Introduction
Bevacizumab (Avastin) is a full-length humanized
murine anti-vascular endothelial growth factor (anti-VEGF)
monoclonal antibody with a molecular mass of 149 kDa, which binds
all isoforms of VEGF (1,2). Bevacizumab functions by inactivating
VEGF, thereby inhibiting endothelial cell activation and
proliferation (3). At present, the
drug is used off-label for the intravitreal treatment of several
neovascular and exudative ocular diseases (4). As VEGF is also important for normal
physiological processes, including cardiac development (5), maintenance of the microvasculature in
a number of organs (6), neural cell
survival (7), vasodilation
(8), trophic support of the
choriocapillaris (9) and
endothelial cell recruitment (10),
inhibition is also associated with a toxic effect in normal issues.
Therefore, the adverse effects of an intravitreal injection of
bevacizumab may occur due to the injection or be drug-related
(11–13). Drug-related adverse events include
inflammation, cataract progression, acute vision loss, central
retinal artery occlusion, anterior ischemic optic neuropathy
(AION), increased blood pressure, deep venous thrombosis and
transient ischemic attack. To date, ischemic retinal and
choriocapillaris changes following the use of intravitreal
anti-VEGF drugs have received a considerable amount of attention
(14,15). However, adverse effects in the
untreated eye following intravitreal bevacizumab injection have not
been reported. The current study presents a clinical case of sudden
vision loss in the untreated eye occurring 10 days after
intravitreal bevacizumab treatment for neovascular glaucoma
(NVG).
Case report
Clinical presentation
A 47-year-old male presented with severe
ophthalmalgia and vision loss in the right eye. The patient had
been diagnosed with proliferative diabetic retinopathy (PDR) 1 year
earlier. The patient provided written informed consent. The patient
underwent pars plana vitrectomy combined with retinal
photocoagulation and pan retinal photocoagulation in the right and
left eyes, respectively. The individual had also undergone Ahmed
glaucoma valve implantation 8 months earlier due to NVG. A general
history revealed that diabetes mellitus type 2 had been diagnosed
10 years previously and that the patient was treated with
subcutaneous insulin injections. The patient’s best-corrected
visual acuity (BCVA) was 20 letters [Early Treatment Diabetic
Retinopathy Study (ETDRS) chart] in the right eye and 80 letters in
the left eye. The intraocular pressure (IOP) was 50 mmHg in the
right eye and 14 mmHg in the left. A slit-lamp examination of the
anterior segment revealed corneal edema, rubeosis of the iris, a
dilated pupil, the loss of the light reflex, lens opacity and a
vitreous hemorrhage in the right eye. A normal anterior segment was
observed in the left eye. The retina of the right eye was
invisible. A clinical examination of the retina of the left eye
revealed a small cotton-wool patch above the disk, hemorrhagic foci
at the posterior pole and a laser spot on the peripheral retina
(Fig. 1A). Optical coherence
tomography (OCT) found an almost normally shaped macula in the left
eye (Fig. 1B). The patient was
administered with an intravitreal injection of bevacizumab (1.25
mg; Genentech/Roche, Basel, Switzerland) in the right eye. Ten days
after the injection, the patient presented with sudden visual loss
in the left eye. The patient’s BCVA was now 22 letters in the right
eye and 25 letters in the left eye. The IOP was 30 mmHg in the
right eye and 14mm Hg in the left eye. The rubeosis of the iris had
disappeared and the vitreous hemorrhage was slightly improved.
However, the retina was still invisible in the right eye. A
biomicroscopic examination of the left eye revealed a swollen optic
disk with unclear boundaries, several retinal hemorrhages and
thinning retinal vessels (Fig. 1C).
The central retinal thickness measured upon OCT examination was 410
μm (Fig. 1D). Fluorescein
angiography (FA) revealed delayed arterial filling with
hyperfluorescence in the optical disk and an enlargement of the
foveal avascular zone (Fig. 1E).
The visual field (VF; Fig. 1F) was
identified to exhibit a quadrantal defect associated with a blind
spot. These symptoms were consistent with a diagnosis of AION
associated with ischemic maculopathy.
