Introduction
Primary Budd-Chiari syndrome (BCS) is a rare
clinical entity characterized by a blocked hepatic venous outflow
tract at various levels from the small hepatic veins to the
inferior vena cava (IVC) (1,2).
Hepatocellular carcinoma (HCC) is one of the major complications of
BCS (3,4). The incidence of HCC associated with
BCS varies between different case studies. In Korea, France and
Japan, 23/159 (14.5%), 11/97 (11.3%) and 3/12 (25%) BCS patients,
respectively, were reported to exhibit complications as a result of
HCC (4–6).
Resection surgery, systemic chemotherapy, target
therapy with sorafenib, radiotherapy and transcatheter arterial
chemoembolization have been reported as therapeutic modalities for
HCC patients with main portal vein invasion or BCS (3,7,8).
However, the long-term outcome is poor, with a median survival of
2–20 months despite therapeutic treatment (3,7,8).
Percutaneous transluminal angioplasty using balloon catheters for
BCS and transcatheter arterial chemoembolization (TACE) for
unresectable HCC are being increasingly reported as suitable
alternative therapeutic modalities (9,10). Few
studies have investigated the clinical efficacy of combining
percutaneous microwave ablation with angioplasty for patients with
BCS complicated by HCC. The current study presents two cases of BCS
associated with HCC within a 4-month period that were treated with
percutaneous microwave ablation and angioplasty. Written informed
consent was obtained from the patients.
Case report
Patient 1
A 43-year-old male with a history of abdominal wall
veins varices for 15 years and hepatosplenomegaly and abdominal
distension for 3 months was admitted to the Affiliated Hospital of
Xuzhou Medical College (Xuzhou, China). The patient reported no
past or present alcohol consumption or a history of diabetes or
hepatitis. At the time of presentation, the patient’s routine
laboratory test results were as follows: Hemoglobin (Hb), 144 g/l
(normal range, 120–160 g/l); white blood cells, 1.7×109
cells/l (normal range, 4–10×109 cells/l); platelet
count, 27×109 cells/l (normal range,
100–300×109 cells/l); total protein, 78 g/l (normal
range, 60–80 g/l); serum albumin, 40.3 g/l (normal range, 34–55
g/l); serum bilirubin, 30.3 μmol/l (normal range, 0–20
μmol/l); aspartate aminotransferase (AST), 26 U/l (normal
range, 0–40 U/l); alanine aminotransferase (ALT), 25 U/l (normal
range, 0–40 U/l); γ-glutamyl transpeptadase (GGT), 22 U/l (normal
range, 0–40 U/l); and alkaline phosphatase (ALP), 71 U/l (normal
range, 42–128 U/l). The α-fetoprotein (AFP) level was 428 ng/ml
(normal range, 0–20 ng/ml). The results also included negative
hepatitis B and C viral serologies. Imaging studies included color
Doppler ultrasonography, magnetic resonance imaging (MRI), computed
tomography (CT), inferior venacavography and hepatic arteriograms.
