Introduction
The myelodysplastic syndromes (MDSs) are a
heterogeneous group of clonal malignant hematopoietic disorders,
which are characterized by ineffective hematopoiesis and a frequent
progression to acute myeloid leukemia (AML). Studies suggest that
the MDSs are a group of stem-cell disorders in which aberrations
within hematopoietic stem cells (HSCs) may lead to the onset of a
number of diseases, including AML (1,2).
Previous data have confirmed that human AML stem cells reside
within the CD34+CD38− compartment of the
leukemic clone, which is also observed in normal HSCs.
DLK1 is a transmembrane protein of the epidermal
growth factor (EGF)-like family that mainly functions as a GF to
maintain proliferating cells in an undifferentiated state (3). DLK1, also known as preadipocyte
factor-1 (pref-1), fetal antigen 1 (FA1), pG2 and ZOG, is expressed
extensively in immature cells and downregulated during fetal
development (4), thus suggesting
that DLK1 is important in stem/progenitor cells. In hematopoiesis,
DLK1 is important in supporting the proliferation of early
progenitor cells (5). The
overexpression of DLK1 has been observed in numerous types of
cancer, including MDS and AML (6,7).
Evidence also suggests that DLK1 may inhibit tumor cell
differentiation and increase tumorigenic potential (8), although the underlying mechanisms
causing these effects remain unclear. Based on this evidence, the
present study aimed to examine the hypothesis that DLK1 plays a
central role in the tumorigenesis of
CD34+CD38− cells. This hypothesis represents
a novel perspective with regard to the differentiation of
CD34+CD38− cells induced by the knockdown of
DLK1 expression. This study has the potential to shed light on the
role of DLK1 in CD34+CD38− cells with regard
to the regulation of the cell cycle and apoptosis, and to provide
mechanistic insights into the progression of malignant tumors.
Materials and methods
Patients
A total of 23 untreated patients (10 females, 13
males) who had been newly diagnosed with MDS according to the World
Health Organization (WHO) criteria were enrolled in the present
study (9). The median age was 56
years (range, 28–76 years). According to the WHO criteria, patients
were classified as follows: refractory cytopenia with multilineage
dysplasia (RCMD; including RCMD with ringed sideroblasts, RCMD-RS;
n=6), refractory anemia with excess blasts-1 (RAEB-1; n=3) and
RAEB-2 (n=14). Written informed consent was obtained from each
patient prior to entering the trial. The study complied with the
acceptable international standards outlined in the Declaration of
Helsinki, and was approved by the Institutional Ethics Committee of
Tianjin Medical University (Tianjin, China).
Sampling of bone marrow cells
Bone marrow was obtained from the posterior iliac
crest and collected in ethylenediaminetetraacetic acid (EDTA)
anticoagulated syringes. Written informed consent was obtained from
each patient prior to bone marrow puncture. The bone marrow samples
were transferred to the laboratory within 4 h of aspiration.
Magnetic sorting of
CD34+CD38− cells
CD34+CD38− cells were obtained
from the mononuclear cell fraction of the MDS bone marrow samples
(Ficoll density gradient separation), followed by immunomagnetic
bead selection with monoclonal murine antihuman CD34 and CD38
antibodies using the autoMACS automated separation system (Miltenyi
Biotech, Mönchengladbach, Germany). The yield and purity of the
positively selected CD34+CD38− cells were
evaluated by flow cytometry (FACSCalibur; Bio-Rad, Hercules, CA,
USA).
Cell culture and transfection
The CD34+CD38− cells were
cultured in X-VIVO 10 medium supplemented with 10% fetal bovine
serum (FBS) and GFs. The GF cocktail, consisting of 100 ng/ml stem
cell factor (Cell Signaling Technology, Inc., Danvers, MA, USA),
100 ng/ml FLT-3 (Reprotech, Rocky Hill, NJ, USA), 20 ng/ml
granulocytic CSF (Reprotech), 20 ng/ml IL-3 (Reprotech) and 20
ng/ml IL-6 (Reprotech), was used. All cultures were maintained at
37°C in a moist atmosphere containing 5% CO2. The cells
were plated in 6-well plates at 2×105 cells/well.
