Introduction
Hepatocellular carcinoma (HCC) is one of the most
serious complications of liver cirrhosis and the third most lethal
cancer worldwide (1). HCC is
particularly frequent in Asia due to a high prevalence of chronic
hepatitis B virus (HBV) infection (2). Radical hepatic resection remains the
only potentially curative treatment for early-stage HCC (3,4).
However, until recently, the results have been disappointing
(5,6). Preventing the post-operative
recurrence and improving the long-term survival in HCC patients
following a hepatectomy are issues that require urgent
investigation.
Octreotide is an octapeptide that pharmacologically
mimics natural somatostatin and has displayed regulatory or
suppressive effects against various tumors (7). Somatostatin is believed to act via
somatostatin receptors (SSTRs) that are expressed by responsive
tumors. In particular, octreotide has a high affinity to SSTR
subtypes 2 and 5. The expression of SSTRs in HCC tissue has been
identified (8,9). In addition, the results of two studies
using SSTR scintigraphy suggest that 40–50% of HCC cases express
SSTRs in vitro and in vivo(10,11).
This suggests that octreotide may be a valuable and promising
approach for HCC treatment.
However, studies have been performed to verify the
anti-tumor effects of octreotide long-acting release (LAR)
treatment on advanced HCC, providing controversial results.
Following the publication of the preliminary study by Kouroumalis
et al(14), three randomized
placebo-controlled studies have reported a significant survival
benefit for long-acting octreotide compared with no treatment in
patients with advanced HCC (11,15,16).
Nevertheless, in the other five trials, no survival benefit was
observed compared with the placebo for long-acting octreotide
(12,13,17–19).
In none of the aforementioned studies was an accurate estimation of
SSTR gene expression in the tumors investigated, knowledge of which
is critical if the therapeutic effects of somatostatin analogues
(SSAs) are to be exploited.
In an attempt to obtain sufficient material for the
pathological diagnosis and gene expression analysis, the present
study was performed in patients with resectable early-stage HCC.
The present study aimed to evaluate SSTR2 and 5 mRNA expression in
a large number of surgically removed HCC tissues by quantifying
specific PCR products with an accurate quantitative (q)PCR method.
Furthermore, SSTR2 and 5 mRNA expression was also quantified in
paired liver cirrhosis tissues to evaluate the expression of SSTR2
and 5 in tumor and cirrhosis tissues. The possibility of using SSTR
expression as an efficacy predictor in HCC patients that are
treated with octreotide LAR was further tested.
Patients and methods
Patients and samples
Tissue specimens of HCC and cirrhotic liver were
randomly obtained from 99 patients who underwent curative liver
resection at the Department of Hepatobiliary Surgery (Beijing Ditan
Hospital, Capital Medical University, Beijing, China) between 2001
and 2004. HCC with underlying HBV-related cirrhosis was diagnosed
by histological examination. No other treatment was administered
prior to the surgery. Immediately after the hepatic resection for
HCC, the tumor and surrounding cirrhotic liver tissue samples (≥1
cm away from the edge of tumor) were harvested and placed in liquid
nitrogen until the RNA extraction procedure. The tissues that were
used in the immunohistochemical studies were obtained from the same
patients. The patient group consisted of 75 males and 24 females
(average age, 58.2 years; range, 28–83 years). The
clinicopathological records of these patients included gender
ratio, age, location, maximum tumor size, serum α-fetoprotein
level, serum HBV DNA level, tumor differentiation and staging.
Tumor differentiation was defined according to the Edmondson
grading system (20). Tumor staging
was defined according to the 7th edition of the Union for
International Cancer Control (UICC) TNM staging system (Table I) (21). Prior to starting the study, ethical
approval was obtained from the ethical committee of Beijing Ditan
Hospital. Informed consent was obtained from each patient who
underwent octreotide treatment.
 | Table ISeventh edition UICC TNM stage of HCC
(2009). |
Table I
Seventh edition UICC TNM stage of HCC
(2009).
| Stage | Tumor | Node | Metastasis |
|---|
| I | T1 | N0 | M0 |
| II | T2 | N0 | M0 |
| IIIA | T3a | N0 | M0 |
| IIIB | T3b | N0 | M0 |
| IIIC | T4 | N0 | M0 |
| IVA | Any T | N1 | M0 |
| IVB | Any T | Any N | M1 |
Adjuvant therapy and follow-up
At one day post-surgery, all the patients were
administered 20 mg octreotide LAR (Sandostatin LAR;
Novartis-Pharma, Beijing, China) through a deep intramuscular
injection every 4 weeks. The planned duration of treatment was 12
months in the absence of disease progression and unacceptable
toxicity. All patients were observed prospectively for
post-operative recurrence with assessment using serum α-fetoprotein
levels, chest x-rays and ultrasonography or computed tomography 1
month after the operation and every 3 months thereafter. The
patients with recurrence were administered a multimodality therapy,
including a second liver resection, radiofrequency ablation or
transarterial chemoembolization (TACE). The treatment decision was
based on the pattern of recurrence and liver function reserve. Full
follow-up data was recorded for all the patients until July 15,
2011.