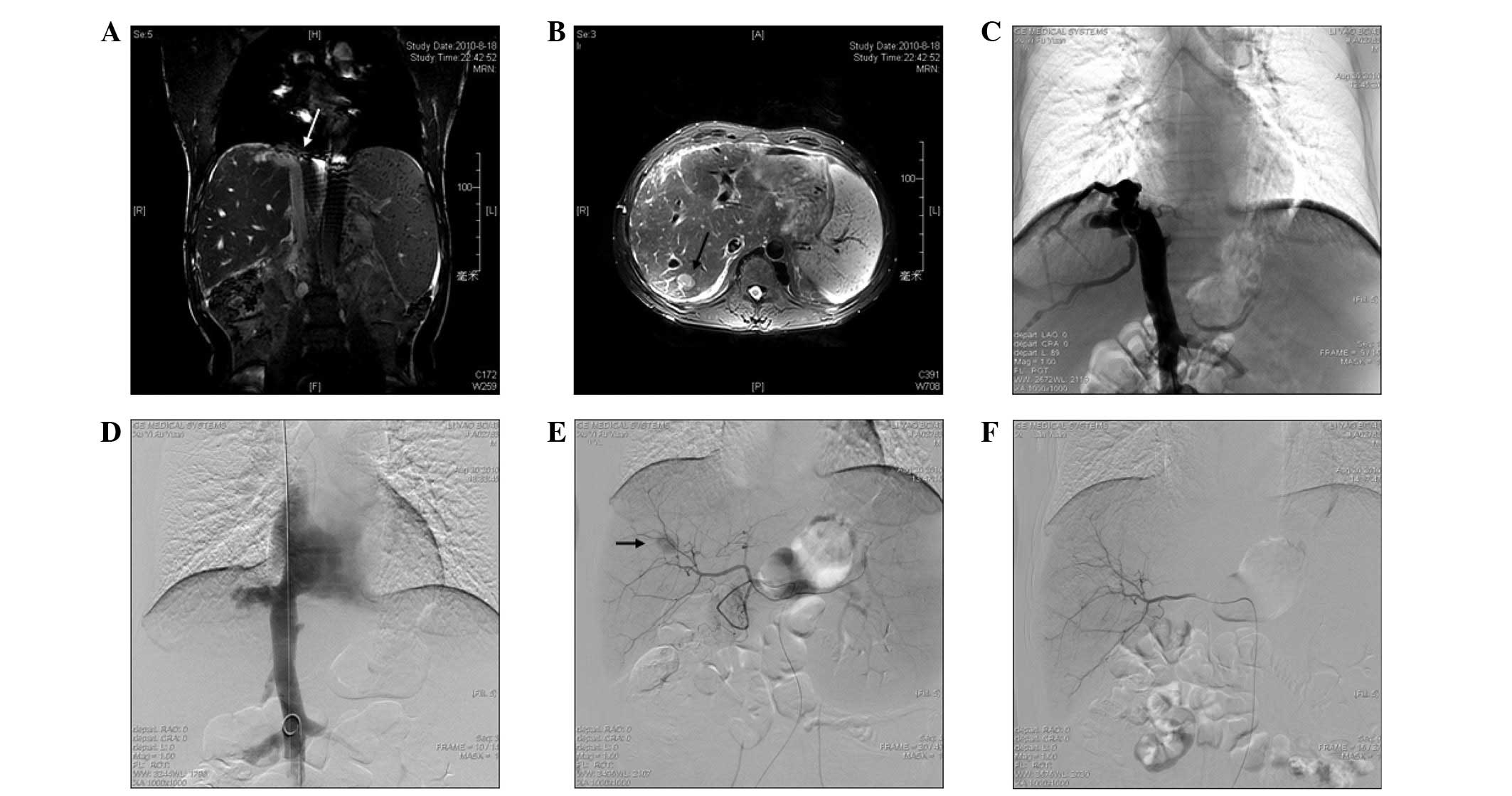
These studies indicated the membranous obstruction of the IVC (BCS)
(Fig. 1A), an enlarged caudate
lobe, patent hepatic and portal veins, azygos and hemiazygos vein
varices and a 2.2×1.7×1.4-cm hypervascular mass, with washout
during the portal venous phase in the superior segment of the right
hepatic lobe, consistent with segment VII (Fig. 1B). The patient was diagnosed with
membranous obstruction of the IVC, associated with HCC due to
elevated levels of AFP and the typical findings on MRI and CT.
