Introduction
The liver is central to body metabolism and when it
is affected by diseases, such as cirrhosis, its complex functions
are invariably impaired. Changes in the metabolism of several
hormones have been observed in liver disease.
It was reported that 8 to 68% of 44 patients with
hepatocellular carcinoma had increased levels of α- and β-human
chorionic gonadotropin (hCG) subunits, calcitonin, parathyroid
hormone (PTH), prolactin (PRL), adrenocorticotropic hormone (ACTH)
and growth hormone (GH). Due to the simultaneous increases of these
hormones in cirrhosis, their production is likely to be due to the
metabolic effects of liver cell dysfunction rather than ectopic
production (1). Corticotrophin
releasing hormone (CRH) has been shown to be secreted by NPL-KC (a
human hepatoma cell line) and behave as a hypothalamic CRH
(2). CRH and ACTH were observed to
be produced by liver and lung metastases in a patient with a
pituitary carcinoma and the clinical presentation of Cushing’s
disease (3).
In males with liver cirrhosis, a reduction in free
testosterone in the serum was shown to be associated with normal
basal lutenising hormone (LH) and follicle-stimulating hormone
(FSH), suggesting impaired function of the
hypothalamic-pituitary-gonadal axis (4). The low testosterone level and the
derangement of the hypothalamic-pituitary function have a role in
the sexual dysfunction and changes in sex hormones that occur in
male patients with cirrhosis (5).
The LH levels of amenorroeic females with various aetiologies of
cirrhosis were shown to have decreased below the normal range in
50% of patients with alcoholic cirrhosis and 42% of patients with
non-alcoholic cirrhosis, and tests revealed that the hypothalamus,
rather than the pituitary, was the site of the disturbance in
gonadotrophin secretion (6).
Gonadotrophin-releasing hormone (GnRH) receptors have been
localised in various differentiated hepatocarcinoma tissues and
their expression in these tissues is associated with the degree of
differentiation (7). Treatment with
a combination of sex hormone suppression and inhibition of their
target receptors has been attempted in the tissues of patients with
hepatocellular carcinoma (8,9).
In patients with cirrhosis, the
hypothalamic-pituitary-adrenal and -gonadal axes and PRL secretion
are impaired. The response of GH to GH-releasing hormone (GHRH) is
also accelerated, and elevated basal and stimulated levels of GH
possibly reflect compensation for the low levels of IGF-1, which is
associated with deteriorating liver function (8). No effects of the aetiology of
cirrhosis on the degree of alteration of the hypothalamic-pituitary
glandular axes have been observed (10,11).
Serum ghrelin, tumour necrosis factor (TNF)-α and
interleukin 6 (IL6) levels have been reported to be significantly
higher in cirrhosis and hepatocellular carcinoma patients, whereas
serum leptin levels were observed to be decreased (12). Amphiregulin (AR), a member of the
epidermal growth factor family has been shown increased in liver
cirrhosis and behaves as a potent pro-regenerative and survival
factor (13). Neurotensin (NT) is
expressed in the human fetal liver, but not in the adult; in the
fibrolamellar carcinoma, (NT) is also expressed but not expressed
in the regerating rat liver (14).
Somatostatin (SRIH), cortistatin and SRIH receptor subtypes are
present in rat Kupfer cells where they may function in an autocrine
manner (15). SRIH analogs (SSTs)
have produced promising results for the treatment of hepatocellular
cancer and a long-acting SST analog (lanreotide; LAN) exhibited the
ability to decrease the S-phase fraction, as well as induce
apoptosis in HepG2 cells in a dose-dependent manner (16). Peptide 23, a newly identified
protein by rat pituitary cells, is stimulated by GH and inhibited
by SRIH. Peptide 23-cDNA has ∼73% homology with human
hepatocellular carcinoma cDNA from human hepatocellular carcinoma
(17).
The majority of liver tumours express
cholecystokinin (CCK-B) receptors and are able to process gastrin
(G) as far as pro-G and G-gly (precursor forms) and this may be
associated with tumour proliferation (18). Gut hormone receptors are
overexpressed in human cancer and allow receptor-targeted tumour
imaging and therapy. A novel promising receptor for these purposes
is the secretin receptor. Secretin receptors are expressed in the
human liver particularly the biliary tract and cholangiocarcinomas
but not the hepatocytes or hepatocellular carcinomas (19). Hepatic failure is associated with
increased pancreatic glucagon levels (true hyperglucagonaemia is
suppressed by glucose) (20).
Methionine enkephalin and other opioid peptides have been shown to
be increased in both cirrhosis and acute liver disease (21). Furthermore, there is evidence that
the central mechanism involved in the brain-liver interaction in
the development of symptoms of hepatic encephalopathy (such as
pruritus and fatigue) involves the opioid system (22). A class of endogenous opioids is
upregulated in liver disease particular to cholestasis, which
contribute to pruritus, hypotension and encephalopathy. Symptoms
associated with cholestasis are reversed or ameliorated by opioid
receptor antagonists. Opioid receptor antagonists have been
reported to relieve multiple symptoms, except for pruritus, and
improve liver function as demonstrated in experimental cholestasis
(23).
7B2 was identified in 1982 by Seidah et al
during the purification of the N-terminal glycol-segment of
proopiomelanocortin (POMC) from pig anterior pituitaries (24). Later, the same investigators
reported the purification of its human pituitary homologue
(25). The two sequences differed
by only one amino acid. 7B2 cDNA cloning from various species has
demonstrated the high evolutionary conservation of the 7B2
molecule, suggesting that 7B2 may be biologically relevant. In
particular, the overall residue identity is extremely high (90–96%)
among mammals, relatively high (67–83%) between mammals and frogs
or fish, and low (17–22%) between vertebrates and invertebrates
(26,27). Initially, 7B2 was revealed to be
located in the pituitary gonadotrophs (26,27)
and to respond to exogenous LH-releasing hormone (LHRH) (26). 7B2 has also been demonstrated to be
located in tissues that are primary neuronal (the brain and adrenal
medulla) or endocrine (pituitary, thyroid and pancreas) tissues, or
those that are known to carry a sub-population of neuroendocrine
cells (gastrointestinal tract). The highest levels are detected in
the anterior lobe of the pituitary, followed by the
neurointer-mediate lobe, hypothalamus, adrenal medulla, thyroid
gland and pancreas (27). In cell
cultures (normal pituitary cells), the secretion of 7B2 appears to
be unaffected by GHRH and CRH, but is increased by LHRH (26,27).