RNA extraction and cDNA synthesis
Total RNA was isolated from the tumor and paired
cirrhotic tissues using TRIzol reagent (Invitrogen, Carlsbad, CA,
USA). The concentration and purity of the RNA samples were
determined using spectrophotometry (BioPhotometer; Eppendorf,
Hamburg, Germany) and quantified by measuring the absorbance at OD
260. RNA quality was assessed by agarose gel electrophoresis.
Following this, the RNA from each sample was reverse-transcribed to
cDNA using the SuperScript III First-Strand Synthesis System for
RT-PCR (Invitrogen) according to the manufacturer’s
instructions.
Synthesis of primers
Using the Primer 3 Analysis Software, version 1.1.0
(simgene.com, Whitehead Institute for Biomedical Research,
Cambridge, MA, USA), the primer sequences were selected to
optimally hybridize and amplify the target cDNA for the qPCR assay.
To avoid amplifying the contaminating genomic DNA, the primers were
designed so that each PCR product covered at least one intron. The
target gene primers sequences used were as follows: SSTR2 forward,
5′-GTC CTC TGC TTG GTC AAG GTG-3′ and reverse, 5′-TGG TCT CAT TCA
GCC GGG ATT-3′; and SSTR5 forward, 5′-GCC TGG GTC CTG TCT CTG TG-3′
and reverse, 5′-TAC CGC CCT CCT GCA CGT-3′. A housekeeping gene was
used as an internal inference to avoid errors arising from the
various efficiencies of the amplification. The primer sequences for
GAPDH were forward, 5′-AGC CAC ATC GCT CAG ACA C-3′ and reverse,
5′-GCC CAA TAC GAC CAA ATC C-3′.
qPCR
qPCR was performed in an ABI Prism 7500 Sequence
Detection System (Applied Biosystems, Foster City, CA, USA). The
reactions were performed in a 20-μl mixture containing 10 μl SYBR
Green PCR Master Mix, 1 μl cDNA Template, 0.5 μl forward primer,
0.5 μl reverse primer and 8 μl ddH2O. The PCR thermal
profile was 2 min at 50ºC followed by 10 min at 95ºC and 40
amplification cycles at 95ºC for 15 sec and 61ºC for 60 sec. The
expression of the target genes was normalized using GADPH as an
endogenous control and a sample number of 1,001 as a calibrator to
correct for differences in the amount of total RNA added to each
reaction. The cycle threshold (Ct) values were analyzed using the
SDS 1.4 software (ABI Prism 7500, SDS User Bulletin; Applied
Biosystems) and the relative mRNA levels were calculated using the
comparative Ct method. The relative quantification (RQ) values were
used to compare the gene expression levels between the various
samples. Each sample was amplified in triplicate to obtain an
average Ct value. A reaction without the cDNA templates was used as
a negative control.
Histopathological studies
Immunohistochemical evaluations were performed using
the standard procedure. The specimens were fixed in 10%
neutral-buffered formalin for at least 12 h, embedded in paraffin,
sectioned (4-μm thick) and processed for immunohistochemistry. For
SSTR2 and 5 expression, the section was incubated with anti-SSTR2
and -SSTR5 rabbit polyclonal antibodies (ab9550 and ab28618,
respectively; Abcam Inc., Cambridge, MA, USA) with a dilution of
1:100 and 1:200, respectively. The negative control slides were
treated with PBS solution as the first antibody under equivalent
conditions. For the secondary developing reagents, a labeled
streptavidin-biotin kit (Shenzhen Jingmei Biology Engineering Co.
Ltd., Shenzhen, China) was used. The slides were developed using
diaminobenzaminidine and counterstained with hematoxylin. A
proportion score (PS) was assigned using the following system: 0,
≤10%; 1, 11–25%; 2, 26–50% and 3, >50%; and a staining intensity
score (IS) was assigned as: 0, none; 1+, weak, 2+, intermediate and
3+, strong. A final total score (TS) was obtained from the sum of
the PS and IS in at least three slides for each enrolled subject
(22).
Statistical analysis
The SPSS 15.0 program (SPSS, Inc., Chicago, IL, USA)
was used for the statistical analyses. The results are expressed as
the mean ± SD. The independent t-test was used to compare the
relative gene copy number between the cirrhosis and HCC specimens.