Angioplasty was the first procedure to be performed
and informed written consent was obtained prior to this. A 5F
sheath was advanced through a percutaneous right femoral vein, then
a 5F pigtail catheter (Cook, Inc., Bloomington, IN, USA) was
inserted over a 0.035-inch guide wire into the IVC, followed by the
inferior vena cavography to confirm the obstruction (Fig. 1C). The 5F pigtail catheter was
inserted trans-femorally into the distal area of the obstruction as
the marker for positioning and a J-type Brockenbrough needle (Cook,
Inc.) was introduced into the proximal region of the obstruction,
using the right jugular vein to cut through the lesion under the
fluoroscopic guidance in the optimal view. When the obstruction was
broken, the needle was exchanged for a 4F catheter. A 260-cm
ultra-stiff guide wire was inserted through the 4F catheter and the
catheter was withdrawn. Following this, a balloon catheter (28–50
mm; Cordis Corporation, Miami, FL, USA) was inserted to dilate the
IVC obstruction twice. Inferior vena cavography was performed
immediately following dilation and revealed good flow into the
atrium (Fig. 1D).
Although the tumor was resectable, the patient
refused surgical resection and was allowed to proceed with TACE. A
celiac arteriography was initially performed to assess the anatomy,
tumor burden and vascularity (Fig.
1E). Selective catheterization of the segmental branch of the
right hepatic artery, which was feeding the lesion, was then
performed using a 2.9F microcatheter (SP). A mixture of 5 ml
iodized oil (Lipiodol; Laboratoire Guerbet, Aulnay-Sous-Bois,
France) and 10 mg pirarubicin hydrochloride was infused into the
feeding artery, followed by selective arterial embolization using
gelatin sponge particles. Angiography was performed immediately and
revealed that the feeding artery had caused the development of an
emboli (Fig. 1F). One week later at
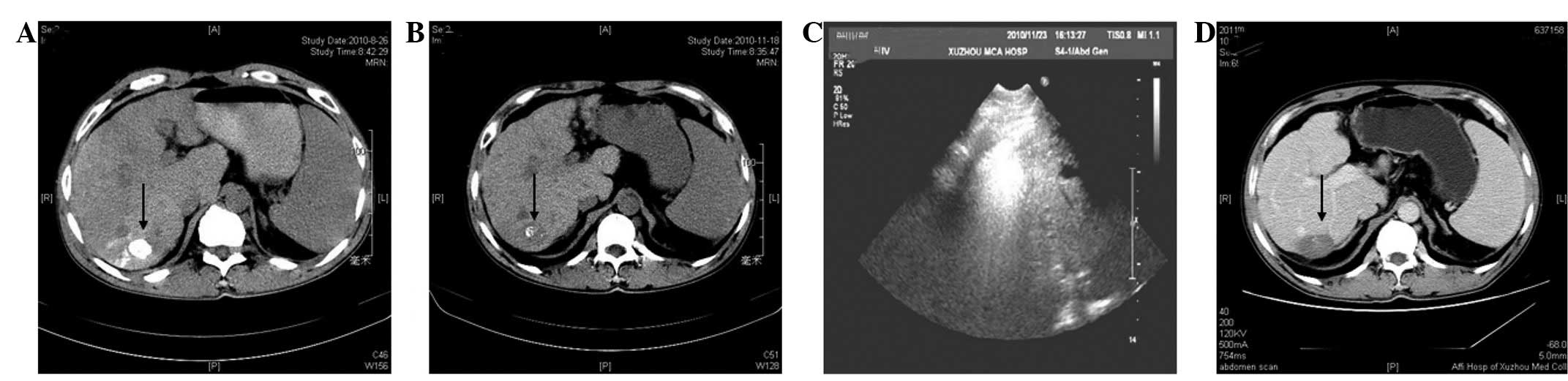
follow-up, a non-contrast CT scan revealed that the complete
iodized oil had been retained inside the tumor (Fig. 2A) and that the serum levels of AFP
had decreased to 26.3 ng/ml. Three months later at follow-up, a
non-contrast hepatic CT scan indicated that the iodized oil deposit
was almost washed out (Fig. 2B) and
that the serum levels of AFP had increased to 112 ng/ml. Further
treatment was required and percutaneous microwave ablation was
scheduled. A KY2000 microwave system with an emission of 915 MHz
(Kangyou Medical Microwave Institute, Nanjing, China) was used on
the patient. The system was equipped with 15-gauge needle
electrodes (diameter, 1.8 mm and length, 20 cm), which were
specifically coated and insulated to prevent tissue adhesion and
had internally cycling water to cool the pole to avoid burning the
skin. Prior to treatment, an appropriate puncture route was
selected for ultrasound. A single antenna was then inserted
percutaneously into the tumor and located at the designated sites
under ultrasound guidance. A power output setting of 60 W for 300
sec was used during the ablations (Fig.
