Introduction
The growth, development, invasion and metastasis of
solid tumors are dependent on angiogenesis. Vascular endothelial
growth factor (VEGF) is the main angiogenesis factor and is able to
increase vasopermeability and promote neovascularization. VEGF
provides essential nutrients for tumor growth and network matrixes
for tumor cell invasion and metastasis, and has important roles in
ascites generation and malignant tumor progression. VEGF has
important physiological roles created mainly when combining with
specific receptors (1–3).
Ovarian cancer is a type of euangiotic tumor, which
has numerous pathological types and no rational clinical symptoms.
It is difficult to identify early, but may exhibit extensive
peritoneal metastasis. Ovarian cancer is highly malignant and has a
poor prognosis. The mortality rate of ovarian cancer is greater
than the total mortality rate of cervical carcinoma and endometrial
carcinoma. It is a predominant gynecological tumor. Evidence
suggests that the abnormal expression of VEGF in ovarian cancer is
closely associated with tumor invasion and metastasis (4–6). In
ovarian tumors with rapid growth, there are vascular distributions,
while slow-growing tumor vessels are only concentrated in
peripheral vessels. Animal experiments have demonstrated that fine
avascular tumors with ovarian cancer metastasizing into the
peritoneum do not grow. However, following neovascularization, the
tumors grow rapidly. VEGF may promote not only angiopoiesis, but
also tumor proliferation by the paracrine or autocrine mechanisms.
Tumor microvessel density (MVD) is a valuable prognostic factor for
advanced ovarian cancer. Hefler et al (5) considered that serum VEGF levels were
closely associated with the prognosis of ovarian cancer. Although
there are studies on the correlation of VEGF with ovarian cancer,
the results of studies concerned with the correlation of the
expression of VEGF and its receptors with the clinical pathology of
ovarian cancer remain inconsistent. In addition, the pathogenesis
remains unclear, and studies investigating the resistance to
ovarian cancer angiopoiesis through the use of VEGF have only just
commenced. Therefore, the aim of the present study was to
investigate the correlation of the expression of VEGF and its
receptors with clinical pathology and MVD in ovarian cancer tissues
by conducting a case-control study, and to further investigate the
role of VEGF in ovarian cancer invasion and metastasis, as well as
in ascites and angiogenesis.
Subjects and methods
Subjects
A total of 48 patients with ovarian cancer were
selected from hospitalized patients receiving surgery at the
General Hospital of Beijing Military Area Command, Beijing, China,
between January 2000 and June 2004. The ages of the patients ranged
between 14 and 70 years old, and the mean age was 48.4 years old.
Among the patients, there were 41 cases of epithelial cancer
(including 24 cases of serous carcinoma, seven cases of mucinous
carcinoma and 10 cases of adenocarcinoma anaplastic) and seven
cases of non-epithelial tumors (including five cases of granulose
cell tumors, one case of dysgerminoma and one case of an endodermal
sinus tumor). Moreover, 14 cases were highly and moderately
differentiated and 24 cases were poorly differentiated. Prior to
surgery, an abdominal CT was performed to identify hepatic
metastasis, and was confirmed by intra-operative exploration.
Standardized cytoreductive surgery was performed. The clinical
staging complied with the staging criteria prepared by the
International Federation of Gynecology and Obstetrics (FIGO) in
1985 (7). Among all the cases,
there were 16 cases in stages I and II and 32 cases in stages III
and IV. All tissue specimens were obtained during surgery, and the
primary lesions of the tumors were collected and fixed with 10%
formalin for routine histological and immunohistochemical
examination. The present study was conducted in accordance with the
declaration of Helsinki and with approval from the Ethics Committee
of Beijing General Army Hospital. Written informed consent was
obtained from all participants.
Immunohistochemical examination
The VEGF antibody was a gift from Professor Yang
Zhihua of the Institute of Oncology, Chinese Academy of Medical
Sciences (Beijing, China). The antibody immunohistochemical
streptavidin-biotin complex (SABC) kit and rabbit anti-human Flt-1
and anti-KDR antibodies were purchased from Wuhan Boster Company
(Wuhan, China). The tissues were conventionally fixed with 10%
formalin, then dehydrated with alcohol, embedded with paraffin wax
and continuously sectioned into slices 4-μm thick. One slice was
used for the pathological examination and the other two slices were
used to detect VEGF and receptor protein expression, respectively.