The χ2 test for the categorical outcome was used to
compare the clinicopathological differences between the defined
groups. The Pearson correlation coefficient was calculated to test
the associations between the continuous variables. The Kaplan-Meier
product-limit method was used to generate survival curves and the
differences between the cohorts were tested using log-rank
statistics. Cox’s proportional hazards regression model was used to
analyze the independent prognostic factors. P<0.05 was
considered to indicate a statistically significant difference. In
the present exploratory study, no adjustments were made for
multiple comparisons.
Results
Relative SSTR2 and 5 mRNA levels in HCC
and paired cirrhosis
mRNA from SSTR2 and 5 was detected in all the HCC
and surrounding cirrhotic liver tissues. qPCR analysis revealed
that SSTR 2 and 5 were expressed at a lower level in the HCC
tissues (1.76±0.92; 95% CI, 1.54–1.99; and 5.36±1.70; 95% CI,
4.94–5.77, respectively) compared with the paired surrounding
cirrhotic liver tissues (3.46±1.45; 95% CI, 3.10–3.81; and
7.25±3.77; 95% CI, 6.32–8.18, respectively; t-value of HCC and
cirrhotic tissues, 8.00 and 3.72, respectively; P<0.05).
Expression of SSTR2 and 5 proteins in HCC
and paired cirrhosis
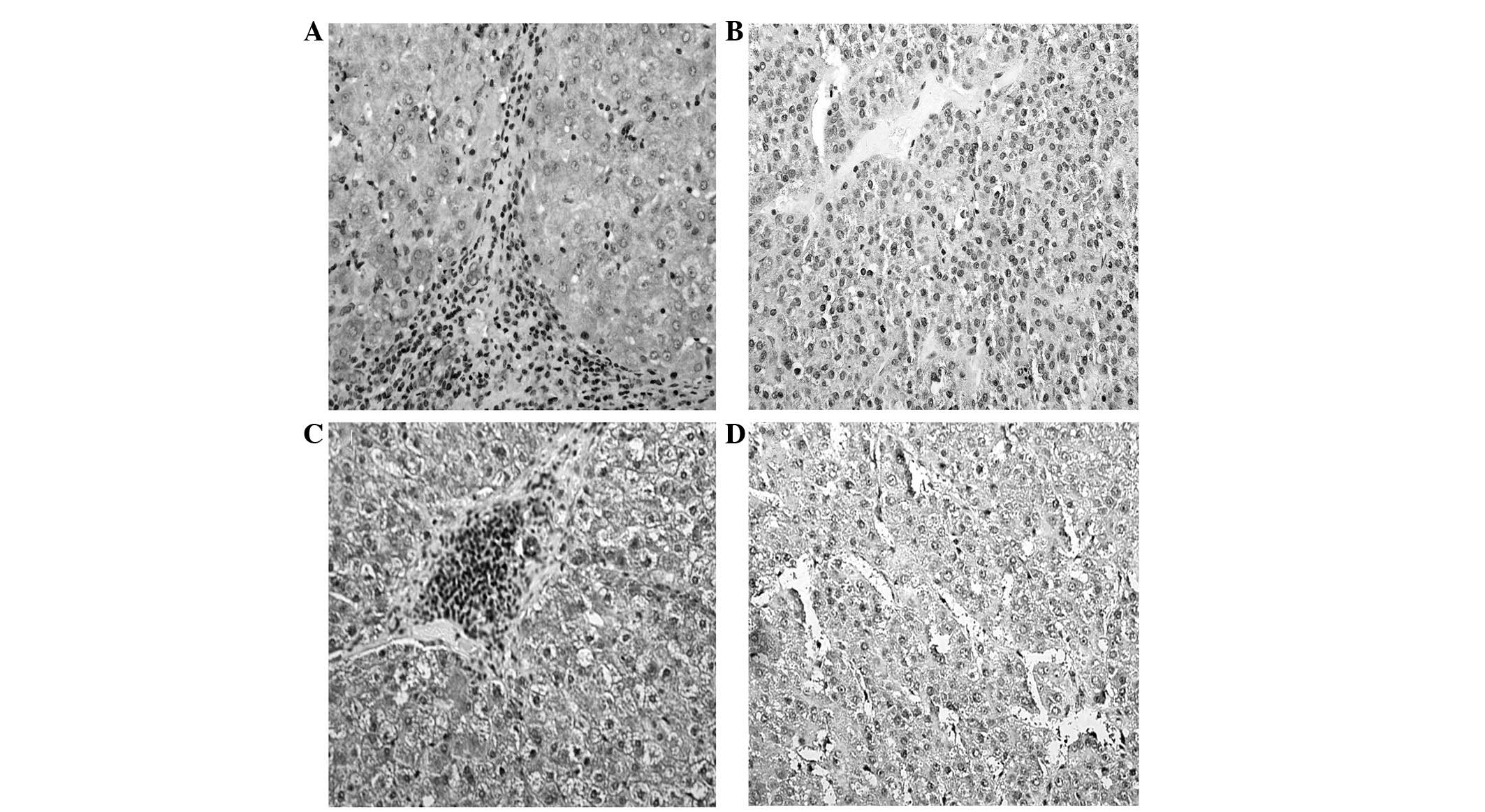
An IHC analysis was performed in order to locate
SSTR2 and 5 protein expression in HCC. Positive immunostaining for
SSTR2 and 5 was localized primarily to the cell membrane and
cytoplasm of the cirrhotic liver cells and the tumor cells
(Fig. 1). SSTR2 expression in the
tumor tissue was lower than in the surrounding cirrhotic liver
tissues at a positive rate of 59.6% (59 of 99 cases) vs. 90.9% (90
of 99 cases; χ2, 26.06; P <0.001). SSTR5 was the most
commonly expressed receptor subtype, with a positive expression
rate in the HCC tissue of 95.5%, identical to that of the paired
cirrhotic tissue. Furthermore, in the HCC samples, SSTR2 expression
detected by qPCR and immunohistochemistry showed a strong
correlation (r=0.312; P=0.002), as did SSTR5 expression (r=0.384;
P=0.001).
