Introduction
Specific mutations in lung cancer appear to be
restricted to specific histologically-defined phenotypes. For
example, mutations of tyrosine kinase signaling pathway genes,
including EGFR(1–3), ALK(4), RET(5,6) and
erbB2(7), are common in
adenocarcinomas, whereas mutations of the nuclear factor erythroid
2-related 2 (NEF2L2; also known as NRF2) gene are
characteristic of squamous cell carcinoma (8–10).
Under homeostatic conditions, Nrf2 is principally
repressed by Kelch-like ECH-associated protein 1 (Keap1), which
functions as an intracellular redox sensor, targeting Nrf2 for
proteasomal degradation. Under oxidant or xenobiotic stress, Keap1
releases Nrf2, which translocates to the nucleus and activates
antioxidant response elements and xenobiotic element genes,
resulting in the protein expression of growth factors and
receptors, drug-metabolizing enzymes and various transcription
factors (11–13). The Keap1 gene mutation has
been previously identified in 3–5% of non-small cell lung cancer
(NSCLC) cases (8,14,15),
however, the correlation between the mutation status and
clinicopathological features was not well defined. We have
previously described NEF2L2 mutation cases (9), and in the present study, the
Keap1 mutation status in 76 surgically-treated NSCLC cases
was investigated.
Patients and methods
Patients
The current study is retrospective and included data
from 76 lung cancer patients who had undergone surgery at the
Department of Surgery, Nagoya City University Hospital (Nagoya,
China). All tumor samples were immediately frozen and stored at
−80°C until assayed. The clinical and pathological characteristics
of the 76 lung cancer patients were as follows; 44 cases were at
stage I, 11 at stage II and 21 at stages III–IV. The mean patient
age was 66.1 years (range, 39–88 years). Among the 76 lung cancer
patients, 46 (60.5%) were diagnosed with adenocarcinoma and 27
(35.5%) suffered from squamous cell carcinoma. The study was
approved by the ethics board of the Nagoya City University Graduate
School of Medicinal Sciences (Nagoya, Chūbu, Japan) and written
consent was obtained from all patients.
PCR for Keap1
Total RNA was extracted from lung cancer tissues
using the Isogen kit (Nippon Gene Co., Ltd., Tokyo, Japan),
according to the manufacturer’s instructions. The RNA concentration
was determined by spectrophotometer and adjusted to a concentration
of 200 ng/ml. In 10 cases, the samples were excluded as the number
of tumor cells was too low to sufficiently extract tumor RNA. The
RNA (1 μg) was reverse transcribed using Superscript II enzyme
(Gibco-BRL, Carlsbad, CA, USA) with 0.5 μg
oligo(dT)12–16 (Amersham Pharmacia Biotech Inc.,
Piscataway, NJ, USA). The reaction mixture was incubated at 42°C
for 50 min and then at 72°C for 15 min. Following this, 1 μl DNA
was used for the PCR analyses. PCR was performed using the LA-Taq
kit (Takara Bio, Inc., Shiga, Japan) in a 25-μl reaction volume.
The primer sequences for the Keap1 gene kinase domain (exon
2–5) were as follows: forward, 5-AACGGTGCTGTCATGTACCA-3 and
reverse, 5-CGCTCTGGCTCATACCTCTC-3 (872 bp). The cycling conditions
were an initial denaturation at 94°C for 5 min, followed by 35
cycles at 94°C for 40 sec, 60°C for 40 sec and 72°C for 55 sec. The
products were purified by the Qiagen PCR purification kit (Qiagen,
Valencia, CA, USA). Amplified cDNAs were separated on 1% agarose
gels and the bands were visualized by ethidium bromide. Images were
captured under ultraviolet transillumination. These samples were
sequenced using the ABI prism 3100 analyzer (Applied Biosystems
Japan Ltd., Tokyo, Japan) and analyzed by BLAST. Chromatograms were
checked by manual review from forward to reverse.
The EGFR, erbB2 and Kras sequencing
methods have previously been described (1,3,7,16).