In each experiment, the negative control group was identical, with
phosphate-buffered saline (PBS) used to replace the first antibody
for staining. For the positive control group, the provided positive
slice was used for staining. Brown granules in the cytoplasm of the
tumor and vascular endothelial cells were treated as positive and
the expression intensity was relative to the standard (7). The observed results were judged by two
blinded pathology experts.
Statistical analysis
Data were processed using the SPSS 10.0 software
package (SPSS Inc., Chicago, IL, USA). The Chi-squared test, exact
probability for four-fold tables and non-parametric rank sum test
(Kruskal-Wallis) were used to compare expression levels. P<0.05
was considered to indicate a statistically significant
difference.
Results
Expression of VEGF, Flt-1 and
KDR/Flk-1
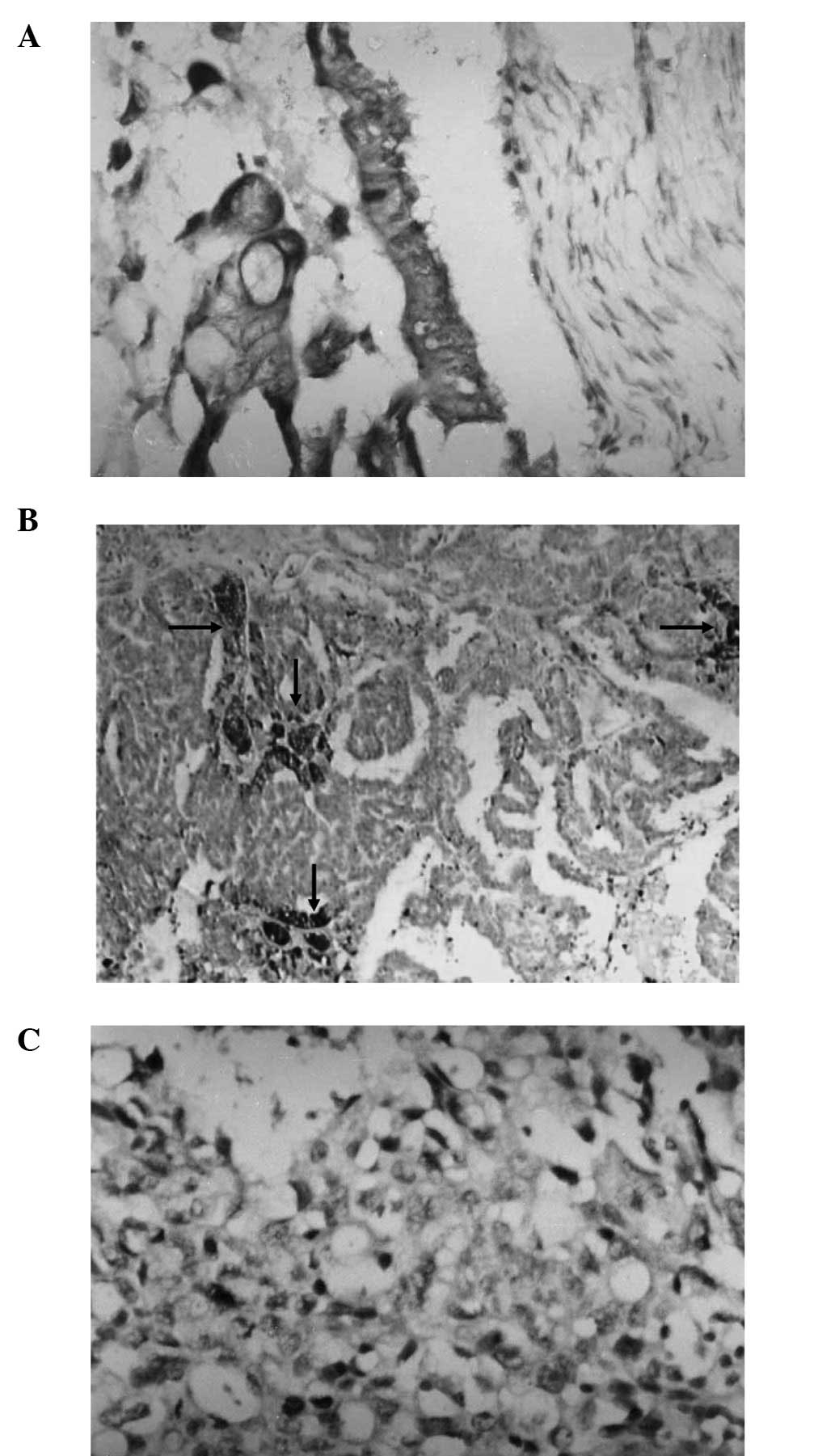
VEGF, Flt-1 and KDR expression was present in the
cytoplasm and vessels of the ovarian cancer tissues, exhibited as
focal or diffuse expression. The expression level of VEGF in
ovarian cancer was 66.7% (32/48). In total, 54.2% (26/48) of the
staining was markedly positive (>++), while 12.5% (6/48) was
weakly positive (+). The expression level of Flt-1 was 58.3%
(28/48), and the expression level of KDR was 43.8% (21/48). The
co-expression level of VEGF and Flt-1 was 92.9% (26/28), and the
co-expression level of VEGF and KDR was 90.5% (19/2; Fig. 1).
Correlation of VEGF, Flt-1 and KDR
expression with clinical pathology and stage
The co-expression levels of VEGF and Flt-1 protein
in the epithelial and non-epithelial tumors were 51.2 and 71.4%,
respectively, and the difference in the pairwise comparison was not
significant (P= 0.58). The co-expression levels of VEGF and Flt-1
in the highly and moderately differentiated and the poorly
differentiated tumors were 28.6 and 64.7%, respectively, and the
difference was significant (P=0.02). In addition, the co-expression
levels of VEGF and Flt-1 in the tumors of FIGO stages I and II and
in stages III and IV were 25.0 and 68.7%, respectively, and the