7B2 is detectable in human plasma. It is present at
remarkably high levels in early childhood, which gradually decrease
to adult levels by 20 years of age and slowly rise again with aging
(28,29). The levels of 7B2 are elevated in
pregnancy, from the second to the fourth trimester, but sharply
decline soon after delivery and return to normal by 4-6 weeks
post-partum (30). Initial studies
of plasma 7B2 level increases in patients suffering from chronic
kidney failure and liver cirrhosis (28,29,31)
have suggested that these organs are involved in 7B2 clearance.
In the present study, the plasma 7B2
immunoreactivity (7B2-IR) levels were measured in patients with
liver disease, mainly cirrhosis of various aetiologies, in a
further attempt to investigate the hepatic handling of this
protein.
Patients and methods
Patients
The present study was also undertaken by Dr L.
Meleagros (Endocrine Unit, Hammersmith Hospital, London, UK). In
total, 18 patients with liver disease, who were under the care of
Dr J. Calam (Department of Medicine, Hammersmith Hospital), were
studied. The study was approved by the Ethics Committee of
Hammersmith Hospital (RPMS, Imperial College London) and written
informed consent was obtained by the patients. Of these patients,
seven (three male and four female), aged 37–67 [54.6±13.5 (SD)]
years and weighing 51–96 [69.5±17.8 (SD)] kg, suffered from liver
cirrhosis of cryptogenic (n=2) or alcoholic (n=5) aetiology. The
remaining 11 patients (four male and seven female), aged 22–76
[56.1±17.6 (SD)] years and weighing 50–100 [67.7±14.8 (SD)] kg,
suffered from miscellaneous liver abnormalities.
The clinical diagnosis was confirmed in the majority
of patients by the histological examination of a percutaneous liver
biopsy or by appropriate radiological investigations (Table I). The total number of patients and
the numbers with jaundice, ascites/oedema and hepatic
encephalopathy are also shown in Table
I.
 | Table I.Characteristics of patients with
liver disease. |
Table I.
Characteristics of patients with
liver disease.
| Diagnosis | Total no. | His | Rad | Jaun | Asc/Oed | Enc |
|---|
| Cirrhosis | 7 | 4 | 3 | 5 | 3 | 5 |
| Primary sclerosing
cholangitis | 1 | 1 | 1 | 0 | 0 | 0 |
| Hepatic
metastases | 6 | 6 | 6 | 1 | 1 | 0 |
| Budd-Chiari
syndrome | 1 | 1 | 1 | 0 | 0 | 1 |
| Fatty liver | 1 | 1 | 1 | 0 | 0 | 0 |
| Amyloid
disease | 1 | 1 | 0 | 0 | 0 | 0 |
| Cholelithiasis | 1 | 0 | 1 | 1 | 0 | 0 |
The medication received by the patients was as
follows (number of patients with cirrhosis vs. without cirrhosis):
folic acid (6 vs. 0), ranitidine (5 vs. 0), prednisolone (1 vs.2),
vitamin K (3 vs. 0), parentrovite (2 vs. 1), lactulose (4 vs. 0),
diuretics (0 vs. 2), heparin (0 vs.1), cyclosporin A (1 vs. 0),
salazopyrine (0 vs. 1), insulin (0 vs. 1), neomycin (1 vs. 0) and
cyproheptadine (0 vs. 1). In addition three patients with and one
without cirrhosis were on protein-restricted diets, and three
cirrhotic patients were on salt-restricted diets. Peripheral venous
blood samples were obtained between 08:00–09:00 hours with the
patients in a seated position. All medication was withheld on the
morning of the study. Samples were collected in heparinised tubes
for the plasma 7B2-IR estimations and were processed as described.
The plasma samples (100 μl aliquots) were assayed for 7B2-IR
in duplicate. Plasma bilirubin, alkaline phosphatase, aspartate
aminotransferase, albumin, prothrombin time, electrolytes, urea and
creatinine were measured at the Chemical Pathology Laboratory of
Hammersmith Hospital.
7B2 radioimmunoassay (RIA)
A sensitive RIA for 7B2-IR was developed in our
laboratory as described previously (32).
Immunisation and preparation of
antisera
A peptide fragment corresponding to residues 23–39
of the authentic 180 amino acid 7B2 molecule was custom synthesised
(Cambridge Research Biochemicals, Cambridge, UK) and conjugated to
bovine serum albumin (BSA; Sigma Chemical Co., Dorset, Poole, UK)
by carbodiimide. The conjugated material was emulsified in complete
Freund’s adjuvant for the primary immunisation and in incomplete
adjuvant for the booster injections. Freund’s adjuvant was prepared
by mixing 8.5 ml n-hexadecane (Koch-Light Laboratories Ltd.) with
1.5 ml Arlasel A (Sigma Chemical Co.) and was then completed by the
addition of heat-killed mycobacteria (1 mg/ml).
Emulsified conjugate (2 ml) containing 80 pg
conjugated peptide was administered to New Zealand white rabbits
via 0.5 ml subcutaneous injections, one into each groin and axilla.
At three months subsequent to the primary immunisation, booster
injections were administered at two-monthly intervals. Each
injection contained 40 μg conjugated 7B2 (23–39) in
2 ml incomplete Freund’s adjuvant (i.e. without mycobacteria). The
rabbits were bled from the marginal ear vein seven to ten days
after each booster injection. Blood was allowed to clot at room
temperature and the serum was separated by centrifugation. Each
harvested serum sample was then evaluated for its ability to bind
125I-7B2 (23–39). For this test, 20 μl undiluted
serum was added to each assay tube (in duplicate) containing assay
buffer and label to a total volume of 700 ml.