Baseline characteristics of the low and
high expression groups
As shown previously, the mean SSTR2 expression in
the HCC samples was 1.76 relative copies. The tumor samples that
expressed SSTR2 above this cutoff were defined as having high SSTR2
expression and those that expressed SSTR2 below this level were
defined as having low expression. Likewise, a cutoff point for
SSTR5 was defined at 5.36 relative copies based upon the mean SSTR5
expression in the HCC tissues. When these defined strata were
applied to the series of 99 HCC specimens, two groups were
categorized based on the co-expression of SSTR2 and 5. A total of
52 (53%) patients had tumors with low SSTR2 and 5 expression and 47
(47%) had tumors with high SSTR 2 and/or 5 expression. Table II shows the baseline
characteristics of the two groups. No differences in the clinical
characteristics, including age, gender, histological grade and
stage, were observed between the groups.
 | Table IIDescriptive characteristics of the low
and high expression groups. |
Table II
Descriptive characteristics of the low
and high expression groups.
| Group, n (%) | | |
|---|
|
| | |
|---|
| Characteristics | Low, (n=52) | High, (n=47) | χ2 | P-value |
|---|
| Gender | | | 0.277 | 0.599 |
| Male | 39 (75) | 36 (77) | | |
| Female | 13 (25) | 11 (23) | | |
| Age, years | | | 0.015 | 0.903 |
| <55 | 13 (25) | 15 (32) | | |
| ≥55 | 39 (75) | 32 (68) | | |
| Child-Pugh
grade | | | 0.020 | 0.886 |
| Grade A | 15 (29) | 18 (38) | | |
| Grade B | 37 (71) | 29 (62) | | |
| AFP(ng/ml) | | | 0.379 | 0.538 |
| ≥400 | 15 (29) | 12 (26) | | |
| <400 | 37 (61) | 35 (74) | | |
|
HBV-DNA(copies/ml) | | | 0.218 | 0.640 |
| ≥500 | 30 (58) | 26 (55) | | |
| <500 | 22 (42) | 21 (45) | | |
| Location | | | 0.707 | 0.400 |
