studied 46 ovarian cancer patients, 40
benign disease patients and a healthy control group and observed
that the release of HE4 occurred earlier than CA125. The levels of
HE4 had significantly increased in early ovarian cancer, while the
levels of 40–50% CA125 did not increase. The present study also
observed that the level of serum HE4 was the highest in serous
carcinoma patients and the difference compared with other types of
ovarian cancer was statistically significant (P<0.01). Drapkin
et al (15) investigated the
expression of HE4 in various types of ovarian cancer organization
using an immunohistochemical method and observed that HE4 was
expressed in 50% of ovarian clear cell carcinomas, 93% of ovarian
serous ovarian cancer and 100% of endometrioid carcinomas of the
ovary. However, it was not expressed in mucinous ovarian cancer and
normal ovarian tissues. Therefore, the diagnostic value of HE4 may
vary with the histopathological type. The present study also showed
that the level of serum HE4 was highest in serous adenocarcinoma
and clear cell carcinoma, compared with the other types of ovarian
cancer, although no statistically significant difference was
observed among mucinous adenocarcinoma, endometrial adenocarcinoma
and undifferentiated carcinoma. Nolen et al (17) studied 65 tumor markers for the
diagnosis of ovarian cancer and identified 34 significant markers.
The diagnostic value of HE4 was the highest, followed by CYFRA
21–1, CA125 and CA-19–9. The sensitivity of the diagnosis of early
ovarian cancer was improved from 74.2 to 91.7% by the combined
detection of HE4 and CA125. It has been suggested that this
combined detection is superior to the single detection of CA125
(18). Although the sensitivity of
the joint detection of HE4 and CA125 is superior compared with the
single detection of HE4, the difference is not significant and the
specificity is lower compared with a single HE4 indicator. This may
be associated with the false positive rate of the single detection
of CA125. The present study showed that the sensitivity and
specificity were 65 and 98.7%, respectively, for the combined
detection of HE4 and CA125. This result was compared with HE4 used
alone and it was observed that the sensitivity had decreased but
the specificity was increased. When combining CA125 with HE4, if
CA125 and HE4 were positive the combination was considered
positive. The sensitivity and specificity of the combination (77.5%
and 88.75%, respectively) were decreased compared with HE4 alone.
Therefore, the present study suggests that the combined detection
of HE4 and CA125 contributes to the differential diagnosis of
benign or malignant pelvic masses, but is not superior to the
single detection of HE4 for the early diagnosis of ovarian
cancer.
Certain studies have investigated whether HE4 may be
used as a marker to monitor disease progress and predict prognoses.
The study of Xu et al (19)
showed that the expression level of serum HE4 was significantly
higher in a preoperative ovarian cancer group compared with
healthy, benign ovarian epithelial tumor and borderline ovarian
tumor groups and the differences were statistically significant
(P<0.05). However, the serum HE4 expression level in
postoperative ovarian cancer patients was significantly lower than
the preoperative level, indicating that the level of serum HE4 may
have play a role in the evaluation of surgical treatment. Although
surgery is the major treatment option in ovarian cancer, its effect
is often compromised by early and insidious extra-pelvic
metastases, such as subphrenic and mesenteric root lesions,
particularly in the superior abdomen region. The early diagnosis
and accurate preoperative assessment of metastasis in ovarian
carcinoma patients is critical for achieving optimal debulking and
improving the five-year survival rate. The present study provides
evidence that preoperative serous HE4 testing in the ovarian cancer
patients may be regarded as an index for estimating the possibility
of optimal cytoreductive surgery. The demarcation criterion was 600
pmol/l, where a value >600 mol/l indicates a lower possibility
of optimal debulking by cytoreductive surgery. HE4 predicted that
the sensitivity of incomplete cytoreductive surgery was 77% and the
specificity was 32%. The present data also showed significant
differences between the Kaplan-Meier survival curves of
HE4-positive and negative patients (P=0.036, log-rank test). This
suggested that the prognosis of ovarian cancer patients with higher
concentrations of serum HE4 was worse than those without serum HE4.
The Cox proportional hazards regression model was also used to
analyze whether HE4 could be used as an independent prognostic
factor, with the following factors: HE4-positive and negative, age,
pathological type (epithelial and non-epithelial), stage (I–II and
III–IV), distant organ transfer, ascites and postoperative residual
focal factors. The results showed that the independent prognostic
factors affecting the survival of ovarian cancer patients were
clinical stage, distant organ metastasis and postoperative residual
foci, while HE4, pathological type, lymph node metastasis and
omentum majus metastasis were not independent prognostic factors
affecting survival. This may be due to the small sample size and
short follow-up period.
Acknowledgements
The present study was supported by a
grant from the Provincial Research Project Funding of Guangxi,
China (Nos. 2010GXNSFD013053 and Z2011217).