The antisera that exhibited >70% binding after 1
h of incubation at room temperature followed by charcoal/dextran
separation were subsequently tested at three dilutions in order to
determine the optimal working dilution. The antiserum used in these
studies was labelled AG7 and used at a final dilution of 1/160,000,
with an affinity constant of 1.9×1011 l/mol with respect
to the synthetic fragment. The antibody was shown to cross-react by
33% with authentic porcine 7B2 on a molar basis. No
cross-reactivities were observed with human insulin, proinsulin,
glucagon, secretin, SRIH, human pancreatic polypeptide, ACTH,
N-terminal of POMC, β-lipoprotein (LPH), β-endorphin, GH, arginine
vasopressin (AVP), oxytocin, ovine corticotrophin releasing factor
(oCRF) and vasoactive intestinal peptide (VIP).
Iodination procedure
The synthetic fragment 7B2 was used for the
preparation of (125I-Tyr4)-7B2 (23–39) by
the standard chloramine T method (33).
Standards
The standards were prepared gravimetrically using
the synthetic 7B2 fragment. Aliquots (10 μl), each
containing 2 pmol 7B2 (23–39), were lyophilised to 10−2
torr and stored in vacuo at −20°C.
Assay conditions
All samples were assayed in duplicate in 2-ml
polysterene tubes (LKB, Luckham Ltd.). A total of 100 μl of
the sample was added to each assay tube. Phosphate buffer (0.4 ml
of 0.6 M, pH 7.4), containing 10 mM EDTA, 7.5 mM sodium azide and
150 mM BSA was used as the assay buffer. The label and antiserum
were made up in the same buffer and 100 μl of each was added
to each assay tube to give a final volume of 700 μl.
Separation
Subsequent to a five-day incubation at 4°C, the
antibody-bound label was separated from the free label by adding
250 μl of a suspension containing 4 mg charcoal (Norit GSX;
Hopkin and Williams) coated with 0.4 mg clinical grade dextran
(Sigma Chemical Co.) to each tube. The tubes were centrifuged at
1600 × g for 20 min at 4°C and the supernatant was aspirated
immediately.
Gamma counting
Following separation, the charcoal pellet and
supernatant were counted in multi-well gamma counters (NE 1600;
Nuclear Enterprises). Since synthetic 7B2 (23–39)
was used as a standard, the results are expressed as 7B2
immunoreactive equivalents (7B2-IE).
7B2-IE changes per 0.9 fmol/assay tube were detected
with 95% confidence limits, with intra- and inter-assay variations
of <15%.
Chromatographic profiles
Samples containing 7B2-IE were subjected to gel
permeation chromatography. A 1.4×90-cm column of Sephadex G-100 was
used to separate the components present in the plasma.
The column was eluted with a 0.06 M phosphate buffer
(pH 7.4) containing 10 mM EDTA, 0.3% BSA and 0.2 M NaCl, at a flow
rate of 3.2 ml/h at 4°C.
The column was pre-calibrated with dextran blue
[molecular weight (MW), 2,000,000], horse heart cytochrome C (MW,
12,384) and a trace amount of NaI125. Dextran blue,
cytochrome C and a trace amount of NaI125 were added to
each sample as internal markers. The elution coefficient (Kav) for
each immunoreactive peak was calculated according to the method
used by Laurent and Killander (34). Gel permeation chromatography of
porcine pituitary extracts showed a major peak (90% of total
immunoreactivity), eluting prior to cytochrome C (32).
Statistical analysis
Statistical analyses were performed using two-tailed
t-tests. P<0.05 was used to indicate a statistically significant
difference.
Results
Patients
The results of the liver function tests and plasma
biochemistry in the patients with and without cirrhosis are
presented in Table II. Among the
cirrhotic patients, significantly raised plasma bilirubin and
aspartate aminotransferase levels, a prolonged prothrombin time and
a reduced plasma albumin level provided evidence of hepatocellular
damage and impaired liver function. Among the non-cirrhotic
patients, the plasma bilirubin level was slightly raised, since 2
patients in this group (one with hepatic metastases and one with
cholelithiasis) had common bile duct obstruction. This group had a
higher, although not statistically significant, mean alkaline
phosphatase level compared with the cirrhotic group, due to
intrahepatic (two patients with primary sclerosing cholangitis and
one with amyloid infiltration) or extra-hepatic (one patient with
hepatic metastases and one with cholelithiasis) cholestasis.
However, the aspartate aminotransferase level was only marginally
increased, while the plasma albumin level and prothrombin time were
within or just above the normal range. Therefore, there was only
minimal hepatocellular damage and impairement of liver function in
the non-cirrhotic group. The plasma creatinine level was higher in
the cirrhotic group compared with the non-cirrhotic group, although
the difference was not significant due to the high intra-group
variation.
 | Table II.Plasma biochemistry and coagulation
status in patients with liver disease. |
Table II.
Plasma biochemistry and coagulation
status in patients with liver disease.