Transfection was performed using Lipofectamine 2000 (Invitrogen
Life Technologies, Carlsbad, CA, USA) according to the
manufacturer’s instructions. The small interfering (si)RNA sequence
targeting DLK1 (siRNA-DLK1) was 5′-GCACCUGCGUGGAUGAUGAU UdTdT-3′
and 5′-dTdTAGTAGTAGGTGCGTCCACG-3′. The siRNA sequence for scrambled
siRNA (siRNA-scr) was 5′-UUGAAGUUAUGUAUCCUCCUU-3′ and 5′-CUGAAG
CUGCUGGGAGUAAUU-3′. Blank transfection served as the control.
Quantitative (q)PCR assay
Total RNA was prepared using TRIzol (Invitrogen Life
Technologies) 48 h after transfection. The total RNA (1 μg)
was reverse transcribed into cDNA using AMV reverse transcriptase
(Takara Bio, Inc., Osaka, Japan) according to the manufacturer’s
instructions. The DLK1 gene was amplified under the following
conditions: 50°C for 2 min and 95°C for 10 min, then 40 cycles at
95°C for 15 sec, followed by 60°C for 60 sec. β-actin served as a
reference. The following oligonucleotide sequences were used: DLK1
forward, 5′-CTGGACGGTGGCCTCTATGAATG-3′ and reverse,
5′-ATCATCCACGCAGGTGCCTC-3′; and β-actin forward,
5′-CACGATGGAGGGGCCGGACTCATC-3′ and reverse,
5′-TAAAGACCTCTATGCCAACACAGT-3′. Each sample was performed in
triplicate and a reverse transcriptase negative control was also
tested to exclude any DNA contamination. The expression ratio was
calculated as 2n, where n is the CT value
difference for each patient normalized by the average CT
difference of the samples from the control subjects
(ΔΔCT method).
Western blotting
Cell lysates were prepared on ice in
radio-immunoprecipitation assay (RIPA) lysis buffer containing 50
mM Tris-Cl at pH 7.5, 150 mM NaCl, 1% Nonidet-P40, 0.5% sodium
deoxycholate, 0.1% sodium dodecyl sulfate and 1–2 mM
phenylmethylsulfonyl fluoride (PMSF). The protein concentrations
were measured using bicinchoninic acid (BCA) assay reagents
(Bio-Rad). A total of 100 μg whole cell lysate protein was
separated by 10% sodium dodecyl sulfate-polyacrylamide gel
electrophoresis (SDS-PAGE), and the separated protein was
transferred onto polyvinylidene difluoride (PVDF) membranes.
Subsequent to blocking by incubation with 5% BSA in Tris-buffered
saline for 1 h at room temperature, the membrane was incubated
using a polyclonal antibody against DLK1 (Cell Signaling
Technology, Inc.) and an anti-GAPDH antibody (Tianjin XiangTian
Technology Co., Ltd., China).
Flow cytometry
At 48 h post-transfection, the cells were collected
and prepared in a suspended solution, then fixed with 250 μl
solution A at room temperature for 10 min. Next, the cells were
incubated with 200 μl solution B at room temperature for 10
min, followed by solution C for 10 min at 4°C in the dark. The cell
cycle was analyzed using FACSCalibur flow cytometry (Bio-Rad).
At 48 h post-transfection, the cells were collected
and washed with cold phosphate-buffered saline (PBS). The cells
were incubated with 5 μl Annexin V and 5 μl propidium
iodide (PI) at room temperature for 15 min in the dark. Cell
apoptosis was analyzed using FACSCalibur flow cytometry
(Bio-Rad).