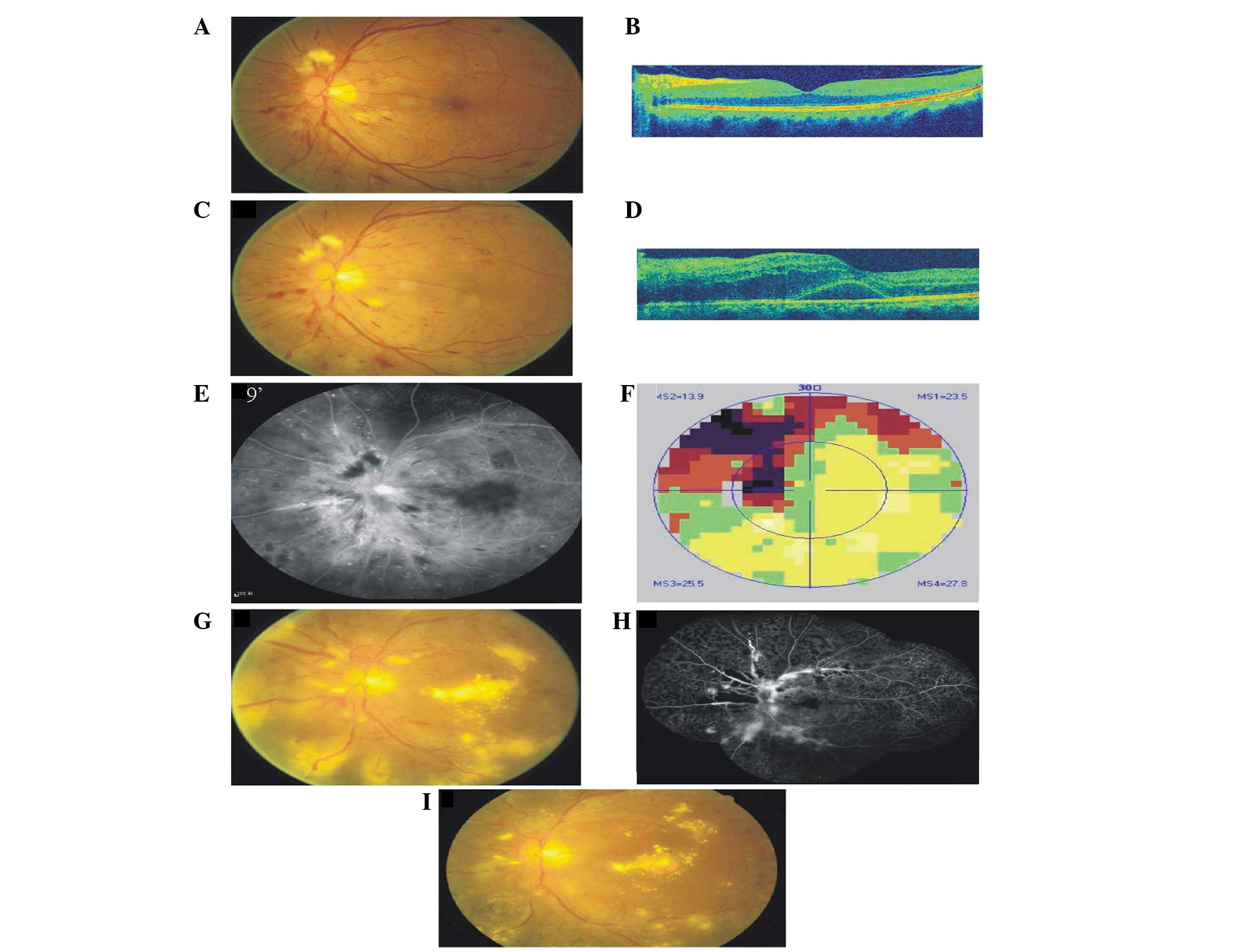 | Figure 1.Color, OCT, FA and VF images of the
left eye. (A and B) One month prior to administration of
intravitreal bevacizumab; (C–F) 10 days post-injection; (G–I) color
images and FA of the left eye 6 months later. (A) Color image
revealing a clear boundary of the disk, a small cottonwool patch
above the disk and a radial hemorrhage at the posterior pole. (B)
OCT revealing an almost normal macula profile. (C) Color image
revealing a swollen optic disk with unclear boundaries, several
retinal hemorrhages and thinning retinal vessels. (D) OCT revealing
macular neurosensory retinal detachment. The CRT was 410 μm.
(E) FA revealing hyperfluorescence in the optic disk and
enlargement of the foveal avascular zone. (F) VF images revealing a
quadrantal defect connected with a physiological blind spot. (G)
Neovessels of 1 PD area above the disk, vascular ectasia and
considerable levels of exudation are present at the posterior pole.