| Variable | Cirrhotic | Non-cirrhotic | P-value |
|---|
| Bilirubin (2–14
μmol/l) | 203.1±81.7
(12–564) | 32.4±14.8
(3–140) | <0.02 |
| Alkaline
phosphatase (30–130 IU/l) | 270.9±64.3
(145–642) | 477.8±125.3
(82–1062) | ns |
| Aspartate
aminotransferase (10–35 U/l) | 167.3±41.6
(38–361) | 57.7±13.8
(18–98) | <0.01 |
| Albumin (35–55
g/l) | 30.3±3.1
(22–46) | 38.2±1.7
(33–50) | <0.05 |
| Prothrombin time
(11–15 seconds) | 20.7±2.2
(14–29) | 15.2±0.5
(13–16) | <0.05 |
| Sodium (136–149
mmol/l) | 135.6±2.8
(121–142) | 138.4±0.8
(134–142) | ns |
| Potassium (3.8–5.2
mmol/l) | 3.9±0.2
(3.1–4.7) | 4.3±0.2
(3.5–5.2) | ns |
| Bicarbonate (24–30
mmol/l) | 24.1±0.8
(20–26) | 25.0±1.2
(18–30) | ns |
| Urea (2.5–6.5
mmol/l) | 7.3±2.0
(2.1–17.1) | 5.3±1.2
(2.9–16.4) | ns |
| Creatinine (55–125
pmol/l) | 148.4±46.8
(53–394) | 85.9±4.3
(53–150) | ns |
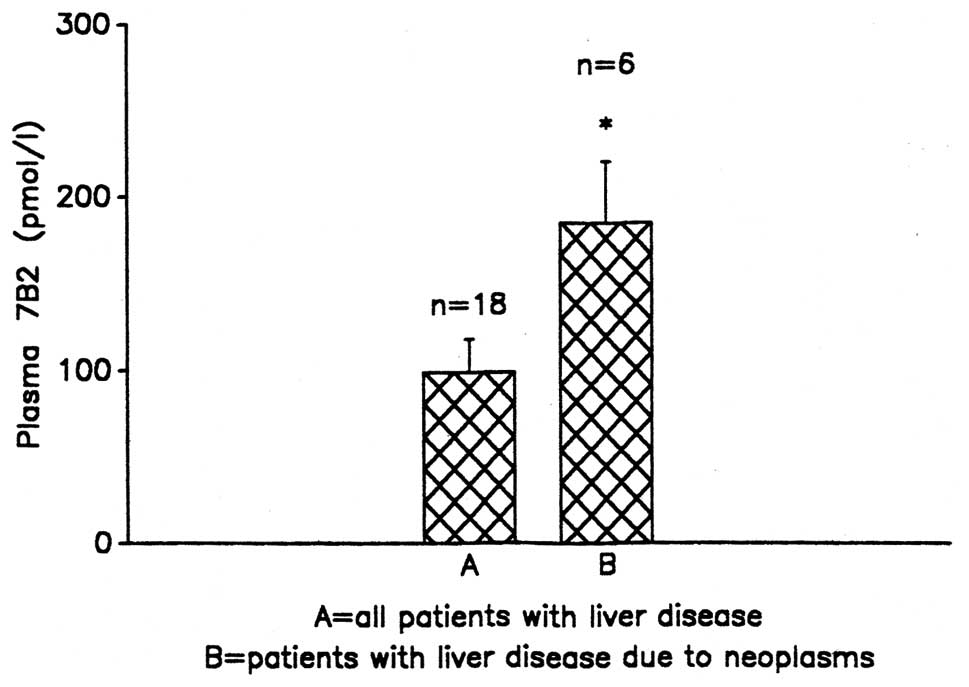
The mean plasma 7B2-IR concentration in liver
disease was 99.44±15.9 pmol/l. In the patients with hepatocellular
damage due to metastatic tumours [Ca bronchus, carcinoid (n=6)],
the 7B2-IR concentrations were higher [185±36.9 pmol/l,
(P<0.05)] compared with the overall subjects with liver damage
(Fig. 1).
When the 11 patients with other causes of liver
damage were screened separately, the mean plasma 7B2-IR
concentration was 56.68±4.5 pmol/l.
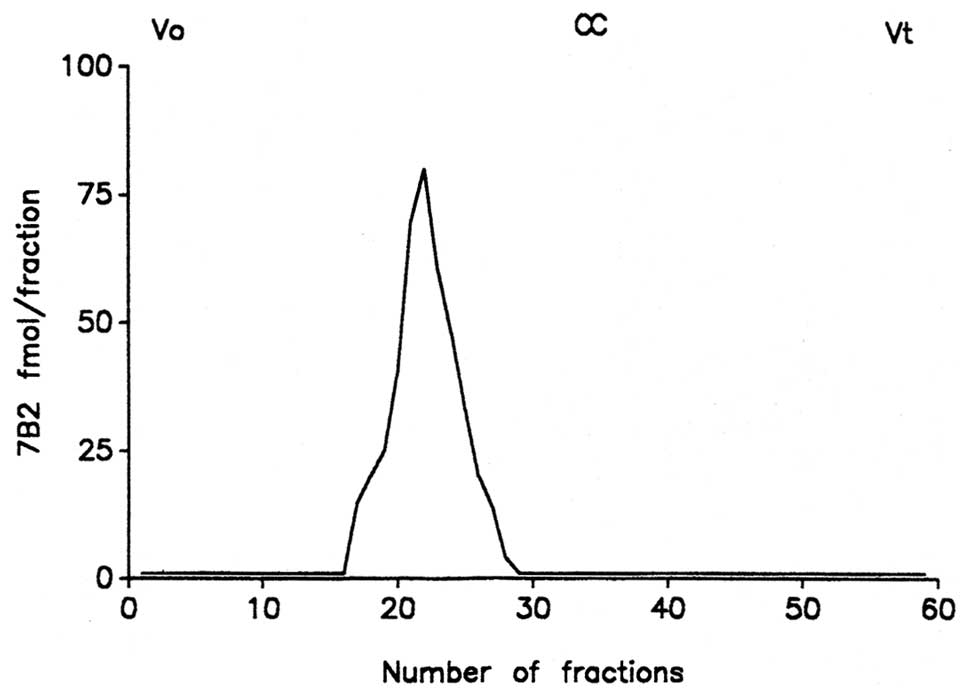
Chromatography
Fig. 2 shows a
representative 7B2 plasma chromatographic profile from the patients
with liver damage.
Discussion
In the present study, it was observed that 7B2-IR
levels were increased in liver disease. In the majority of the
patients with hepatocellular damage, the 7B2-IR levels ranged
between 1.5- and 3-fold of the normal range compared with the
control subjects. These data are in accordance with previous
studies that have demonstrated increased 7B2 plasma levels in
patients with cirrhosis (28).
Kobori (35) also reported
increased 7B2-IR in the plasma of 13 patients with cirrhosis.
In the present study, it was also evident that in
individual patients with liver disease due to metastatic tumours
(Ca bronchus, metastatic carcinoid), 7B2-IR was increased by
>3–5-fold compared with the normal range. This agrees with the
results of Suzuki et al (36) who showed that 7B2-IR is mainly
encountered in neoplastic conditions, particularly endocrine or
other tumours of the gastrointestinal tract. It is therefore
difficult to conclude whether 7B2 is produced by the damaged
hepatocyte or if it is released by the tumour itself, particularly
for patients with liver damage due to metastatic tumours.