Statistical analysis
SPSS 16.0 (SPSS, Inc., Chicago, IL, USA) was used to
perform the statistical analysis. The data are expressed as the
mean ± SD. The paired t-test analysis of variance was used to
analyze the significance between groups. The least significant
difference method of multiple comparisons with parental and control
groups was used when the probability for the analysis of variance
was statistically significant. P<0.05 was considered to indicate
a statistically significant difference.
Results
Inhibition of DLK1 expression with
siRNA
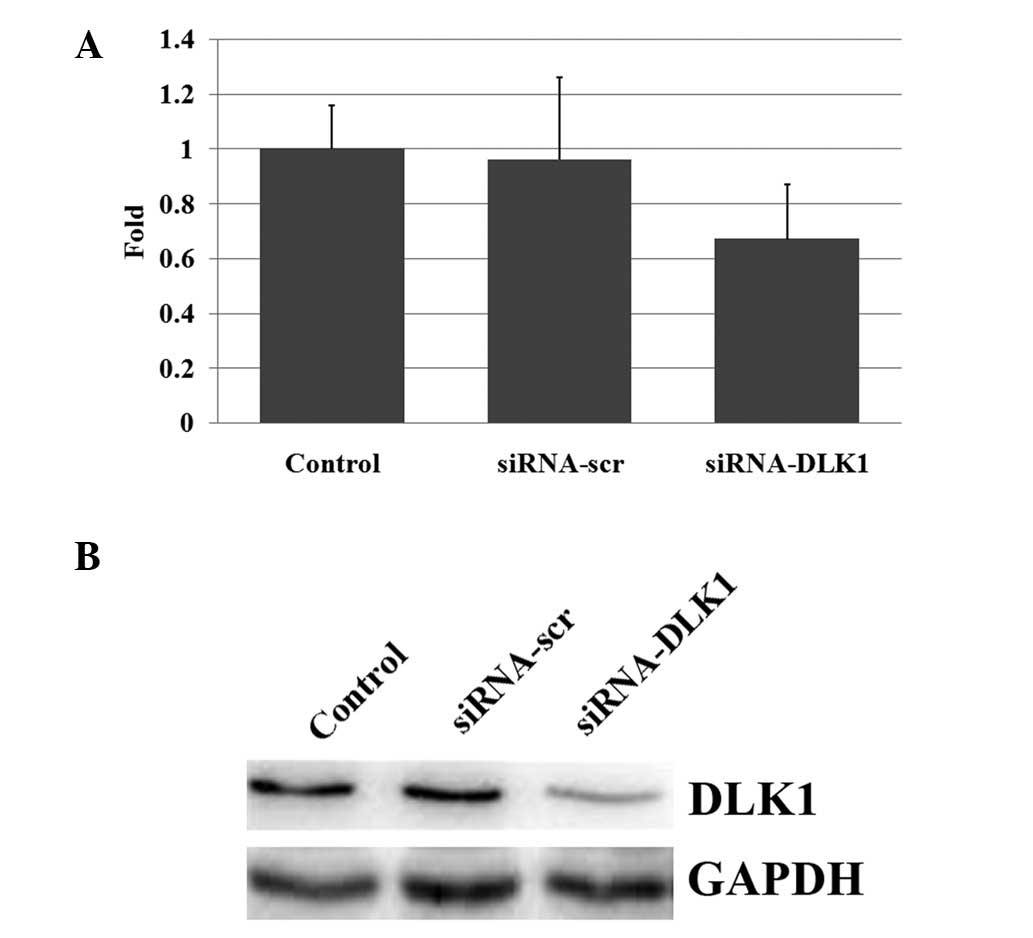
Following the transfection of the siRNA-DLK1, the
mRNA and protein expression levels of DLK1 were inhibited. qPCR
amplification revealed significantly decreased levels of DLK1 mRNA
expression due to the inhibition caused by the siRNAs (Fig. 1A). A western blot analysis
additionally revealed significantly decreased levels of DLK1
protein expression (Fig. 1B).