| Left Lobe | 22 (42) | 14 (30) | | |
| Right Lobe | 30 (58) | 33 (70) | | |
| Tumor size
(cm) | | | 0.054 | 0.817 |
| ≤3 | 36 (69) | 29 (62) | | |
| >3 | 16 (31) | 18 (38) | | |
| Microvascular
invasion | | | 0.379 | 0.538 |
| Present | 15 (29) | 12 (26) | | |
| Absent | 37 (71) | 35 (74) | | |
| Edmondson-Steiner
grade | | | 0.244 | 0.621 |
| I–II | 19 (37) | 17 (36) | | |
| III–IV | 33 (63) | 30 (64) | | |
| TNM-7 stage | | | 0.347 | 0.556 |
| I | 31 (60) | 30 (64) | | |
| II | 21 (40) | 17 (36) | | |
Prognostic roles
The drug was well tolerated, as 97 (98%) of 99
patients were administered LAR octreotide for the full 12 months
(13 doses). A total of 10 patients from the low expression group
and 17 from the high expression group did not experience recurrence
during follow-up. With the exception of three patients who
survived, the remaining 24 patients succumbed; the non-tumorous
causes of mortality were liver failure (9 patients), heart disease
(4 patients), cerebral vascular accident (4 patients), car accident
(3 patients) and miscellaneous (4 patients). The overall survival
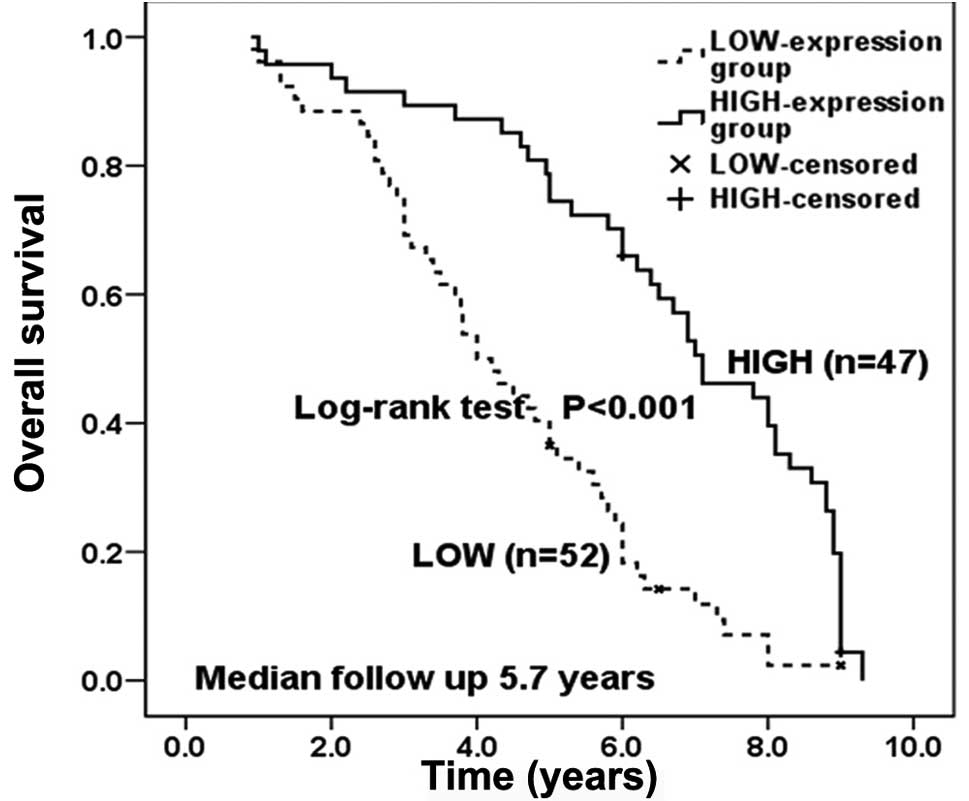
times were significantly longer in the high expression group than
the low expression group (Fig. 2).
The median overall survival was 7.1 years (95% CI, 5.9–8.3) in the
high expression group and 4.0 years (95% CI, 3.3–4.7) in the low
expression group. The cumulative 1-, 3-, and 5-year overall
survival rates were 98, 89 and 74%, respectively, for the high
expression group and 96, 69 and 36%, respectively, for the low
expression group.
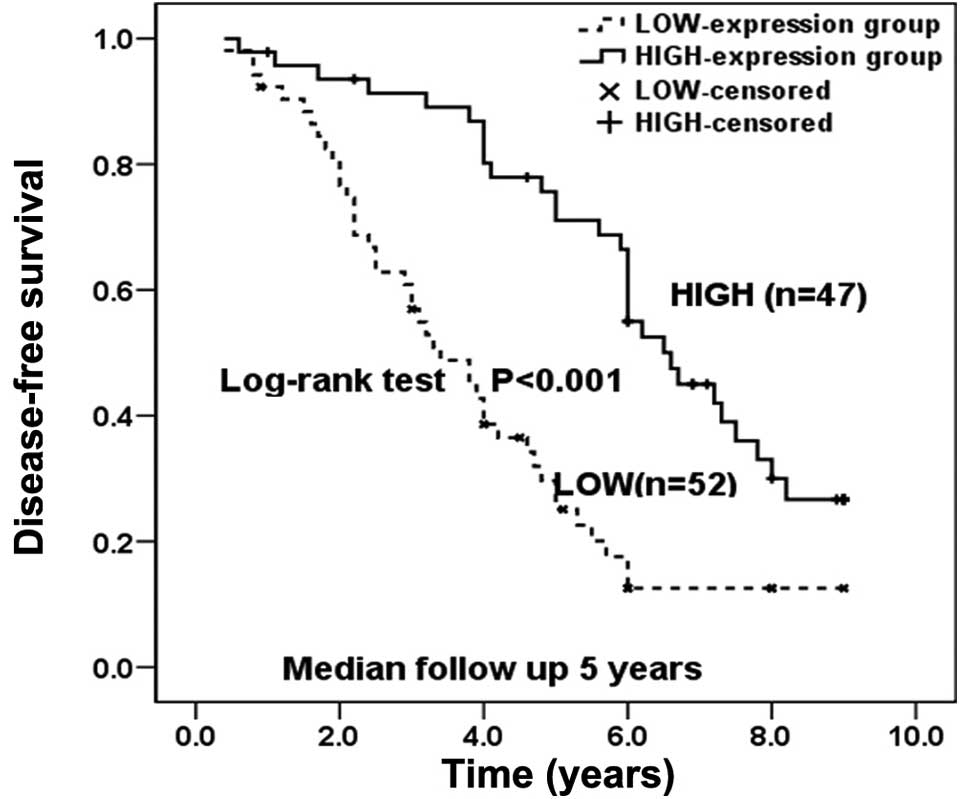
The disease-free survival results were also
significantly improved in the high expression group compared with
the low expression group (Fig. 3).