The immunocytochemical analysis of tumour tissues
has revealed more extensive associations between 7B2 and
neuroendocrine tumours than the titration of its circulating
levels. 7B2 has been detected in the majority of benign, and
approximately half of malignant, pancreatic tumours, particularly
insulin-producing tumours (36–39).
Notably, insulinomas containing only proinsulin and not the active
mature form were shown to also lack 7B2 (38). 7B2-IR has been detected in bronchial
carcinoids and small-cell lung carcinomas that are associated with
liver metastases (36,39–41).
Various other neuropeptides have been investigated
in liver diseases and alterations in their levels have been
observed. Neurotensin (NT) is expressed in the human fetal liver,
but not in adults. NT is also expressed in fibrolamellar carcinoma,
although it is not expressed in the regenerating rat liver
(42). NT has also been shown to be
produced by a fibrolamellar hepatoma (14). The majority of liver tumours express
cholecystokinin (CCK-B) receptors and are able to process gastrin
(G) as far as pro-G and G-gly (the precursor forms), which may be
associated with tumour proliferation (43,44).
Gut hormone receptors are overexpressed in human cancer and allow
receptor-targeted tumour imaging and therapy. A novel promising
receptor for these purposes is the secretin receptor. Secretin
receptors are expressed in the human liver, particularly in the
biliary tract and cholangiocarcinomas, but not in hepatocytes or
hepatocellular carcinomas (19).
Hepatic failure is associated with increased pancreatic glucagon
levels (true hyperglucagonaemia suppressed by glucose) (20). Methionine-enkephalin and other
opioid peptides have been shown to be increased in cirrhosis and
acute liver disease (23).
Additionally, there is evidence that the central mechanism involved
in the brain-liver interaction in the development of the symptoms
of hepatic encephalopathy (such as pruritus and fatigue) involves
the opioid system (23,44). A class of endogenous opioids is
upregulated in liver disease, particularly cholestasis, which
contribute to pruritus, hypotension and encephalopathy. Symptoms
associated with cholestasis are reversed or at least ameliorated by
mu-opioid receptor antagonists. Opioid receptor antagonists have
been reported to relieve multiple symptoms, with the exception of
pruritus, and improve liver function, as demonstrated in
experimental cholestasis (23,44).
In a multiple primary hepatic carcinoid tumour of the liver,
neuron-specific enolase (NSE) and chromogranin were identified
immunocytochemically in all tumour cell types. The S-100 protein,
human choriogonadotrophin and serotonin were also observed
(45). It has been suggested
previously that 7B2-IR is co-localised with several chromogranins
in the secretory granules (44,45)
and that it belongs to the granin family (46). It is therefore possible that, in
patients whose disease is due to primary or secondary liver
neoplasms, 7B2-IR levels may be even higher than in cirrhosis of
other aetiologies, due to the overproduction of 7B2 by the tumour
cells, as observed in several liver neoplasms that have been
positively stained for chromogranin peptides (47,48).
In neuroendocrine cells, 7B2 functions as a specific
chaperone for proprotein convertase 2 (proPC2) (49). It has been proposed that 7B2 serves
as an intracellular proPC2 chaperone and prevents the premature
activation of the zymogen during its transit in the regulated
secretory pathway. It appears that pro7B2 attaches to proPC2 in the
endoplasmic reticulum (ER). This attachment is facilitated by the
relatively alkaline conditions of this compartment. The inactive
complex is transported to the trans-Golgi network (TGN) where
pro7B2 is cleaved into an N-terminal protein and a C-terminal
peptide. ProPC2 is then autocatalytically cleaved after the
prodomain as the complex is transported into secretory granules. In
these organelles, the prodomain and 7B2 fragments dissociate from
the enzyme, which then becomes fully activated. Thus, 7B2 regulates
PC2 activation (49). The 7B2 and
PC2 proteins are packaged into secretory granules. It is unclear
whether proPC2 requires an association with 7B2 polypeptides in
order to be sorted into these organelles. Although not yet
established, 7B2 may be a component of the PC2 aggregates that are
sorted into the secretory granules (50,51).
Since 7B2 has been grouped with the chromogranins
and secretogranins into the so-called granin family of proteins,
one of its presumed functions is to facilitate the sorting of
neuroendocrine proteins from the secretory granules (46). By studying POMC-expressing
non-pituitary tumours, corticotrophin-like-intermediate lobe
peptide (CLIP), a product of corticotrophin cleavage by PC2, was
detected only in the tumours that expressed PC2 (39,51),
and it may be assumed that these tumours also contain 7B2.
7B2 has also been detected in pituitary tumours
(52,53). It is also noteworthy that, unlike
PC2-null mice which are viable, 7B2-null mutants die early in life
from Cushing’s disease due to ACTH hypersecretion by the
intermediate lobe, suggesting the possible involvement of 7B2 in
secretory granule formation and secretion regulation (54). The 7B2-null mice with Cushing’s
disease may be saved early in life by an adrenalectomy (55). Neither our plasma ACTH tumours of
Cushing’s patients (26,56), nor those studied by Natori et
al (57,58) allow the conclusion that 7B2-IR
levels in plasma are increased in Cushing’s patients, and
corticotrophic adenomas have not shown increased 7B2 secretion
in vitro, although 7B2 is concomitantly secreted with ACTH
by AtT-20 tumourous cells in vitro (56). It has been suggested that the lethal
phenotype that is exhibited by 7B2-null mice with a complex
Cushing’s disease-like pathology, is due to intermediate lobe ACTH
hypersecretion as a consequence of the interruption of PC2-mediated
peptide processing, as well as undefined consequences of the loss
of 7B2.
Sarac et al (59) reported that 7B2-null mice exhibit a
multisystem disorder that includes severe pathoanatomical and
histopathological alterations to vital organs, including the heart
and spleen, but most notably the liver, in which massive steatosis
and necrosis are observed. Metabolic derangements in the glucose
metabolism result in glycogen and fat deposition in the liver under
conditions of chronic hypoglycaemia. Liver failure is also likely
to contribute to abnormalities in blood coagulation and blood
chemistry, such as lactic acidosis. It has been suggested that a
hypoglycaemic crisis coupled with respiratory distress and
intensive internal thrombosis is likely to result in the rapid
deterioration and death of the 7B2-null mice (59).