difference was significant (P= 0.005). The co-expression level of
VEGF/KDR was not associated with the tumor pathological type,
extent of differentiation or clinical stage (Table I).
 | Table I.Correlation of VEGF, Flt-1 and KDR
expression with clinical significance in ovarian cancer. |
Table I.
Correlation of VEGF, Flt-1 and KDR
expression with clinical significance in ovarian cancer.
| Category | Cases, n | VEGF
| Flt-1
| KDR
| VEGF/Flt-1
| VEGF/KDR
|
|---|
| n (%) | P-value | n (%) | P-value | n (%) | P-value | n (%) | P-value | n (%) | P-value |
|---|
| Pathological
type | | | | | | | | | | | |
| Epithelial
tumor | 41 | 27 (65.8) | 0.88 | 24 (58.8) | 0.73 | 17 (41.5) | - | 21 (51.2) | - | 16 (39.0) | - |
| Non-epithelial
tumor | 7 | 5 (71.4) | | 4 (57.1) | | 4 (57.1) | 0.73 | 5 (71.4) | 0.58 | 3 (42.8) | 0.80 |
| Tissue
differentiation | | | | | | | | | | | |
| G1, G2 | 14 | 8 (57.1) | | 6 (42.9) | | 5 (35.7) | | 4 (28.6) | | 3 (21.4) | |
|
G3/undifferentiated | 34 | 24 (70.6) | 0.59 | 22 (64.7) | 0.18 | 16 (47.1) | 0.48 | 22 (64.7) | 0.02 | 16 (47.1) | 0.09 |
| FIGO stages | | | | | | | | | | | |
| I, II | 16 | 9 (56.2) | | 6 (43.7) | | 7 (43.8) | | 4 (25.0) | | 4 (25.0) | |
| III, IV | 32 | 23 (71.9) | 0.27 | 22 (68.7) | 0.77 | 14 (43.8) | 0.95 | 22 (68.7) | 0.005 | 15 (46.9) | 0.16 |
Correlation of VEGF, Flt-1 and KDR
expression with clinical metastasis and ascites
The expression levels of VEGF and Flt-1 in the
patients with lymph node metastasis were 85.7 and 78.6%,
respectively, evidently higher than those in the patients without
lymph node metastasis; the two differences were significant
(P=0.009; P=0.02). The Flt-1 expression level of the 18 cases with
ascites <1000 ml was 45.2%, evidently lower than that of the
patients with ascites ≥1000 ml (78.3%); the difference was
significant (P=0.02). For the KDR expression level, there was a
significant difference between the patients with hepatic metastasis
and the patients without hepatic metastasis (P= 0.02), while the
co-expression level of VEGF and KDR in the patients with hepatic
metastasis was significantly increased (P=0.005; Table II).