References
|
1.
|
Shaaban A and Rezvani M: Ovarian cancer:
detection and radiologic staging. Clin Obstet Gynecol. 52:73–93.
2009. View Article : Google Scholar
|
|
2.
|
Chen WQ, Zhang SW and Zou XN: Cancer
incidence and mortality in China, 2006. Chin J Cancer Res. 23:3–9.
2011.(In Chinese).
|
|
3.
|
Nolen BM and Lokshin AE: Protein
biomarkers of ovarian cancer: the forest and the trees. Future
Oncol. 8:55–71. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Li J, Dowdy S, Tipton T, et al: HE4 as a
biomarker for ovarian and endometrial cancer management. Expert Rev
Mol Diagn. 9:555–566. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Kirchhoff C, Habben I, Ivell R and Krull
N: A major human epididymis-specific cDNA encodes a protein with
sequence homology to extracellular proteinase inhibitors. Biol
Reprod. 45:350–357. 1991. View Article : Google Scholar
|
|
6.
|
Kirchhoff C: Molecular characterization of
epididymal proteins. Rev Reprod. 3:86–95. 1998. View Article : Google Scholar
|
|
7.
|
Galgano MT, Hampton GM and Frierson HF Jr:
Comprehensive analysis of HE4 expression in normal and malignant
human tissues. Mod Pathol. 19:847–853. 2006.PubMed/NCBI
|
|
8.
|
Moore RG, Brown AK, Miller MC, Skates S,
Allard WJ, Verch T, Steinhoff M, Messerlian G, DiSilvestro P,
Granai CO and Bast RC Jr: The use of multiple novel tumor
biomarkers for the detection of ovarian carcinoma in patients with
a pelvic mass. Gynecol Oncol. 108:402–408. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Köbel M, Kalloger SE, Boyd N, McKinney S,
Mehl E, Palmer C, Leung S, Bowen NJ, Ionescu DN, Rajput A, Prentice
LM, Miller D, Santos J, Swenerton K, Gilks CB and Huntsman D:
Ovarian carcinoma subtypes are different diseases: implications for
biomarker studies. PLoS Med. 5:e2322008.PubMed/NCBI
|
|
10.
|
Deraco M, Baratti D, Laterza B, et al:
Advanced cytoreduction as surgical standard of care and
hyperthermic intraperitoneal chemotherapy as promising treatment in
epithelial ovarian cancer. Eur J Surg Oncol. 37:4–9. 2011.
View Article : Google Scholar
|
|
11.
|
Chua TC, Liauw W, Robertson G and Morris
DL: Second-line treatment of first relapse recurrent ovarian
cancer. Aust N Z J Obstet Gynaecol. 50:465–471. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Berry NB, Cho YM, Harrington MA, Williams
SD, Foley J and Nephew KP: Transcriptional targeting in ovarian
cancer cells using the human epididymis protein 4 promoter. Gynecol
Oncol. 92:896–904. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Moore RG, McMeekin DS, Brown AK,
DiSilvestro P, Miller MC, Allard WJ, Gajewski W, Kurman R, Bast RC
Jr and Skates SJ: A noval multiple marker bioassay utiliazing HE4
and CA125 for the prediction of ovarian cancer in patients with a
pelvic mass. Gynecol Oncol. 112:40–46. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Wang K, Gan L, Jeffery E, Gayle M, Gown
AM, Skelly M, Nelson PS, Ng WV, Schummer M, Hood L and Mulligan J:
Monitoring gene expression profile changes in ovarian carcinomas
using cDNA microarray. Gene. 229:101–108. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Drapkin R, von Horsten HH, Lin Y, Mok SC,
Crum CP, Welch WR and Hecht JL: Human epididymis protein 4 (HE4) is
a secreted glycoprotein that is overexpressed by serous
andendometrioid ovarian carcinomas. Cancer Res. 65:2162–2169. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
16.
|
Montagnana M, Lippi G, Ruzzenente O,
Bresciani V, Danese E, Scevarolli S, Salvagno GL, Giudici S,
Franchi M and Guidi GC: The utility of serum human epididymis
protein 4 (HE4) in patients with a pelvic mass. J Clin Lab Anal.
23:331–335. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Nolen B, Velikokhatnaya L, Marrangoni A,
De Geest K, Lomakin A, Bast RC Jr and Lokshin A: Serum biomarker
panels for the discrimination of benign from malignant cases in
patients with an adnexal mass. Gynecol Oncol. 117:440–445. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
18.
|
Andersen MR, Goff BA, Lowe KA, Scholler N,
Bergan L, Drescher CW, Paley P and Urban N: Use of a symptom index,
CA125, and HE4 to predict ovarian cancer. Gynecol Oncol.
116:378–383. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19.
|
Xu CL, Yang YH and Wang HL: The value of
serum human epididymis protein 4 epithelial in the diagnosis of
ovarian cancer. Chinese Journal of Gynecology and Obstetrics.
26:684–686. 2010.
|