2C). Nine months later, contrast-enhanced CT imaging results
revealed no areas of contrast material enhancement in the lesion
following microwave ablation (Fig.
2D). During 24 months of follow-up, the patient was free of
symptoms; the IVC was patent and the AFP serum levels and liver
function test results were normal.
Patient 2
A 56-year-old male patient with a 12-year history of
vein varices on the abdominal wall and lower extremities, leg
pigmentation for 10 years and leg ulcers for 2 years was admitted
to the Affiliated Hospital of Xuzhou Medical College. The
individual was treated by splenectomy in another hospital due to
hypersplenism 1 month prior to admission, but experienced no
clinical improvement. Seven days prior to admission to the
Affiliated Hospital of Xuzhou Medical College, the patient began to
complain of pain and swelling in the right lower extremity. Upon
admission, the routine laboratory tests results were as follows:
Hb, 125 g/l; white blood cells, 4.97×109 cells/l;
platelet count, 259×109 cells/l; total protein, 70.6
g/l; serum albumin, 30.4 g/l; serum bilirubin, 20.5 μmol/l;
AST, 53 U/l; ALT, 15 U/l; GGT, 95 U/l; and ALP, 176 U/l. The serum
AFP levels were 6.7 ng/ml. Color Doppler ultrasonography and MRI
revealed ascites and BCS (segment obstruction of the IVC and three
hepatic veins, as well as massive thromboses in the IVC),
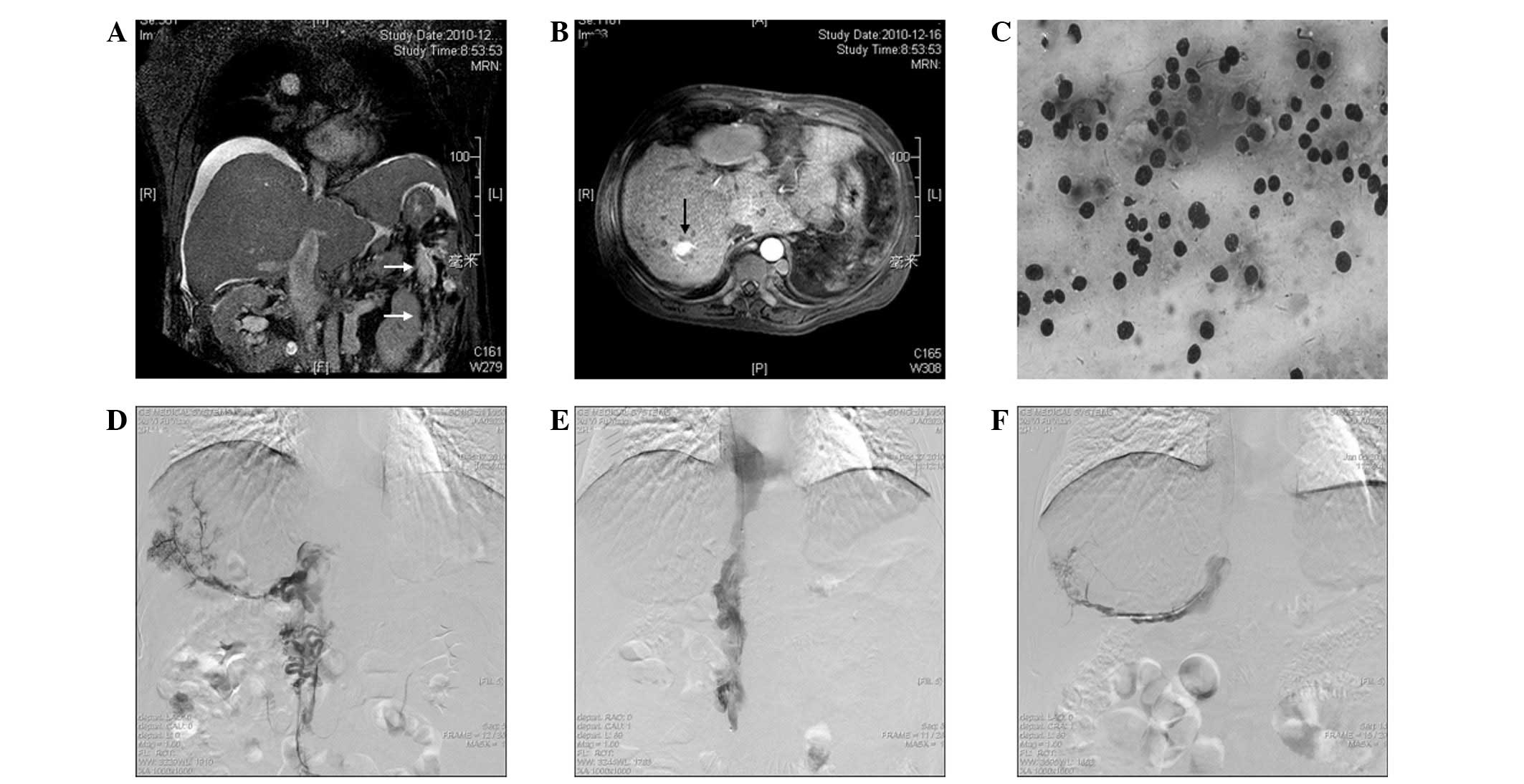
associated with HCC due to elevated levels of AFP (Fig. 3A). Magnetic resonance venography
revealed a 2.3×2.0×1.5-cm well-demarcated and hypervascular tumor
located in segment VII of the liver (Fig. 3B). A biopsy confirmed the diagnosis
of HCC (Fig. 3C).
A thrombolysis catheter was placed into the right