 | Table II.Correlation between the expression of
VEGF and its receptors, and hepatic metastasis and ascites in
ovarian cancer. |
Table II.
Correlation between the expression of
VEGF and its receptors, and hepatic metastasis and ascites in
ovarian cancer.
| Category | Cases, n | VEGF
| Flt-1
| KDR
| VEGF/Flt-1
| VEGF/KDR
|
|---|
| n (%) | P-value | n (%) | P-value | n (%) | P-value | n (%) | P-value | n (%) | P-value |
|---|
| Lymph node | | | | | | | | | | | |
| (+) | 28 | 24 (85.7) | | 20 (71.4) | | 15 (53.4) | | 20 (71.4) | | 13 (46.4) | |
| (−) | 20 | 8 (40.0) | 0.009 | 8 (40.0) | 0.02 | 6 (30.0) | 0.1 | 6 (30.0) | 0.005 | 6 (30.0) | 0.25 |
| Hepatic
metastasis | | | | | | | | | | | |
| With | 8 | 7 (87.5) | | 6 (75.0) | | 7 (87.5) | | 6 (75.0) | | 7 (87.5) | |
| Without | 40 | 25 (62.5) | 0.36 | 22 (55.0) | 0.5 | 14 (35.0) | 0.02 | 20 (50.0) | 0.39 | 12 (30.0) | 0.005 |
| Ascites (ml) | | | | | | | | | | | |
| ≥1000 | 30 | 24 (75.0) | | 21 (70.0) | | 16 (53.3) | | 21 | | 13 | |
| <1000 | 18 | 8 (44.4) | 0.01 | 7 (38.9) | 0.04 | 5 (27.8) | 0.09 | 5 | 0.005 | 6 | 0.49 |
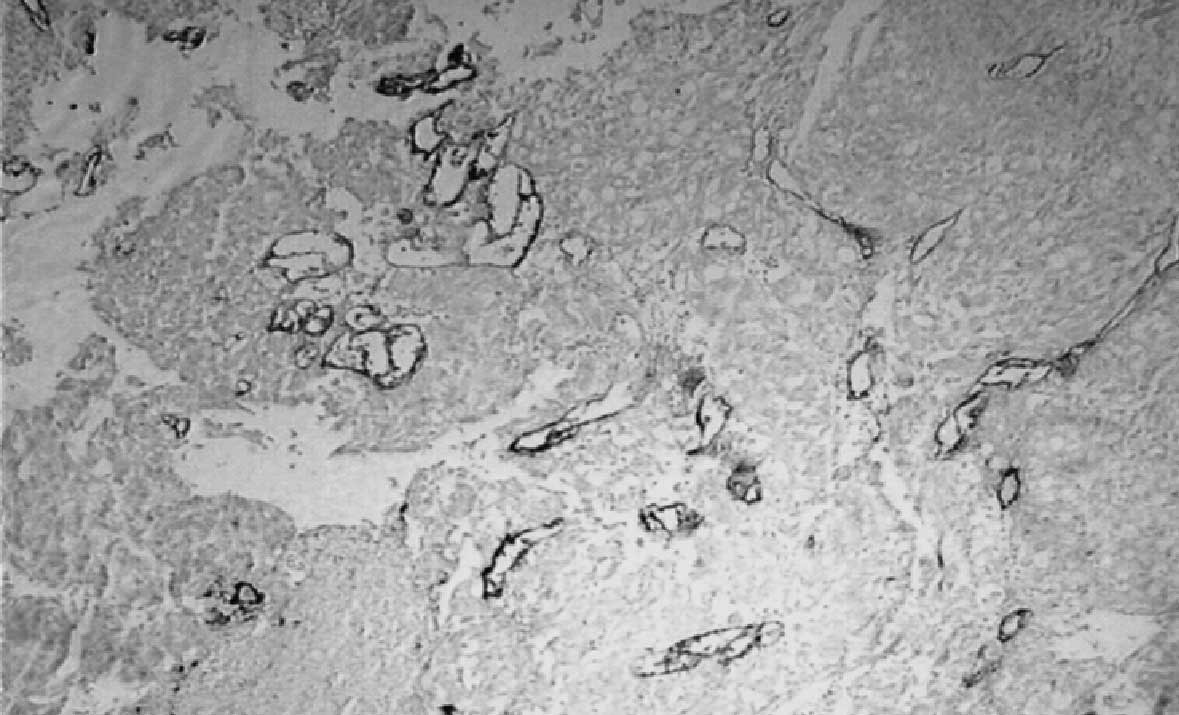
Correlation of VEGF and receptor
expression with MVD
Among the 32 VEGF-positive patients, the mean MVD
was 19.51±8.69. Compared with the mean MVD of the 16 VEGF-negative
patients (12.68±4.04), the difference was significant (P=0.01). The
mean MVD of the Flt-1-positive patients was 19.19±9.53. Compared
with mean MVD of the Flt-1-negative patients (17.16±6.74), the
difference was not significant (P=0.54). The mean MVD of the
KDR-positive patients was 21.64±8.63, and that of the KDR-negative
patients was 17.06±7.87. There was a significant difference between
these two groups (P=0.03; Table
III; Fig. 2).
 | Table III.Correlation between the expression of
VEGF and its recepteors with MVD in ovarian cancer. |
Table III.
Correlation between the expression of
VEGF and its recepteors with MVD in ovarian cancer.
| | | MVD
| |
|---|