Therefore, DLK1 expression was effectively inhibited by RNA
interference.
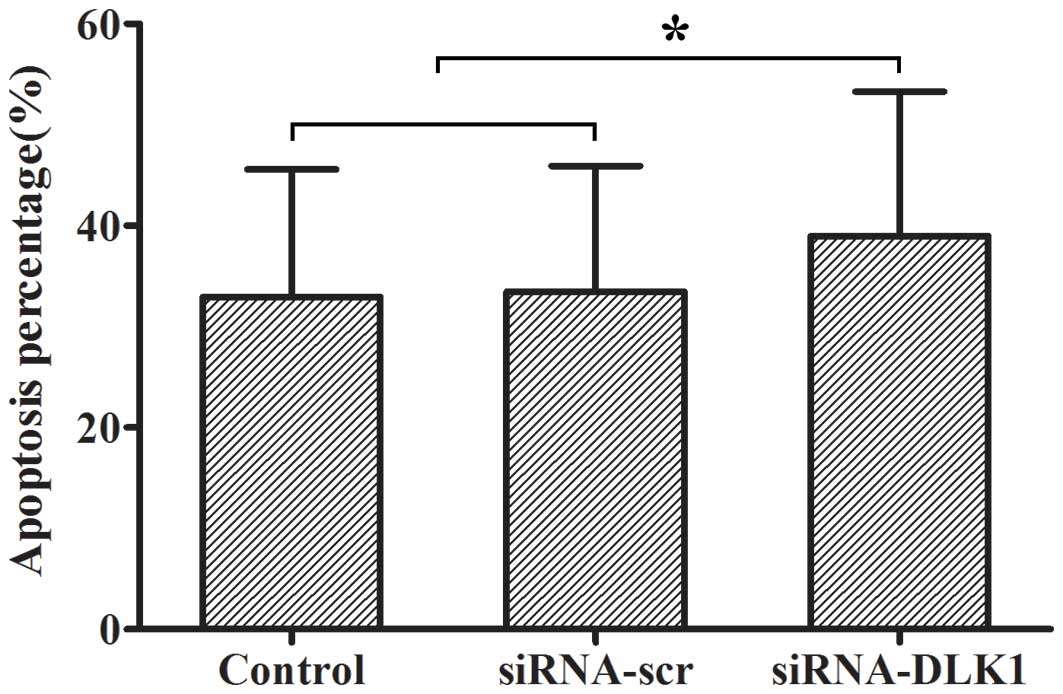
Apoptosis in DLK1 knockdown cells
The rate of apoptosis was measured by flow
cytometry. The apoptotic rate was increased when DLK1 expression
was knocked down compared with the siRNA-scr and control groups
(38.97±14.32 vs. 33.48±12.44 and 32.94±12.64%, P<0.05; Fig. 2).