All the aforementioned observations and knowledge
make 7B2 an intriguing molecule, whose definite function remains to
be elucidated in view of the fact that it appears to exist in cells
where PC2 activity is not evident (60), thus suggesting a bigger biological
role for 7B2 compared with PC2.
The family of proprotein convertases has been
implicated in tumourigenesis and metastasis in animal models. In a
study assessing PC1, PC2 and 7B2 in primary colon cancers, PC1 and
PC2 mRNA, protein expression and protein cleavage profiles were
observed to be altered in liver colorectal metastases compared with
unaffected and normal livers. Active PC1 protein was overexpressed
in tumours, which correlated with its mRNA profile. Moreover, the
enhanced PC2 processing pattern in the tumours was correlated with
the overexpression of its specific binding protein 7B2. These
results were corroborated by immunocytochemistry. The authors
suggested that the exclusive presence of 7B2 in metastatic tumours
may represent a new target for early diagnosis, prognosis and/or
treatment (61).
Liver dysfunction may be due to various pathologies
and drug interactions, and all the aforementioned observations
suggest that 7B2 may be have a more biologically important role
than PC2. The biological role of 7B2 appears to be important for
animal survival, and more studies should be undertaken to identify
a definite role for this protein, not only in liver disease, but
also in other clinical entities.
References
|
1.
|
Scheuer A, Grün R and Lehman FG: Peptide
hormones in liver cirrhosis and hepatocellular carcinoma. Oncodev
Biol Med. 2:1–10. 1981.PubMed/NCBI
|
|
2.
|
Parkes DG, Yamamoto GY, Vaughan JM and
Vale WW: Characterization and regulation of
corticotrophin-releasing factor in the human hepatoma NPLC-KC cell
line. Neuroendocrinology. 57:663–669. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
Nawata H, Higuchi K, Ikuyama S, Kato K,
Ibayashi H, Mimura K, Sueshi K, Zingami H and Imura H:
Corticotrophin-releasing hormone- and adrenocorticotropin-producing
pituitary carcinoma with metastases to the liver and lung in a
patient with Cushing’s disease. J Clin Endocrinol Metab.
71:1068–1073. 1990.PubMed/NCBI
|
|
4.
|
Mowat NA, Edwards CR, Fischer R, McNeilly
AS, Green JR and Dawson AM: Hypothalamic-pituitary-gonadal function
in men with cirrhosis of the liver. Gut. 17:345–350. 1976.
View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Van Thiel DH, Lester R and Vaitukaitis J:
Evidence for a defect in pituitary secretion of luteinizing hormone
in chronic alcoholic men. J Clin Endocrinol Metab. 47:499–507.
1978.PubMed/NCBI
|
|
6.
|
Wang YJ, Wu JC, Lee SD, Tsai YT and Lo KJ:
Gonadal dysfunction and changes in sex hormones in postnecrotic
cirrhotic men: a matched study with alcoholic men.
Hepatogastroenterology. 38:531–534. 1991.PubMed/NCBI
|
|
7.
|
Bell H, Raknerud N, Falch JA and Haug E:
Inappropriately low levels of gonadotrophins in amenorrhoeic women
with alcoholic and non-alcoholic cirrhosis. Eur J Endocrinol.
132:444–449. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Zietz B, Lock G, Plach B, Drobnik W,
Grossmann J, Schölmerich J and Straub RH: Dysfunction of the
hypothalamic-pituitary-glandular axes and relation to Child-Pugh
classification in male patients with alcoholic and virus-related
cirrhosis. Eur J Gastroenterol Hepatol. 15:495–501. 2003.PubMed/NCBI
|
|
9.
|
Zhang J, Huang G and Huang W: Gonadotropin
releasing hormone and its receptor in the tissue of human
hepatocellular carcinoma. Zhonghua Yi Xue Za Zhi. 78:343–346.
1998.(In Chinese).
|
|
10.
|
Manesis EK, Giannoulis G, Zoumboulis P,
Vafiadou I and Hadziyannis SJ: Treatment of hepatocellular
carcinoma with combined suppression and inhibition of sex hormones:
a randomized, controlled trial. Hepatology. 21:1535–1542.
1995.PubMed/NCBI
|
|
11.
|
Eaveri R, Ben-Yehudah A and
Lorberboum-Galski H: Surface antigens/receptors for targeted cancer
treatments: the GnRH receptor/binding site for targeted
adenocarcinoma therapy. Curr Cancer Drug Targets. 4:673–687. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Ataseven H, Bahcecioglu IH, Kuzu N, et al:
The levels of grelin, leptin, TNF-alpha, and IL-6 in liver
cirrhosis and hepatocellular carcinoma due to HDV infection.
Mediators Inflamm. 2006:783802006. View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Berasain C, Castillo J, Perugorria MJ,
Prieto J and Avila MA: Amphiregulin: a new growth factor in
hepatocarcinogenesis. Cancer Lett. 254:30–41. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Ehrenfried JA, Zhou Z, Thompson JC and
Evers BM: Expression of the neurotensin gene in fetal human liver
and fibrolamellar carcinoma. Ann Surg. 220:484–489. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Xidakis C, Mastrodimou N, Notas G, Renieri
E, Kolios G, Kouroumalis E and Thermos K: RT-PCR and
immunocytochemistry studies support the presence of somatostatin,
cortistatin and somatostatin receptor subtypes in rat Kupfer cells.
Reg Pept. 143:78–82. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
16.
|
Raderer M, Hejna MH, Muller C, Kornek GV,
Kurtaran A, Virgolini I, Fiebieger W, Hamilton G and Scheithauer W:
Treatment of hepatocellular cancer with the long acting
somatostatin analog lanreotide in vitro and in vivo.
Int J Oncol. 16:1197–1201. 2000.PubMed/NCBI
|
|
17.
|
Katsumata N, Chakraborty C, Myal Y,
Schroedter IC, Murphy LJ, Shiu RP and Friesen HG: Molecular cloning
and expression of peptide 23, a growth hormone-releasing
hormone-inducible pituitary protein. Endocrinology. 136:1332–1339.