| Protein | Expression | Cases, n | Mean | Range | Kruskal-Wallis
P-value |
|---|
| VEGF | Positive | 32 | 19.51±8.69 | 7.2–46.0 | |
| Negative | 16 | 12.68±4.04 | 4.1–21.6 | 0.01 |
| KDR | Positive | 21 | 21.64±8.63 | 10.2–46.0 | |
| Negative | 27 | 17.06±7.87 | 4.1–37.8 | 0.03 |
| Flt-1 | Positive | 28 | 19.19±9.53 | 4.1–46.0 | |
| Negative | 20 | 17.16±6.74 | 8.2–29.4 | 0.54 |
Discussion
VEGF, also known as vascular permeability factor, is
a multi-functional factor, with a highly conservative protein
structure. When its glycoprotein monomers are combined by disulfide
bonds to form a dimer, it becomes biologically active. The Flt-1
and KDR proteins are VEGF-specific receptors, belonging to the type
III tyrosine kinase receptors. VEGF has important physiological
roles created mainly when combining with specific receptors
(3). A number of studies have
demonstrated that VEGF is the main positive regulator in the
process of tumor angiogenesis, and that it is involved in the
occurrence and development of tumors by promoting angiogenesis, as
well as being associated with the degree of malignancy of tumors
(8–10). The growth of solid tumors is divided
into the non-vascular pre-invasion stage and the vascularization
invasion growth stage. In the vascularization stage, tumors begin
to grow rapidly. For the growth of the germinal center and tumors,
oxygen and nutritional supplies are required from the vessels,
otherwise hypoxia and necrosis will occur. In order to maintain the
unlimited invasive growth of malignant tumors, the tumors must
continuously and extensively conduct angiopoiesis. Numerous study
results have demonstrated that VEGF is the main positive regulator
in the process of tumor angiogenesis and that it is involved in the
occurrence and development of tumors by promoting angiogenesis
(10). The present
immunohistochemical results showed that the majority of tissue
samples expressing VEGF receptor exhibit positive VEGF expression.