The median disease-free survival was 6.6 years (95%CI, 5.2–8.0) in
the high expression group and 3.4 years (95% CI, 2.5–4.3) in the
low expression group. The cumulative 1-, 3-, and 5-year
disease-free survival rates were 97, 89 and 71%, respectively, for
the high expression group and 90, 55 and 25%, respectively, for the
low expression group. The overall incidence of tumor recurrence was
significantly lower in the high expression group than in the low
expression group (63.83 vs. 82.69%; χ2=4.54;
P=0.033).
To evaluate the potential of using SSTR expression
in determining the post-operative prognosis of HCC patients, a
multivariate analysis was conducted using a Cox proportional hazard
regression model. All the clinicopathological characteristics and
the SSTR2 and 5 mRNA levels were included in the model. The Cox
multivariate analysis revealed that SSTR2 expression levels may be
used as an independent prognostic marker for operable and TNM-7
stage HCC (Tables III and
IV).
 | Table IIIUnivariate and multivariate analysis
of overall survival of patients with HCC. |
Table III
Univariate and multivariate analysis
of overall survival of patients with HCC.
| Univariate
analysis | Multivariate
analysis |
|---|
|
|
|
|---|
| Variable | P-value | RR | 95% CI | P-value | RR | 95% CI |
|---|
| Gender (male vs
female) | 0.407 | 0.808 | 0.487–1.338 | | | |
| Age, years (≥55 vs.
<55) | 0.831 | 0.957 | 0.636–1.438 | | | |
| Child-Pugh grade (A
vs. B) | 0.976 | 0.993 | 0.644–1.532 | | | |
| AFP, ng/ml (>400
vs. <400) | 0.952 | 0.988 | 0.657–1.485 | | | |
| Location (left vs.
right) | 0.270 | 0.789 | 0.518–1.202 | | | |
| Tumor size, cm
(>3 vs. ≤3) | 0.238 | 1.285 | 0.847–1.951 | | | |
| HBV-DNA, copies/ml
(≥500 vs. <500) | 0.002 | 1.936 | 1.265–2.962 | 0.321 | 1.271 | 0.792–2.040 |
| Microvascular
invasion (present vs. absent) | 0.000 | 2.410 | 1.539–3.773 | 0.094 | 1.506 | 0.933–2.431 |
|
Edmondson-Steiner-grade (I–II vs.
III–IV) | 0.002 | 1.986 | 1.287–3.064 | 0.282 | 1.328 | 0.792–2.226 |
| TNM-7 stage (I vs.
II) | 0.000 | 2.579 | 1.692–3.932 | 0.002 | 2.303 | 1.499–3.539 |
| SSTR-2
expression | 0.000 | 0.472 | 0.372–0.598 | 0.000 | 0.531 | 0.372–0.758 |
| SSTR-5
expression | 0.000 | 0.754 | 0.663–0.857 | 0.682 | 0.965 | 0.814–1.144 |
 | Table IVUnivariate and multivariate analysis
of disease-free survival for patients with HCC. |
Table IV
Univariate and multivariate analysis
of disease-free survival for patients with HCC.
| Univariate
analysis | Multivariate
analysis |
|---|
|
|
|
|---|
| Variable | P-value | RR | 95% CI | P-value | RR | 95% CI |
|---|
| Gender (male vs.
female) | 0.488 | 0.817 | 0.462–1.446 | | | |
| Age, years (≥55 vs.
<55) | 0.270 | 1.299 | 0.816–2.070 | | | |
| Child-Pugh grade (A
vs. B) | 0.737 | 0.920 | 0.566–1.496 | | | |
| AFP, ng/ml (>400
vs. <400) | 0.879 | 1.037 | 0.651–1.652 | | | |
| Location (left vs.
right) | 0.294 | 0.776 | 0.482–1.247 | | | |
| Tumor size, cm
(>3 vs ≤3) | 0.321 | 1.268 | 0.793–2.025 | | | |
| HBV-DNA, copies/ml
(≥500 vs. <500) | 0.007 | 1.920 | 1.198–3.076 | 0.131 | 1.494 | 0.888–2.514 |
| Microvascular
invasion (present vs. absent) | 0.006 | 1.972 | 1.216–3.196 | 0.816 | 1.065 | 0.626–1.812 |
|
Edmondson-Steiner-grade (I–II vs.