1995.PubMed/NCBI
|
|
18.
|
Caplin M, Khan K, Savage K, Rode J, Varro
A, Michaeli D, Grimes S, Brett B, Pounder R and Dhillon A:
Expression and processing of gastrin in hepatocellular carcinoma,
fibrolamellar carcinoma and cholangiocarcinoma. J Hepatol.
30:519–526. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
19.
|
Körner M, Hayes GM, Rehmann R, Zimmermann
A, Scolz A, Wiedenmann B, Miller LJ and Reubi JC: Secretin
receptors in the human liver: expression in billiary tract and
cholangiocarcinoma, but not in hepatocytes or hepatocellular
carcinoma. J Hepatol. 45:825–835. 2006.
|
|
20.
|
Christofides ND: Pancreatic glucagons.
Radioimmunoassay of Gut Regulatory Peptides. Bloom SR and Long GL:
WB Saunders Company Ltd; pp. 741982
|
|
21.
|
Thorton JR and Losowsky MS: Methionine
enkephalin is increased in plasma in acute liver disease and is
present in bile and urine. J Hepatol. 8:53–59. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
22.
|
Yudaydin C: The central opioid system in
liver disease and its complications. Metab Brain Dis. 16:79–83.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
23.
|
Davis M: Cholestasis and endogenous
opioids: liver disease and exogenous opioid pharmacokinetics. Clin
Pharmacokinet. 46:825–850. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
24.
|
Hsi KL, Seidah NG, De Serres G and
Chrétien M: Isolation and NH2-terminal sequence of a novel porcine
anterior pituitary poly-peptide. Homology to proinsulin, secretin
and Rous sarcoma virus transforming protein TVFV60. FEBS Lett.
147:261–266. 1982. View Article : Google Scholar
|
|
25.
|
Seidah NG, Hsi KL, De Serres G, Rochemont
J, Hamelin J, Antakly T, Cantin M and Chrétien M: Isolation and
NH2-terminal sequence of a highly conserved human and porcine
pituitary protein belonging to a new superfamily.
Immunocytochemical localization in pars distalis and pars nervosa
of the pituitary and in the supraoptic nucleus of the hypothalamus.
Arch Biochem Biophys. 225:525–534. 1983.
|
|
26.
|
Venetikou MS: 7B2: In vitro and in vivo
studies on a new pituitary protein. PhD thesis. Senate House;
London, UK: 1992
|
|
27.
|
Mbikay M, Seidah NG and Chrétien M:
Neuroendocrine protein 7B2: structure, expression and functions.
Biochem J. 357:329–342. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
28.
|
Natori S, Iguchi H, Nawata H, Kato K,
Ibayashi H and Chretien M: Age-related change in plasma
concentration of 7B2 (a novel pituitary polypeptide) in normal
humans. Life Sci. 41:977–981. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
29.
|
Iguchi H, Natori S, Ou Y, Nawata H, Kato
K, Ibayashi H and Bloom SR: Plasma levels of 7B2 (a novel pituitary
polypeptide) and its molecular forms in plasma and urine in
patients with chronic renal failure: possible degradation by the
kidney. Reg Pept. 21:263–270. 1988. View Article : Google Scholar
|
|
30.
|
Leonhardt UT, Stevenson JC, Ghatei MA,
Lischka A, MacDonald DW, Whitehead MI and Bloom SR: Elevated 7B2
levels during normal human pregnancy. Am J Obstet Gynecol.
158:1141–1144. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
31.
|
Suzuki H, Kobori H, Ohtake R, Hashigami Y,
Suzuki Y, Shimoda Sl and Bloom SR: Circulating concentrations of
immunoreactive peptide 7B2 in certain pathophysiological conditions
and response to oral glucose load. Clin Chem. 34:410–413.
1988.PubMed/NCBI
|
|
32.
|
Suzuki H, Christofides ND, Chretien M,
Seidah NG, Polak JM and Bloom SR: A novel pituitary protein (7B2)
in the rat urogenital tract. Biomed Res. 6:139–143. 1985.
|
|
33.
|
Hunter WH and Greenwood FC: Preparation of
iodine-131 labelled human growth hormone of high specific activity.
Nature. 194:495–496. 1962. View
Article : Google Scholar : PubMed/NCBI
|
|
34.
|
Laurent TC and Killander J: A theory of
gel filtration and its experimental verification. J Chromatogr A.
14:317–330. 1964. View Article : Google Scholar
|
|
35.
|
Kobori H: Immunoreactive 7B2
concentrations in plasma and cerebrospinal fluid in
pathophysiological conditions and the responses to oral glucose
load, intravenous LHRH, TRH and arginine infusion. Nihon Naibunpi
Gakkai Zasshi. 65:1135–1148. 1989.(In Japanese).
|
|
36.
|
Suzuki H, Ghatei MA, Williams SJ,
Uttenthall LO, Facer P, Bishop AE, Polak JM and Bloom SR:
Production of pituitary protein 7B2 immunoreactivity by endocrine
tumours and its possible diagnostic value. J Clin Endocrinol Metab.
63:758–765. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
37.
|
Suzuki H, Christofides ND, Chretien M,
Seidah NG, Polak JM and Bloom SR: Developmental changes in
immunoreactive content of novel pituitary protein 7B2 in human
pancreas and its identification in pancreatic tumours. Diabetes.
36:1276–1279. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
38.
|
Azzoni C, Yu JY, Baggi MT, D’Adda T,
Timson C, Polak JM and Bordi C: Studies on co-localization of 7B2
and pancreatic hormones in normal and tumoural islet cells.
Virchows Arch A Pathol Anat Histopathol. 421:457–466. 1992.
View Article : Google Scholar : PubMed/NCBI
|
|
39.
|
Vieau D, Linard CG, Mbikay M, Lenne F,
Chretien M, Luton JP and Bertagna X: Expression of the
neuroendocrine cell marker 7B2 in human ACTH secreting tumours.