The consistent co-expression of VEGF and the VEGF receptor
indicates that in ovarian cancer, VEGF not only indirectly promotes
tumor cell growth by affecting the receptor on the vascular
endothelial cells to induce angiopoiesis, but also directly
promotes tumor cell growth by affecting the receptor on the tumor
cells. Verheul et al indicated that VEGF is involved in
tumor occurrence and development using the paracrine or autocrine
mechanisms (11). VEGF is also
closely related to ovarian tumor cell proliferation. The PCR
results of Shen et al demonstrated that VEGF was unrelated
to the pathological type, but closely associated with the extent of
differentiation. The VEGF expression level in poorly-differentiated
cancers was 100% (markedly positive level, 83.3%), and the
expression level in the highly- and moderately-differentiated
cancers was 95.1% (markedly positive level, 34.8%; P=0.0004)
(12). Clinical stage is associated
with the degree of the malignancy of the tumors. In the present
study, the expression of VEGF and its receptors was unrelated to
the clinical stage of the tumor, which was not the expected result.
This was possibly due to the small number of selected cases; thus
it is necessary to conduct further future studies with larger
sample sizes. In addition, it was observed in the present study
that the co-expression level of VEGF and its receptor Flt-1 was
significantly higher in poorly-differentiated tumors compared with
highly- and moderately-differentiated tumors. The more advanced the
tumor clinical stage was, the higher the co-expression level of
Flt-1 and VEGF; this is consistent with the expected result. It
appears that VEGF interacts with the Flt-1 receptor to promote
malignant transformation and tumor progression.
One of the clinical characteristics of ovarian
cancer is that it readily forms a large quantity of ascites. In the
majority of cases, when ovarian cancer is identified, extensive
metastasis in the abdominopelvic cavity has occurred, which
increases the difficulty of performing surgery and thus affects the
patient prognosis. The mechanisms and factors that affect ascites
formation and abdominopelvic cavity metastasis in ovarian cancer
remain unclear. A large number of animal experiments have suggested
that, as a specific endothelial cell mitogen, VEGF is the main
angiogenesis factor. VEGF is able to increase vasopermeability,
promote neovascularization and is important in tumor vascular
endothelial cell proliferation and migration, as well as ascites
generation (13,14). Fujimoto et al (15) indicated that tumorous ascites
formation is due to tumor-derived VEGF affecting the tumor vessels
and host vessels via paracrine effects, which increase
vasopermeability and cause mass extravasation of the plasma protein
fluid. In the present study, the VEGF and Flt-1 receptor expression
levels were increased in patients with a large quantity of ascites
and positive peritoneal cytology, while KDR expression was not
correlated with ascites, indicating that VEGF promotes malignant
ascites generation, possibly by combining with the Flt-1
receptor.
During tumor growth, the levels of angiogenesis
factors secreted by the tumor cells are markedly increased, thus
significantly increasing tumor angiogenesis and speeding up tumor
progression, which manifests as the features of metastasis (?).
Studies suggest that avascular, resting cells in micrometastatic
foci are able to remain latent in the body for a longer period of
time. The proliferation rate of tumor cells in the dormancy period
is the same as that of the tumor growth, and the main difference is
that the death rate of the former is increased, causing
angiogenesis to decrease (7). It
has been proposed that VEGF expression is associated with the
malignant behavior of ovarian cancer. Patients with high VEGF
expression more frequently undergo lymph node and hepatic
metastasis compared with VEGF-negative patients (14,16).
In the present study, the VEGF expression level in the patients
with lymph node metastasis was 85.7%, significantly higher than
that in the patients without lymph node metastasis (40%; P= 0.0009)
However, there was no significant difference between the patients
with hepatic metastasis, which was possibly associated with the
smaller number of cases of patients with hepatic metastasis. The
Flt-1 expression level in the patients with lymph node metastasis
was 71.3%, significantly higher than that in the patients without
lymph node metastasis (40%; P= 0.02). VEGF may have an important
role in lymph node metastasis when combined with Flt-1. In
addition, the KDR expression level and the co-expression level of
VEGF and KDR in patients with hepatic metastasis was significantly
higher compared with patients without hepatic metastasis (P=0.02
and P=0.005, respectively), indicating that VEGF promoted the
hepatic metastasis of ovarian cancer by combining with KDR and was
associated with the hematogenous metastasis of ovarian cancer.