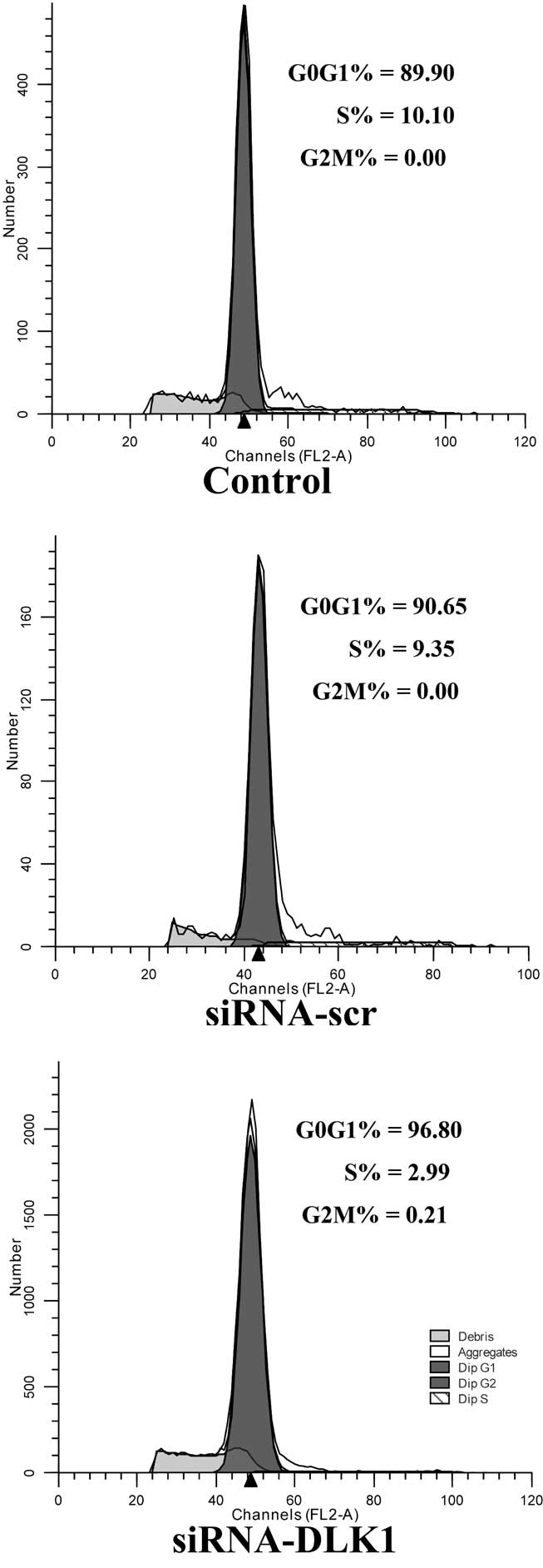
Cell cycle progression in DLK1-knockdown
cells
The flow cytometry assay revealed that the cells
were stimulated to enter the G0/G1 phase and
inhibited from entering the S phase of the cell cycle when DLK1
expression was knocked down compared with the siRNA-scr and control
groups (G0/G1 phase, 95.81±3.87 vs.
91.29±10.39 and 91.22±10.82%, P<0.05; S phase, 3.90±3.61 vs.
8.41±10.33 and 8.38±10.65%, P<0.05; Fig. 3).
Discussion
The present study examined the effect of knocking
down the expression of DLK1 on tumorigenesis in
CD34+CD38− cells. DLK1 (pref-1) is a
transmembrane and secreted protein, which is a member of the
EGF-like family, homologous to Notch/Delta/Serrate. DLK-1 contains
a signal peptide followed by 6 EGF-like repeats, a transmembrane
domain and a short intracellular tail (3). The DLK1 gene is located within an
imprinted region of chromosome 14q32 and expressed only from the
paternal allele in normal cells (10). The upregulation of DLK1 has been
previously observed in CD34+ cells from patients with
MDS (11,12). Sakajiri et al (6) analyzed hematopoiesis in DLK1-knockout
mice and suggested that DLK1 contributed to granulocyte,
megakaryocyte and B-cell clonogenic growth, and was required for
the generation of splenic B cells.
The elevated expression of DLK1 has been observed in
several tumor types, including MDS and AML. However, the role of
DLK1 and its mechanism in tumor growth remains to be fully
elucidated (13). If DLK1 maintains
tumor cells in a stem cell-like state, the relatively long lifespan
of stem cells will allow undifferentiated tumor cells to accumulate
and the stable genetic and epigenetic changes that ultimately
result in tumor malignancy to be perpetuated. The role of DLK1 in
tumor progression requires investigating in further detail.
The cell cycle analysis of the present study
revealed that DLK1-expressing CD34+CD38−
cells exhibited a slower progression through the
G0/G1 phase into the S phase compared with
the controls. In addition, the apoptotic rate of the
CD34+CD38− cells was elevated when the
expression of DLK1 was inhibited. These findings signify the
importance of the DLK1 gene in inhibiting cell differentiation and
reducing the rate of cellular apoptosis.