Results
Keap1 gene mutation status in Japanese
lung cancer patients
Of the 76 patients, 19 (25.0%) had EGFR
mutations within the kinase domain, including 8 exon 19 deletions,
10 L858R and 1 G719S. In addition, 3 patients had Kras
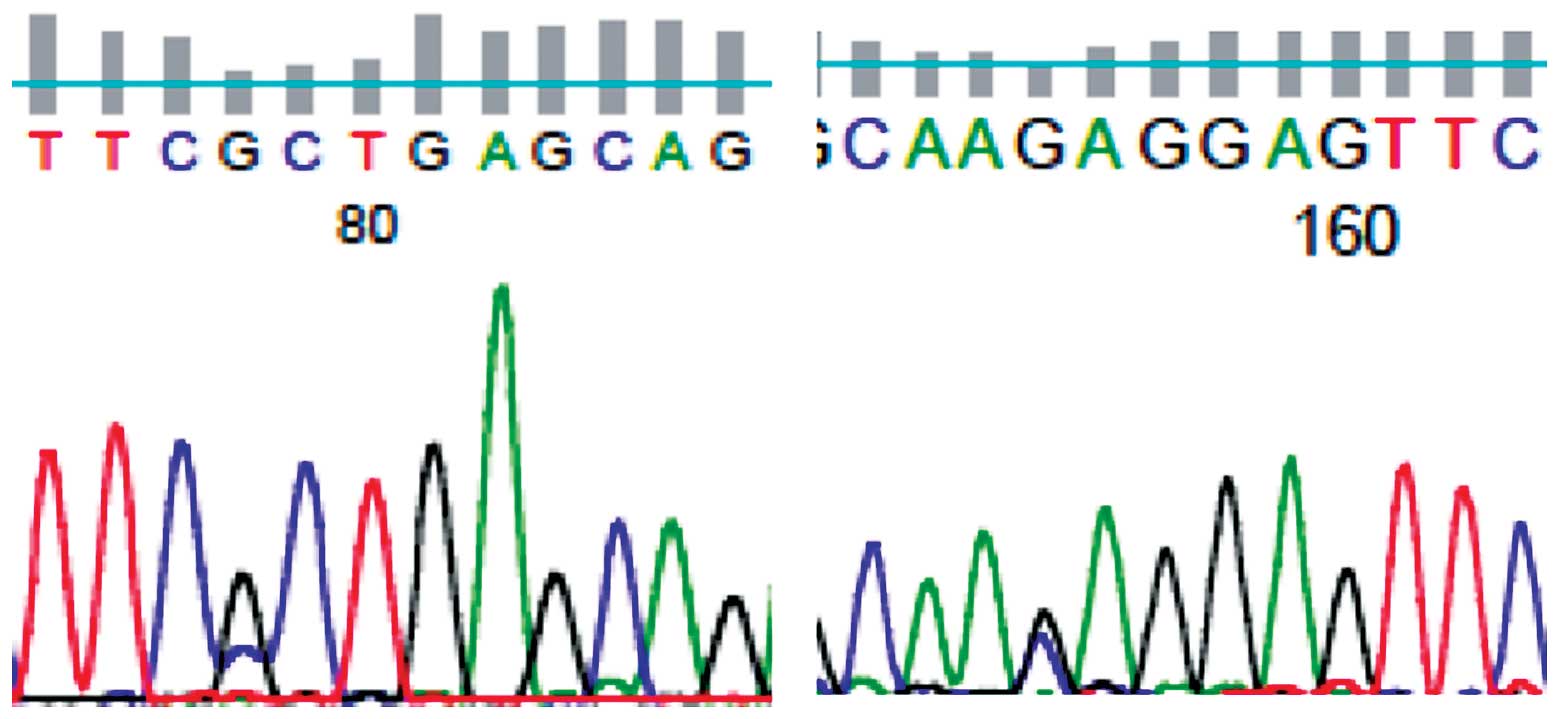
mutations at codons 12 or 13. The Keap1 mutation was
identified in 2/76 (2.6%) NSCLC patients (Fig. 1); 1 A191P (571 G to C, alanine to
proline; stage IIIa) and 1 E218Q (652 G to T, glutamate to
glutamine; stage IIb). The two patients were male, had a history of
smoking and suffered from adenocarcinoma.
Within these NSCLC cases, the EGFR, Kras,
erbB2 and NRF2 mutations existed exclusively. The
survival of the patients with or without the Keap1 mutations
was not shown to be significantly different (log-rank test,
P=0.2919).