As a morphological basis of tumor growth and
development, angiogenesis provides nutrition and oxygen for tumor
cells to promote rapid proliferation, and microvessels are present
at high-densities. In the present study, F8 was selected as a
vascular endothelial marker, and the results showed that the mean
MVD of patients with VEGF expression was 19.51±8.69, significantly
higher compared with patients without VEGF expression (12.68±4.049;
P<0.02). The corresponding MVD of the patients with VEGF
expression was high. Furthermore, it was also revealed that the MVD
of the patients with positive KDR expression was 21.64±8.63,
significantly higher than that of the KDR-positive patients
(17.06±7.87; P=0.035), while there was no significant difference in
the mean MVD between the patients with and without Flt-1
expression, indicating that VEGF promotes ovarian tumor
angiogenesis mainly by interacting with KDR. Shalaby et al
(17) showed that for mice lacking
the gene encoding KDR/Flk-1, embryonic endothelial cell
differentiation was hindered and vessels could not be formed.
Homologous mice lacking the gene encoding Flt-1 were unable to
inhibit endothelial cell differentiation. Although angiopoiesis
occurred, the function of the formed vessels was seriously damaged.
One study (7) demonstrated that KDR
is important in endothelial cell differentiation, mitosis and
angiogenesis, and that it was the main angiogenesis regulator,
whereas Flt-1 receptor was mainly involved in interactions between
endothelial cells or between the endothelium and ECM, presenting
clear vascularization and increased vasopermeability, which was
consistent with the conclusions of the present study.
VEGF participates in multiple mechanisms by
combining with the corresponding receptors. VEGF promotes ovarian
cancer cell growth, angiopoiesis and distant metastasis. VEGF and
its receptors are able to act as indicators for predicting the
potential for tumor metastasis and malignant ascites. In addition,
the vascular dependence of malignant tumor growth and metastasis
indicates that it is feasible to inhibit these processes by
inhibiting tumor angiopoiesis, thus revealing a second field of
tumor treatment (18–20). Anti-angiogenesis treatments are able
to not only block tumor growth, but also more markedly inhibit
tumor metastasis. The present study demonstrated the significance
of the role of VEGF in tumors. With VEGF and its receptors as
targets, it is feasible to prepare corresponding antagonists and
inhibitors to suppress VEGF synthesis and secretion, hinder the
combination of VEGF with its receptors or inhibit receptor
expression to block the promotion of tumor growth and
metastasis.
References
|
1.
|
Nsihida N, Yano H, Komai K, Nishida T,
Kamura T and Kojiro M: Vascular endothelial growth factor-C and
vascular endothelial growth factor receptor 2 are related closely
to the prognosis of patients with ovarian carcinoma. Cancer.
101:1364–1374. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
2.
|
Karavasilis V, Malamou-Mitsi V, Briasoulis
E, Tsanou E, Kitsou E and Pavlidis N: Clinicopathologic study of
vascular endothelial growth factor, thrombospondin-1, and
microvessel density assessed by CD34 in patients with stage III
ovarian carcinoma. Int J Gynecol Cancer. 16(Suppl 1): 241–246.
2006. View Article : Google Scholar
|
|
3.
|
Gómez-Raposo C, Mendiola M, Barriuso J,
Casado E, Hardisson D and Redondo A: Angiogenesis and ovarian
cancer. Clin Transl Oncol. 11:564–571. 2009.
|
|
4.
|
Diniz Bizzo SM, Meira DD, Lima JM, Mororó
Jda S, Casali-da-Rocha JC and Ornellas MH: Peritoneal VEGF burden
as a predictor of cytoreductive surgery outcome in women with
epithelial ovarian cancer. Int J Gynaecol Obstet. 109:113–117.