Clin Endocrinol (Oxf). 36:597–603. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
40.
|
Iguchi H, Hara N, Hayashi I, Ohta M, Bloom
SR and Chrétien M: Elevation of a novel pituitary protein (7B2) in
the plasma in small cell carcinoma of the lung. Eur J Cancer Clin
Oncol. 25:1225–1232. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
41.
|
Roebroek AJ, Martens GJ, Duits AJ,
Schalken JA, van Bokhoven A, Wagenaar SS and van de Ven WJ:
Differential expression of the gene encoding the novel pituitary
polypeptide 7B2 in human lung cancer cells. Cancer Res.
49:4154–4158. 1989.PubMed/NCBI
|
|
42.
|
Kapuscinski M, Schulkes A, Read D and
Hardy KJ: Expression of neurotensin in endocrine tumors. J Clin
Endocrinol Metab. 70:100–106. 1990. View Article : Google Scholar
|
|
43.
|
Ito H, Masuda H, Tahara E and Wittekind C:
Carcinoid tumor of the liver. Dtsch Med Wochenschr. 114:623–627.
1989.(In German).
|
|
44.
|
Marzioni M, Fava G and Benedetti A:
Nervous and neuroendocrine regulation of the pathophysiology of
cholestasis and of biliary carcinogenesis. World J Gastroenterol.
12:3471–3480. 2006.PubMed/NCBI
|
|
45.
|
Osamura RY, Chrétien M and Marcinkiewicz
M: Ultrastructural localization of secretory granule constituent
chromogranin and 7B2. Pathol Res Pract. 183:617–619. 1988.
View Article : Google Scholar : PubMed/NCBI
|
|
46.
|
Ozawa H and Takata K: The granin family -
its role in sorting and secretory granule formation. Cell Struct
Funct. 20:415–420. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
47.
|
Qin LX and Tang ZY: The prognostic
molecular markers in hepatocellular carcinoma. World J
Gastroenterol. 8:385–392. 2002.PubMed/NCBI
|
|
48.
|
Geller SA, Dhall D and Alsabeh R:
Application of immunocytochemistry to liver and gastrointestinal
neoplasms: liver, stomach, colon, and pancreas. Arch Pathol Lab
Med. 132:490–499. 2008.PubMed/NCBI
|
|
49.
|
Braks JA and Martens GJ: 7B2 is a
neuroendocrine chaperone that transiently interacts with prohormone
convertase PC2 in the secretory pathway. Cell. 78:263–273. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
50.
|
Shennan KI, Taylor NA and Docherty K:
Calcium- and pH-dependent aggregation and membrane association of
the precursor of the prohormone convertase PC2. J Biol Chem.
269:18646–18650. 1994.PubMed/NCBI
|
|
51.
|
Jan G, Taylor NA, Scougall KT, Docherty K
and Shennan KI: The propeptide of the prohormone convertase PC2
acts as a transferable aggregation and membrane-association signal.
Eur J Biochem. 257:41–46. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
52.
|
Lloyd RV, Jin L, Qian X, Scheithauer BW,
Young WF Jr and Davis DH: Analysis of the chromogranin A
post-translational cleavage product pancreastatin and the
prohormone convertase PC2 and PC3 in normal and neoplastic human
pituitaries. Am J Pathol. 146:1188–1198. 1995.
|
|
53.
|
Takumi I, Steiner DF, Sanno N, Teramoto A
and Osamura RY: Localization of prohormone convertases 1/3 and 2 in
the human pituitary gland and pituitary adenomas: analysis by
immunocytochemistry, immunoelectron microscopy, and laser scanning
microscopy. Mod Pathol. 11:232–238. 1998.
|
|
54.
|
Westphal CH, Muller L, Zhou A, Zhu X,
Bonner-Weir S, Schambelan M, Steiner DF, Lindberg I and Leder P:
The neuroendocrine protein 7B2 is required for peptide hormone
processing in vivo and provides a novel mechanism for pituitary
Cushing’s disease. Cell. 96:689–700. 1999.PubMed/NCBI
|
|
55.
|
Laurent V, Kimble A, Peng B, Zhu P, Pintar
JE, Steiner DF and Lindberg I: Mortality in 7B2 null mice can be
rescued by adrenalectomy. Involvement of dopamine in ACTH
hypersecretion. Proc Natl Acad Sci USA. 99:3087–3092. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
56.
|
Venetikou MS, Burrin JM, Ghatei MA and
Bloom SR: ACTH and 7B2 release by atT-20 cells in vitro: effect of
CRF, VIP, AVP and SRIF during perifusion and static culture. FEBS
J. 275(Suppl): 2622008.
|
|
57.
|
Natori S, Iguchi H, Nawata H, Kato K,
Ibayashi H, Nagasaki H and Chrétien M: Evidence for the release of
a novel pituitary polypeptide (7B2) from the growth
hormone-producing pituitary adenoma of patients with acromegaly. J
Clin Endocrinol Metab. 66:430–437. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
58.
|
Natori S, Iguchi H, Ohasi M, Nakao R,
Bloom SR and Nawata H: Effect of octapeptide analogue (SMS 201–995)
on plasma 7B2 (a neuroendocrine polypeptide) levels in patients
with acromegaly. Clin Endocrinol (Oxf). 32:49–55. 1990.
|
|
59.
|
Sarac MS, Zieske AW and Lindberg I: The
lethal form of Cushing’s in 7B2 null mice is induced by multiple
metabolic and hormonal abnormalities. Endocrinology. 143:2324–2332.
2002.
|
|
60.
|
Seidel B, Dong W, Savaria D, Zheng M,
Pintar JE and Day R: Neuroendocrine protein 7B2 is essential for
proteolytic conversion and activation of proprotein convertase 2 in
vivo. DNA Cell Biol. 17:1017–1029. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
61.
|
Tzimas GN, Chevet E, Jenna S, Nguyên DT,
Khatib AM, Marcus V, Zhang Y, Chrétien M, Seidah NG and Metrakos P:
Abnormal expression and processing of the proprotein convertase PC1
and PC2 in human colorectal liver metastases. BMC Cancer.
17:1492005. View Article : Google Scholar : PubMed/NCBI
|