III–IV) | 0.030 | 1.691 | 1.053–2.715 | 0.953 | 1.017 | 0.581–1.779 |
| TNM-7 stage (I vs.
II) | 0.000 | 3.263 | 2.011–5.297 | 0.000 | 3.158 | 1.858–5.367 |
| SSTR-2
expression | 0.000 | 0.475 | 0.362–0.624 | 0.004 | 0.542 | 0.359–0.818 |
| SSTR-5
expression | 0.000 | 0.708 | 0.607–0.827 | 0.318 | 0.902 | 0.737–1.105 |
Discussion
The expression of SSTRs in tumor cells allows new
possibilities for the treatment and diagnosis of patients with HCC.
Five subtypes of SSTRs, SSTR1–5, have been cloned and belong to a
distinct group within the super family of G-protein-coupled
receptors with seven transmembrane regions (23). As measured by autoradiography, 41%
of HCCs have been shown to be SSTR-positive, predominantly subtype
2, which is consistent with the study by Bläker’s et
al(9,11). In the study, HCCs displayed
differential, individual expression patterns, as well as variable
SSTR expression levels. The overall expression rate of SSTR1, 2, 3,
4, and 5 was 46, 41, 64, 0 and 75%, respectively. SSTR occupancy
represents the basis for in vivo tumor targeting, and is a
significant consideration in determining the clinical efficacy of
somatostatin therapy.
Pharmacological studies have already shown that SSA
octreotide acts mainly via two SSTRs (SSTR2 and 5) expressed on
responsive tumors (24). In the
present study, qPCR was used to identify the differential SSTR
expression profiles between HCC and the surrounding non-tumorous
cirrhotic tissues. The present data revealed a wide range of SSTR2
and 5 expression in the tumor and cirrhosis samples. However,
downregulation was noted in the HCC specimens. Similarly, Reynaert
et al were able to demonstrate the presence of SSTRs in the
majority of HCC and adjacent cirrhotic liver tissues using the PCR
technique (8). In another study,
Xie et al also identified that ~60% of HCCs expressed SSTRs,
as well as the non-tumor cirrhotic liver tissues (25).
In the present study, the HCC specimens had a 1.95-
and 1.35-fold reduction in SSTR2 and 5 mRNA levels, respectively,
as compared with the adjacent cirrhotic liver tissues. Similar to
this observation, Reynaert et al also identified that in two
of six patients, the surrounding cirrhotic liver tissues expressed
SSTR5 mRNA more clearly than the tumors of these patients. As they
did not use a qPCR method, they were not able to draw firm
conclusions with regard to the variation in mRNA expression
(8). This observation corresponded
with the findings made in pancreatic and colorectal cancer studies
(26,31,32).
In contrast to normal tissue or benign lesions, there is a loss of
SSTR2 gene expression in pancreatic carcinoma and advanced
colorectal cancer and their respective metastases (26,31–33).
SSTR2 expression was selectively lost in 90% of the human
pancreatic carcinomas and derived pancreatic cell lines.
Reintroducing SSTR2 in human pancreatic cancer cells by stable
expression resulted in a constitutive activation of SSTR2 and an
inhibition of cell growth in the absence of an exogenous ligand.
These effects resulted from an increased expression and secretion
of the somatostatin ligand, thus leading to a negative autocrine
loop. The negative feedback loop may also exist in liver cancer.
Additionally, insulin-like growth factor-1 (IGF-1), which is
produced by hepatocytes as an endocrine hormone, has been shown to
play a pathogenic role in cancer, and octreotide has been shown to
negatively control serum IGF-1 levels, possibly via SSTR2 and
SSTR5, and a direct downregulation of IGF gene expression (35). Apoptosis has also been shown to be
induced by SSTR2 in human pancreatic cancer cells expressing
mutated p53 that were devoid of endogenous SSTR2, following the
correction of the deficit by the expression of SSTR2 (36). The absence of SSTR2 and SSTR5 may
explain the lack of local response to octreotide therapy in certain
advanced liver cancers.
In the normal liver, hepatocytes and HSCs have been
shown to be negative for all five SSTRs (8). During the preneoplastic stage leading
to HCC, SSTR gene expression levels in liver tissues appear to be
altered regularly (9,34). SSTR expression levels increase with
the progress of disease conditions, i.e. from normal liver tissues,
hepatitis, liver cirrhosis and reaching a peak in the precancerous
lesions (25). Once HCC has
developed, transcription of the SSTR gene does not increase
further, supporting its major involvement in the preneoplastic
stage. Downregulation of SSTR transcription may result in a loss of
a tumor suppressive effect of SSTRs in human HCC.