2010.PubMed/NCBI
|
|
5.
|
Hefler LA, Zeillinger R, Grimm C, et al:
Preoperative serum vascular endothelial growth factor as a
prognostic parameter in ovarian cancer. Gynecol Oncol. 103:512–517.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Zhao Y, Zong ZH and Xu HM: RhoC expression
level is correlated with the clinicopathological characteristics of
ovarian cancer and the expression levels of ROCK-I, VEGF, and MMP9.
Gynecol Oncol. 116:563–571. 2010. View Article : Google Scholar
|
|
7.
|
Weidner N, Folkman J, Pozza F, et al:
Tumor angiogensis: a new significant and independent prognostic
indicator in early stage breast carcinoma. J Natl Cancer Inst.
84:1875–1887. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Brustmann H: Vascular endothelial growth
factor expression in serous ovarian carcinoma: relationship with
topoisomerase II alpha and prognosis. Gynecol Oncol. 95:16–22.
2004. View Article : Google Scholar
|
|
9.
|
Mabuchi S, Kawase C, Altomare DA, et al:
Vascular endothelial growth factor is a promising therapeutic
target for the treatment of clear cell carcinoma of the ovary. Mol
Cancer Ther. 9:2411–2422. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Smerdel MP, Waldstrøm M, Brandslund I,
Steffensen KD, Andersen RF and Jakobsen A: Prognostic importance of
vascular endothelial growth factor-A expression and vascular
endothelial growth factor polymorphisms in epithelial ovarian
cancer. Int J Gynecol Cancer. 19:578–584. 2009. View Article : Google Scholar
|
|
11.
|
Verheul HM, Hoekman K, Jorna AS, Smit EF
and Pinedo HM: Targeting vascular endothelial growth factor
blockade: ascites and pleural effusion formation. Oncologist.
5:45–50. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Shen GH, Ghazizadeh M, Kawanami O, et al:
Prognostic significance of vascular endothelial growth factor
expression in human ovarian carcinoma. Br J Cancer. 83:196–203.
2000.PubMed/NCBI
|
|
13.
|
Herr D, Sallmann A, Bekes I, et al: VEGF
induces ascites in ovarian cancer patients via increasing
peritoneal permeability by downregulation of Claudin 5. Gynecol
Oncol. 127:210–216. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Rudlowski C, Pickart AK, Fuhljahn C, et
al: Prognostic significance of vascular endothelial growth factor
expression in ovarian cancer patients: a long-term follow-up. Int J
Gynecol Cancer. 16(Suppl 1): 183–189. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Fujimoto J, Sakaguchi H, Aoki I, Khatun S
and Tamaya T: Clinical implications of expression of vascular
endothelial growth factor in metastatic lesions of ovarian carcers.
Br J Cancer. 85:313–316. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
16.
|
Bolat F, Gumurdulu D, Erkanli S, et al:
Maspin overexpression correlates with increased expression of
vascular endothelial growth factors A, C and D in human ovarian
carcinoma. Pathol Res Pract. 1:379–387. 2008. View Article : Google Scholar
|
|
17.
|
Shalaby F, Rossant J, Yamaguchi TP, et al:
Failure of blood-island formation and vasculogenesis in
Flk-1-deficient mice. Nature. 376:62–66. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
18.
|
Wang FQ, Barfield E, Dutta S, Pua T and
Fishman DA: VEGFR-2 silencing by interference RNA (siRNA)
suppresses LPA-induced epithelial ovarian cancer (EOC) invasion.
Gynecol Oncol. 115:414–423. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19.
|
Yen CF, Lee CL, Murk W, Han CM and Liao
SK: Reducing peritoneal vascular endothelial growth factor
concentration and inhibiting cancer scattering in a mouse model of
laparoscopy. Am J Obstet Gynecol. 198:423.e1–423.e7. 2008.
|
|
20.
|
Yagi Y, Fushida S, Harada S, et al:
Biodistribution of humanized anti-VEGF monoclonal
antibody/bevacizumab on peritoneal metastatic models with
subcutaneous xenograft of gastric cancer in mice. Cancer Chemother
Pharmacol. 66:745–753. 2010. View Article : Google Scholar : PubMed/NCBI
|