Discussion
In the current study, two Keap1 mutations
were identified in 76 Japanese NSCLC patients. The Keap1
mutation was exclusively identified without EGFR, erbB2 or
NRF2 mutations. Keap1 mutations were predominantly
identified in patients with a history of heavy smoking and advanced
adenocarcinoma. This population was also hypothesized to exhibit a
lower incidence of EGFR gene mutations (1–3).
The Keap1 gene is a negative regulator of the
cell adaptive response to radical oxidant species and xenobiotics,
which is mediated by the NRF2 transcription factor. More recently,
a role has emerged for NRF2 in cancer and a number of studies have
identified that NRF2 constitutive upregulation is associated with
cancer development and progression (17–19).
High levels of nuclear NRF2 facilitate cancer cell growth and
survival as a result of the transactivation of cytoprotective genes
(17–19). Thus, studies on the deregulation of
the KEAP1/NRF2 pathway have enhanced our understanding of the
molecular mechanisms associated with cancer. We have previously
reported that mutations of NRF2 (NFE2L2) were
identified in squamous carcinoma cases (9), which was consistent with results shown
by additional studies (8,10). In NSCLC, the overexpression of
nuclear NRF2 is principally attributable to genetic and epigenetic
alterations and the loss of function of its receptor, Keap1
(11,17,20). A
previous study demonstrated that low or absent Keap1 expression is
common in NSCLC (56%), largely in adenocarcinomas (8). However, the authors identified only
one Keap1 mutation (exon 2–5) in 31 of the tumors examined,
including 20 with nuclear NRF2 expression, indicating that the
Keap1 mutation is not the main mechanism of protein loss or
reduction. These observations are inconsistent with previous
studies reporting Keap1 mutations in 8 and 19% of two NSCLC
cohorts, predominantly with adenocarcinomas (11,17).
Keap1 mutations are associated with a poor
prognosis in individuals with NSCLC (14). In addition, low or absent Keap1
expression is associated with a poor outcome (8). A number of studies have demonstrated
that nuclear NRF2 activation promotes cell survival in malignant
cells (17,18,21)
and may explain the shorter survival of NSCLC. The inactivation of
putative tumor suppressor genes affects the growth and progression
of tumors. As a mutation of Keap1 is uncommon, its mechanism
in NSCLC remains unknown and may be associated with other
Keap1-binding proteins that have antiapoptotic and proliferative
functions, including prothymosin a (22).
In the present study, Keap1 mutations were
only identified in patients with adenocarcinoma, but not squamous
cell carcinomas. The results indicated that Keap1 mutations
in Japanese individuals with NSCLC are not common, with observed
frequencies demonstrated to be even lower compared with our
previous in vitro analysis in lung cancer cell lines (50%)
(11). Present observations
revealed that a mutation of the Keap1 gene as a mechanism of
tumorigenesis is unlikely to be associated with the majority of
Japanese NSCLC cases. However, the completely exclusive
EGFR, NRF2 and Kras mutation statuses are
likely to be useful for the development of patient-specific therapy
for NSCLC. Further studies are required to confirm the mechanisms
of Keap1 mutations to determine the sensitivity or
resistance of therapy for lung cancer.
Acknowledgements
The present study was supported by Grants-in-Aid for
Scientific Research, Japan Society for the Promotion of Science
(nos. 23659674, 21390394 and 21591820) and a Grant for Cancer
Research of Programs for Developing the Supporting System for
Upgrading Education and Research (2009) from the Ministry of
Education, Culture, Sports, Science and Technology of Japan. The
authors would like to thank Akiha Kuramoto and Miki Mochizuki for
their technical assistance.
References
|
1
|
Paez JG, Janne PA, Lee JC, et al: EGFR
mutations in lung cancer: correlation with clinical response to
gefitinib therapy. Science. 304:1497–1500. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Lynch TJ, Bell DW, Sordella R, et al:
Activating mutations in the epidermal growth factor receptor
underlying responsiveness of non-small-cell lung cancer to
gefitinib. New Eng J Med. 350:2129–2139. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Sasaki H, Endo H, Konishi A, et al: EGFR
mutation status in Japanese lung cancer patients: genotyping
analysis using LightCycler. Clin Cancer Res. 11:2924–2929. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Soda H, Choi YL, Enomoto M, et al:
Identification of the transforming EML4-ALK fusion gene in
non-small-cell lung cancer. Nature. 448:561–566. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Lipson D, Capelletti M, Yelensky R, et al:
Massively parallel sequencing assay identifies novel ALK and RET
gene fusions from colorectal and lung cancer biopsies. Nat Med.