Extremely little is known about the molecular
mechanism and signal transduction pathway of DLK1 that is involved
in cell differentiation. Thus, these regulatory mechanisms require
further investigation.
In conclusion, the present study indicates that the
suppression of DLK1 expression in CD34+CD38−
cells results in a less aggressive phenotype. Impaired
differentiation and reduced hematopoietic cell production are
important features of hematopoiesis in MDS. These results support
the further investigation of the role of DLK1 in abnormal
hematopoiesis in MDS.
Acknowledgements
This study was supported by grants
from the Natural Science Foundation of China (30971286, 30971285
and 81170472), the Chinese Medical Association of Molecular Biology
Clinical Application Research Special Funds (CAMB042010), The
‘Eleventh Five-year Plan’ National Science and Technology Support
Plan (2008BA161B00) and the Health Industry Research Special
Project (201002024).
References
|
1.
|
Corey SJ, Minden MD, Barber DL, Kantarjian
H, Wang JC and Schimmer AD: Myelodysplastic syndromes: the
complexity of stem-cell diseases. Nat Rev Cancer. 7:118–129. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
2.
|
Nimer SD: MDS: a stem cell disorder - but
what exactly is wrong with the primitive hematopoietic cells in
this disease? Hematology Am Soc Hematol Educ Program. 2008:43–51.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
Laborda J: The role of the epidermal
growth factor-like protein dlk in cell differentiation. Histol
Histopathol. 15:119–129. 2000.PubMed/NCBI
|
|
4.
|
Floridon C, Jensen CH, Thorsen P, et al:
Does fetal antigen 1 (FA1) identify cells with regenerative,
endocrine and neuroendocrine potentials? A study of FA1 in
embryonic, fetal, and placental tissue and in maternal circulation.
Differentiation. 66:49–59. 2000. View Article : Google Scholar
|
|
5.
|
Moore KA, Pytowski B, Witte L, Hicklin D
and Lemischka IR: Hematopoietic activity of a stromal cell
transmembrane protein containing epidermal growth factor-like
repeat motifs. Proc Natl Acad Sci USA. 94:4011–4016. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Sakajiri S, O’kelly J, Yin D, et al: DLK1
in normal and abnormal hematopoiesis. Leukemia. 19:1404–1410. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Astuti D, Latif F, Wagner K, et al:
Epigenetic alteration at the DLK1-GTL2 imprinted domain in human
neoplasia: analysis of neuroblastoma, phaeochromocytoma and Wilms’
tumour. Br J Cancer. 92:1574–1580. 2005.PubMed/NCBI
|
|
8.
|
Li L, Forman SJ and Bhatia R: Expression
of DLK1 in hematopoietic cells results in inhibition of
differentiation and proliferation. Oncogene. 24:4472–4476. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Vardiman JW, Thiele J, Arber DA, et al:
The 2008 revision of the World Health Organization (WHO)
classification of myeloid neoplasms and acute leukemia: rationale
and important changes. Blood. 114:937–951. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Khoury H, Suarez-Saiz F, Wu S and Minden
MD: An upstream insulator regulates DLK1 imprinting in AML. Blood.
115:2260–2263. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Hofmann WK, de Vos S, Komor M, Hoelzer D,
Wachsman W and Koeffler HP: Characterization of gene expression of
CD34+ cells from normal and myelodysplastic bone marrow.
Blood. 100:3553–3560. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Pellagatti A, Cazzola M, Giagounidis A, et
al: Gene expression profiles of CD34+ cells in
myelodysplastic syndromes: involvement of interferon-stimulated
genes and correlation to FAB subtype and karyotype. Blood.
108:337–345. 2006.PubMed/NCBI
|
|
13.
|
Kim Y: Effect of retinoic acid and
delta-like 1 homologue (DLK1) on differentiation in neuroblastoma.
Nutr Res Pract. 4:276–282. 2010. View Article : Google Scholar : PubMed/NCBI
|