(H) FA revealing hyperfluorescence on the disk and superotemporal
and inferior areas. There was no non-perfusion in the nasal area.
(I) Color images of the left eye 7 months later. The area of
neovessels above the disk was reduced to 1/4 PD, vascular ectasia
was observed and the level of exudation was reduced. CRT, central
retinal thickness; OCT, optical coherence tomography; FA,
fluorescein angiography; VF, visual field; PD, papilla disk. |
Treatment
Compound anisodine was injected around the
superficial temporal artery, using 2 ml each time, 10 times in
total, and methylprednisolone (20 mg) was periorbitally injected
once. Six months later, the patient’s BCVA had improved to 44
letters in the left eye. A clinical examination identified
neovessels of 1 papilla disk (PD) area above the disk, the caliber
of the vein was different and considerable exudation was observed
at the posterior pole (Fig. 1G and
H). Laser photocoagulation treatment was administered
immediately. The time of exposure was 0.15 sec, the diameter of the
spot was 300–500μm, the energy was 340 mW and the number of
spots was 1,244. At the last check-up, the patient’s BCVA was 44
letters, the area of neovessels above the disk was reduced to 1/4
PD, vascular ectasia was observed and the level of exudation was
reduced (Fig. 1I).
Discussion
A growing number of neovascular ocular diseases are
currently being treated with bevacizumab and side effects are
reported frequently. In the current case study, we hypothesized
that acute ischemia occurred in the patient’s left eye as a result
of the intravitreal administration of bevacizumab in the right
eye.
The intravitreal half-life of bevacizumab in the
eyes is ∼4.3 days and has been detected in the serum at low
concentrations in rabbits (16).
The Fc receptor (FcRn) of the antibody is able to cross the
blood-retina barrier (17),
modulating IgG transport and protecting against its catabolism,
thereby leading to a longer serum half-life.
VEGF is a master regulator of angiogenesis.
Sufficient concentrations of VEGF must be maintained in the eye to
sustain normal functions. Retinal pigment epithelium (RPE)-secreted
VEGF has been identified to be critical for ocular development and
plays a prominent role in maintaining the choriocapillaris
(18,19). Chronic VEGF inhibition leads to
choriocapillary dysfunction and results in macular ischemia.
Sinapis et al (20) previously reported that the maximum
concentration of intravitreal bevacizumab (1.25 mg/0.05 ml) in the
injected eye occurred at day 1, whereas in the untreated eye and
the serum, the maximum concentration was identified at day 8. In
the present study, we hypothesized that the sudden loss of vision
in the untreated eye at 10 days post-injection resulted from the
gradual distribution of intravitreal bevacizumab from the right eye
to the left. This may be through the use of the blood circulation,
and may lead to retinal ischemia, particularly macular ischemia.
The disappearance of the iris rubeosis and partially dissolved
vitreous hemorrhage in the injected eye demonstrates the efficacy
of bevacizumab. Whether the retinal ischemia in the injected eye
improved following treatment remains unknown as the retina was
invisible. In the present case, FA and VF examinations revealed
acute retinal ischemia. Further investigation is required to
understand the adverse effects associated with using low
concentrations of bevacizumab. As the retinal ischemia has not
improved and the vessels are under the influence of diatetes
mellitus, the neovessels appear above the disk. Although the laser
treatment was efficient for the neovessels, further observation is
still required. The present case study demonstrates that
intravitreal bevacizumab is an effective treatment for neovascular
ocular diseases, however, it is also associated with adverse events
that must be taken into consideration, particularly as bevacizumab
is an off-label treatment. Photocoagulation remains an effective
treatment for PDR.
References
|
1.
|
Kaiser PK: Antivascular endothelial growth
factor agents and their development: therapeutic implications in
ocular diseases. Am J Ophthalmol. 142:660–668. 2006. View Article : Google Scholar
|
|
2.
|
Simó R and Hernández C: Intravitreous
anti-VEGF for diabetic retinopathy: hopes and fears for a new
therapeutic strategy. Diabetologia. 51:1574–1580. 2008.PubMed/NCBI
|
|
3.
|
Burger RA: Experience with bevacizumab in
the management of epithelial ovarian cancer. J Clin Oncol.