18:382–384. 2012. View
Article : Google Scholar : PubMed/NCBI
|
|
6
|
Yokota K, Sasaki H, Okuda K, et al:
KIF5B/RET fusion gene in surgically treated adenocarcinoma of the
lung. Oncol Rep. 28:1187–1192. 2012.PubMed/NCBI
|
|
7
|
Sasaki H, Shimizu S, Endo K, et al: EGFR
and erbB2 mutation status in Japanese lung cancer patients. Int J
Cancer. 118:180–184. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Solis LM, Behrens C, Dong W, et al: Nrf2
and Keap1 abnormalities in non-small cell lung carcinoma and
association with clinicopathologic features. Clin Cancer Res.
16:3743–3753. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Sasaki H, Hikosaka Y, Okuda K, et al:
NFE2L2 gene mutation in male Japanese squamous cell carcinoma of
the lung. J Thorac Oncol. 5:786–789. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Shibata T, Ohta T, Tong KI, et al: Cancer
related mutations in Nrf2 impair its recognition by Keap1-Clu3 E3
ligase and promote malignancy. Proc Natl Acad Sci USA.
105:13568–13573. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Singh A, Misra V, Thimmulappa RK, et al:
Dysfunction KEAP1-NRF2 interaction in non-small-cell lung cancer.
PloS Med. 3:e4202006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hayes JD and McMahon M: NRF2 and KEAP1
mutations: permanent activation of an adaptive response in cancer.
Trends Biochem Sci. 34:176–188. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Thimmulappa RK, Mai KH, Srisuma S, et al:
Identification of Nrf2- regulated genes induced by the
chemopreventive agent sulforaphane by oligonucleotide microarray.
Cancer Res. 62:5196–5203. 2002.
|
|
14
|
Takahashi T, Sonobe M, Menju T, et al:
Mutations in Keap1 are a potential prognostic factor in resected
non-small cell lung cancer. J Surg Oncol. 101:500–506.
2010.PubMed/NCBI
|
|
15
|
Too NJ, Kim HR, Kim YR, An CH and Lee SH:
Somatic mutations of the KEAP1 gene in common solid cancers.
Histopathology. 60:943–952. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Sasaki H, Hikosaka Y, Kawano O, et al:
Evaluation of Kras mutation and copy number gain in non-small cell
lung cancer. J Thorac Oncol. 6:15–20. 2011. View Article : Google Scholar
|
|
17
|
Ohta T, Iijima K, Miyamoto M, et al: Loss
of Keap1 function activates Nrf2 and provides advantages for lung
cancer cell growth. Cancer Res. 68:1303–1309. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Singh A, Boldin-Adamsky S, Thimmukaooa RK,
et al: RNAi-mediated silencing of nuclear factor
erythroid-2-related factor 2 gene expression in non-small cell lung
cancer inhibits tumor growth and increases efficacy of
chemotherapy. Cancer Res. 68:7975–7984. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Cho JM, Manandhar S, Lee HR, Park HM and
Kwak MK: Role of the Nrf2-antioxidant system in cytotoxicity
mediated by anticancer cisplatin: implication to cancer cell
resistance. Cancer Lett. 260:96–108. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Padmanabhan B, Tong KI, Ohta T, et al:
Structural basis for detects of Keap1 activity provoked by its
point mutations in lung cancer. Mol Cell. 21:689–700. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Shibata T, Kokubu A, Gotoh M, et al:
Genetic alteration of Keap1 confers constitutive Nrf2 activation
and resistance to chemotherapy in gallbladder cancer.
Gastroenterokogy. 135:1358–1368. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Sasaki H, Nonaka M, Fujii Y, et al:
Expression of the prothymosin-a gene as a prognostic factor in lung
cancer. Surg Today. 31:936–938. 2001. View Article : Google Scholar : PubMed/NCBI
|