accessory hepatic vein and IVC through the left femoral vein
(Fig. 3D). A bolus of 10 ml mixed
urokinase (10,000 units) was injected every 4 h to dissolve the
thrombus with full-dose heparin. Serial venography revealed gradual
resolution of the clot in the right accessory hepatic vein and IVC,
however, a large amount of thrombus remained in the iliac and
femoral veins. On day 6, a 5F pigtail catheter was inserted into
the distal area of the IVC obstruction through the left femoral
vein as a marker for positioning; a J-type Brockenbrough needle
(Cook, Inc.) was introduced into the proximal section of the IVC
obstruction via the right jugular vein to cut through the lesion
under fluoroscopic guidance. Following the rupture of the IVC
occlusion, a 5F thrombolysis catheter was inserted into the right
femoral vein through the IVC. Thrombolysis was continued over the
course of the next 4 days. Following complete resolution of the
fresh thrombus in the right accessory hepatic vein, IVC and right
iliac and femoral veins, a 25–50-mm balloon catheter (Cook, Inc.)
was inserted and located at the segmental obstruction of the IVC.
The balloon was then dilated to obtain full expansion of the IVC.
Angiography of the IVC and iliofemoral vein was performed
immediately following the procedure. A patent IVC (Fig. 3E) and right iliofemoral vein was
observed following angioplasty. Due to an area of chronic
thrombosis and stenosis, which was observed at the intrahepatic
portion of the IVC and the right accessory hepatic vein,
catheter-directed thrombolysis was continued over the next 9 days.
On day 19, the right accessory hepatic vein was almost clearly
visible upon angiography (Fig. 3F).
The patient received a total dose of 11.4×106 units
urokinase over the 19 days.