25:2902–2908. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Magdelaine-Beuzelin C, Pinault C, Paintaud
G and Watier H: Therapeutic antibodies in ophthalmology: old is new
again. MAbs. 2:176–180. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Lambrechts D and Carmeliet P: Genetics in
zebrafish, mice and humans to dissect congenital heart disease:
insights in the role of VEGF. Curr Top Dev Biol. 62:189–224. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Lee S, Chen TT, Barber CL, Jordan MC,
Murdock J, Desai S, Ferrara N, Nagy A, Roos KP and Iruela-Arispe
ML: Autocrine VEGF signaling is required for vascular homeostasis.
Cell. 130:691–703. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Nishijima K, Ng YS, Zhong L, et al:
Vascular endothelial growth factor-A is a survival factor for
retinal neurons and a critical neuroprotectant during the adaptive
response to ischemic injury. Am J Pathol. 171:53–67. 2007.
View Article : Google Scholar
|
|
8.
|
Wei W, Chen ZW, Yang Q, et al:
Vasorelaxation induced by vascular endothelial growth factor in the
human internal mammary artery and radial artery. Vascul Pharmacol.
46:253–259. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Saint-Geniez M, Kurihara T, Sekyama E, et
al: An essential role for RPE-derived solube VEGF in the
maintenance of the choriocapillaris. Proc Natl Acad Sci USA.
106:18751–18756. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Csaky KG, Baffi JZ, Byrnes GA, et al:
Recruitment of marrow-derived endothelial cells to experimental
choroidal neovascularization by local expression of vascular
endothelial growth factor. Exp Eye Res. 78:1107–1116. 2004.
View Article : Google Scholar
|
|
11.
|
Fung AE, Rosenfeld PJ and Reichel E: The
International Intravitreal Bevacizumab Safety Survey: using the
internet to assess drug safety worldwide. Br J Ophthalmol.
90:1344–1349. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Hosseini H and Razeghinejad MR: Anterior
ischemic optic neuropathy after intravitreal injection of
bevacizumab. J Neuroophthalmol. 29:160–161. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Battaglia Parodi M, Iacono P, Cascavilla
ML, Zucchiatti I, Kontadakis DS, Vergallo S and Bandello F:
Sequential anterior ischemic optic neuropathy and central retinal
artery and vein occlusion after ranibizumab for diabetic macular
edema. Eur J Ophthalmol. 20:1076–1078. 2010.
|
|
14.
|
Kim KS, Chang HR and Song S: Ischemic
change after intravitreal bevacizumab (Avastin) injection for
macular oedema secondary to non-ischemic central retinal vein
occlusion. Acta Ophthalmol (Copenh). 86:925–927. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
von Hanno T, Kinge B and Fossen K: Retinal
artery occlusion following intravitreal anti-VEGF therapy. Acta
Ophthalmol. 88:263–266. 2010.PubMed/NCBI
|
|
16.
|
Bakri SJ, Snyder MR, Reid JM, Pulido JS
and Singh RJ: Pharmacokinetics of intravitreal bevacizumab
(Avastin). Ophthalmology. 114:855–859. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Kim H, Fariss RN, Zhang C, Robinson SB,
Thill M and Csaky KG: Mapping of the neonatal Fc receptor in the
rodent eye. Invest Ophthalmol Vis Sci. 49:2025–2029. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
18.
|
Aiello LP, Northrup JM, Keyt BA, Takagi H
and Iwamoto MA: Hypoxic regulation of vascular endothelial growth
factor in retinal cells. Arch Ophthalmol. 113:1538–1544. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
19.
|
Blaauwgeers HG, Holtkamp GM, Rutten H,
Witmer AN, Koolwijk P, Partanen TA, Alitalo K, Kroon ME, Kijstra A,
van Hinsbergh VWM and Schlingermann RO: Polarized vascular
endothelial growth factor secretion by human retinal pigment
epithelium and localization of vascular endothelial growth factor
receptors on the inner choriocapillaris. Evidence for a trophic
paracrine relation Am J Pathol. 155:421–428. 1999.
|
|
20.
|
Sinapis CI, Routsias JG, Sinapis AI,
Sinapis D, Agrogiannis G, Pantopoulou A, Theocharis SE, Baltatzis
S, Patsouris E and Perrea DN: Pharmacokinetics of intravitreal
bevacizumab (Avastin(R)) in rabbits. Clin Ophthalmol. 5:697–704.
2011. View Article : Google Scholar : PubMed/NCBI
|