As the patient refused to undergo resection or
orthotopic liver transplantation, percutaneous microwave ablation
was performed. The treatment was performed under ultrasound
guidance with the patient under intravenous anesthesia. The
microwave unit used in this case was the same type as in patient 1.
An appropriate puncture route was selected for ultrasound, and
local anesthesia with 1% lidocaine was administered. Next, a single
antenna was percutaneously inserted into the tumor and placed at
designated sites under ultrasound guidance. A power output of 40 W
for 120 sec, 50 W for 420 sec and 60 W for 60 sec was used during
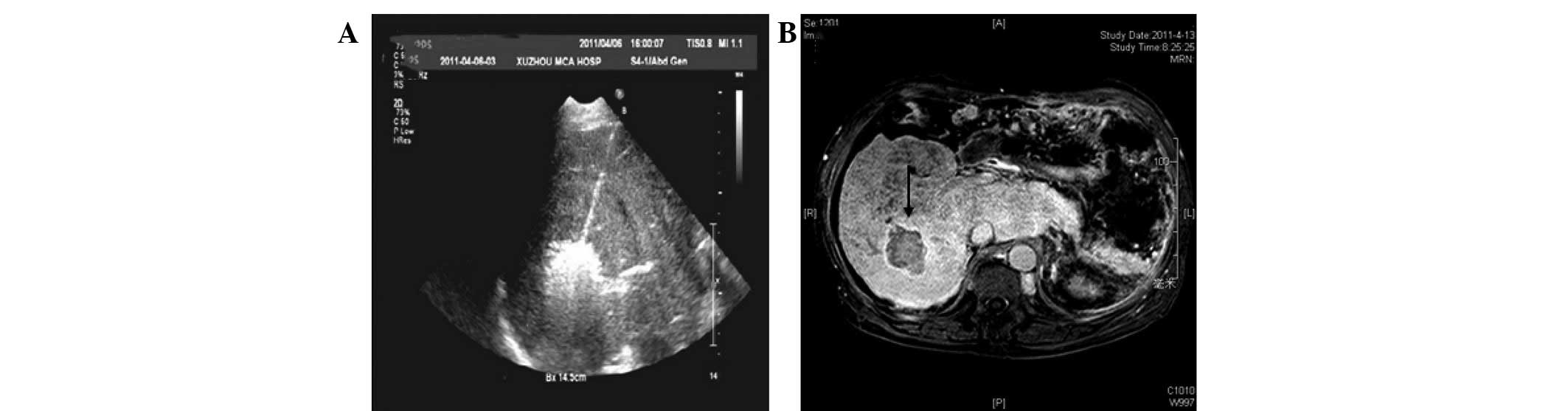
microwave ablation (Fig. 4A). No
major complications occurred during or following the surgery.
Gadolinium-enhanced MRI performed one week after the percutaneous
microwave coagulation therapy revealed a hypointensive area with a
hyperintensive rim and unenhanced area within the treated region
(Fig. 4B). During the 16 months of
follow-up, imaging revealed no recurrence and the patient’s liver
function results were almost normal.
Discussion
The current study presents two cases of primary BCS
complicated by HCC that were successfully treated with percutaneous
transluminal angioplasty and percutaneous microwave ablation,
without any complications. TACE was used to treat the HCC in one
case and catheter-directed thrombolysis was used to treat accessory
hepatic vein, IVC and lower extremity thromboses in the other
case.
There are three main types of BCS: Type I, occlusion
of the IVC; type II, occlusion of the hepatic veins; and type III,
occlusion of the IVC and the hepatic veins. The incidence of HCC
combined with BCS varies between the types of BCS. Type I BCS is
more prone to inducing HCC and the incidence ranges between 10.7
and 43.5% (6,11–14).
In the present case study, the two patients suffered from BCS
combined with HCC. Patient 1 was of type I and patient 2 was of
type III BCS. To date, the underlying mechanisms involved in HCC
induction by BCS remain to be determined. In a previous study,
Shrestha hypothesized that hepatic vena cava disease is an
independent risk factor of HCC (14).
BCS combined with HCC is different from benign
regenerative nodules of the liver in BCS. Brancatelli et al
(15) previously demonstrated that
the diameters of benign regenerative nodules in the liver are
smaller (0.5–4 cm) than HCCs; the quantity of nodules were higher
and they were distributed diffusely in the left and right lobe of
liver, the major lesion density was higher than the ambient normal
hepatic tissues, as indicated by CT plain scans. Large regenerative
nodules were bright on T1-weighted magnetic resonance images and
presented the same enhancement characteristics following
intravenous bolus administration of gadolinium contrast material.
Vilgrain et al (16)
analyzed 23 cases of liver nodules in BCS, as confirmed by
pathohistology. Specifically, 4 cases had an average maximal
diameter of 7.3 cm for HCC lesions and a quantity of 1–3 lesions;
19 cases exhibited benign regenerative nodules with an average
diameter of maximal lesions of 3.3 cm. The quantity of nodules was
high, with >10 in 15 cases. In the present case study, although
the diameters of the liver nodules were <3 cm in the two
patients, all nodules were solitary. The final diagnosis of patient
1 was based on the results of CT, digital subtraction angiography
(DSA) and AFP (>400 ng/mL). Patient 2 was diagnosed with HCC
based on the results of the cytological examination.
Angioplasty is widely accepted as the main treatment
procedure for BCS (9,17). Satisfactory results may be achieved
using thrombolytic therapy for the occlusion of the IVC or the
hepatic veins (18). Balloon
dilation in the IVC was conducted in one case and thrombolysis by
catheter and balloon dilation in the IVC was conducted in the
other. In the two cases, the IVC was unblocked successfully and
complications did not occur.
The therapeutic treatment of BCS combined with HCC
includes TACE and surgery. Gwon et al (5) reported survival rates of 3 and 5 years
in 64 and 50.4% of cases, respectively, when using TACE for the
treatment of BCS combined with HCC. Following the administration of
TACE to patient 1, reduced iodized oil was found in the HCC lesions
during the follow-up. Therefore, percutaneous microwave ablation
was considered to be a suitable treatment. A radical cure may be
achieved in smaller HCCs (≤4 cm) by employing percutaneous
microwave ablation. The 5-year survival rate for this technique has
been shown to be similar to that in patients undergoing surgical
treatment (19, 20). In patient 1 and 2, the average
diameter of the HCC nodules was <3 cm, which was suitable for
percutaneous microwave ablation. No recurrence was identified in
patients 1 and 2 during 24 and 16 months of follow-up,
respectively, indicating the efficacy of this treatment.
In the two present cases, angioplasty was performed
followed by percutaneous microwave ablation; following removal of
the IVC or hepatic vein blockage, liver congestion was relieved and
the absorption of the ascites was facilitated. This protocol
prevents complications, including liver hemorrhage, which may be
caused by percutaneous microwave ablation.
Objectively, the limitations of this protocol were
as follows: i) The number of cases in the present study is low; and
ii) the long-term effects of the protocol remain to be observed.
However, in general, the combination of angioplasty with
percutaneous microwave ablation is likely to represent a safe and
effective method for treating BCS associated with HCC.
References
|
1.
|
Janssen HL, Garcia-Pagan JC, Elias E,
Mentha G, Hadengue A and Valla DC; European Group for the Study of
Vascular Disorders of the Liver: Budd-Chiari syndrome: a review by
an expert panel. J Hepatol. 38:364–371. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
2.
|
Rajani R, Melin T, Björnsson E, et al:
Budd-Chiari syndrome in Sweden: epidemiology, clinical
characteristics and survival - an 18-year experience. Liver Int.
29:253–259. 2009.PubMed/NCBI
|
|
3.
|
Jang JW, Yoon SK, Bae SH, Choi JY, Chung
KW and Sun HS: Rapidly progressing Budd-Chiari syndrome complicated
by hepatocellular carcinoma. Korean J Intern Med. 18:191–195.
2003.PubMed/NCBI
|
|
4.
|
Moucari R, Rautou PE, Cazals-Hatem D, et
al: Hepatocellular carcinoma in Budd-Chiari syndrome:
characteristics and risk factors. Gut. 57:828–835. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Gwon D II, Ko GY, Yoon HK, et al:
Hepatocellular carcinoma associated with membranous obstruction of
the inferior vena cava: incidence, characteristics and risk factors
and clinical efficacy of TACE. Radiology. 254:617–626. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Matsui S, Ichida T, Watanabe M, et al:
Clinical features and etiology of hepatocellular carcinoma arising
in patients with membranous obstruction of the inferior vena cava:
in reference to hepatitis viral infection. J Gastroenterol Hepatol.
15:1205–1211. 2000. View Article : Google Scholar
|
|
7.
|
Kage M: Budd-Chiari syndrome and
hepatocellular carcinoma. J Gastroenterol. 39:706–707. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Worns MA, Weinmann A, Pfingst K, et al:
Safety and efficacy of sorafenib in patients with advanced
hepatocellular carcinoma in consideration of concomitant stage of
liver cirrhosis. J Clin Gastroenterol. 43:489–495. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Xu K, Feng B, Zhong H, et al: Clinical
application of interventional techniques in the treatment of
Budd-Chiari syndrome. Chin Med J (Engl). 116:609–615.
2003.PubMed/NCBI
|
|
10.
|
Chen CY, Li CW, Kuo YT, et al: Early
response of hepatocellular carcinoma to transcatheter arterial
chemoembolization: choline levels and MR diffusion constants -
initial experience. Radiology. 239:448–456. 2006. View Article : Google Scholar
|
|
11.
|
Kew MC, McKnight A, Hodkinson J, Bukofzer
S and Esser JD: The role of membranous obstruction of the inferior
vena cava in the etiology of hepatocellular carcinoma in Southern
African blacks. Hepatology. 9:121–125. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Orloff MJ, Isenberg JI, Wheeler HO, Daily
PO and Girard B: Budd-Chiari syndrome revisited: 38 years’
experience with surgical portal decompression. J Gastrointest Surg.
16:286–300. 2012.PubMed/NCBI
|
|
13.
|
Shrestha SM, Okuda K, Uchida T, et al:
Endemicity and clinical picture of liver disease due to obstruction
of the hepatic portion of the inferior vena cava in Nepal. J
Gastroenterol Hepatol. 11:170–179. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Shrestha SM: Liver cirrhosis and
hepatocellular carcinoma in hepatic vena cava disease, a liver
disease caused by obstruction of inferior vena cava. Hepatol Int.
3:392–402. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Brancatelli G, Federle MP, Grazioli L,
Golfieri R and Lencioni R: Benign regenerative nodules in
Budd-Chiari syndrome and other vascular disorders of the liver:
radiologic-pathologic and clinical correlation. Radiographics.
22:847–862. 2002. View Article : Google Scholar
|
|
16.
|
Vilgrain V, Lewin M, Vons C, et al:
Hepatic nodules in Budd-Chiari syndrome: imaging features.
Radiology. 210:443–450. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Wu T, Wang L, Xiao Q, et al: Percutaneous
balloon angioplasty of inferior vena cava in Budd-Chiari
syndrome-R1. Int J Cardiol. 83:175–178. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
18.
|
Ding PX, Li YD, Han XW and Wu G: Agitation
thrombolysis for fresh iatrogenic IVC thrombosis in patients with
Budd-Chiari syndrome. J Vasc Surg. 52:782–784. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19.
|
Bruix J, Hessheimer AJ, Forner A, Boix L,
Vilana R and Llovet JM: New aspects of diagnosis and therapy of
hepatocellular carcinoma. Oncogene. 25:3848–3856. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
20.
|
Liang P and Wang Y: Microwave ablation of
hepatocellular carcinoma. Oncology. 72(Suppl 1): 124–131. 2007.
View Article : Google Scholar
|