Notably, the results of the present study were not
compatible with the investigation performed by Xie et
al(25), in which an
overexpression of SSTRs was identified in HCC. The main reason for
this was that in the study by Xie et al, the controlled
cirrhotic liver and HCC tissues were not obtained from the same HCC
patient, but from other patients with non-tumor liver cirrhosis.
Furthermore, the present study also analyzed SSTR expression in
non-tumor cirrhotic liver tissues from 10 cirrhotic patients with
chronic hepatitis B and obtained the same results as Xie et
al (data not shown).
Octreotide is an octapeptide that mimics natural
somatostatin pharmacologically and possesses potent antineoplastic
activity in several human cancers (7). The use of octreotide as a monotherapy
has been observed to prolong survival in patients with unresectable
HCC (11,14–16).
Furthermore, in an animal study, nude mice were administered with
octreotide for 35 days following a surgical removal of human HCC
xenografts. Compared with the control group, octreotide at doses of
100 and 200 μg/kg/day significantly inhibited the growth rate of
second primary tumors, decreased lung metastasis and prolonged the
life span (27). Although other
controlled trials have reported no survival benefit for long-acting
octreotide compared with placebos (12,13,17–19),
certain researchers believe it is possible that octreotide LAR may
benefit a subgroup of patients whose tumors express high levels of
SSTRs (19).
In the present study, to verify this hypothesis, the
patients with HCC were divided into two defined groups according to
the SSTR2 and 5 expression levels. The two groups were compared for
clinicopathological data and survival results. The statistical
analysis revealed that the patients in the two groups were of
similar age and had a similar tumor morphology distribution. This
observation corresponds to the findings observed by Bläker et
al(9), where the expression of
the SSTRs appeared to be independent of tumor stage and/or
differentiation, which was also similar to the findings in
gastrointestinal and pancreatic endocrine tumors (28).
The present study showed a significant difference in
the survival rates between the low and high expression groups. The
overall and disease-free survival outcomes were significantly
improved in the high expression group compared with the low
expression group. The multivariate model also revealed that SSTR2
expression levels may be used as an independent prognostic marker
of HCC, as well as tumor TNM-7 stage. Similar results have been
reported in other types of cancers, including neuroblastomas
(29) and breast (30), pancreatic and colorectal cancers
(31,32). The overexpression of SSTR2 has also
displayed anti-tumor effects and significantly increased the
sensitivity of SSTR treatment in a number of experimental
SSTR-negative cancer cell lines and xenografts (33,34).
Although this analysis requires validation from larger prospective
series, SSTR gene expression levels, particularly those for SSTR2,
appear to be viable molecular markers to appropriately select HCC
patients for post-operative octreotide LAR therapy.
To the best of our knowledge, the present study is
the first to investigate the association of SSTR gene expression
levels with the long-term prognosis of patients with early-stage
HCC undergoing post-operative octreotide LAR treatment. The
downregulation of SSTR transcription may result in the loss of
tumor suppression. SSTR mRNA expression was shown to correlate with
survival in patients with early-stage HBV-related HCC who were
treated with octreotide LAR. The inhibitory effects of SSAs on
tumor growth may be mediated by SSTR expression.
Acknowledgements
The authors would like to thank Dr Beibei Wang and
Dr Guoli Li (Institute of Infectious Diseases, Capital Medical
University) for their assistance with the experiments.
References
|
1
|
Bosch FX, Ribes J, Cléries R and Díaz M:
Epidemiology of hepatocellular carcinoma. Clin Liver Dis.
9:191–211. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Yuen MF, Hou JL and Chutaputti A; Asia
Pacific Working Party on Prevention of Hepatocellular Carcinoma.
Hepatocellular carcinoma in the Asia pacific region. J
Gastroenterol Hepatol. 24:346–353. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
El-Serag HB, Siegel AB, Davila JA, et al:
Treatment and outcomes of treating of hepatocellular carcinoma
among Medicare recipients in the United States: a population-based
study. J Hepatol. 44:158–166. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Yeh CN, Chen MF, Lee WC and Jeng LB:
Prognostic factors of hepatic resection for hepatocellular
carcinoma with cirrhosis: univariate and multivariate analysis. J
Surg Oncol. 81:195–202. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Nagasue N, Ono T, Yamanoi A, et al:
Prognostic factors and survival after hepatic resection for
hepatocellular carcinoma without cirrhosis. Br J Surg. 88:515–522.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Izumi N: Prediction and prevention of
intrahepatic recurrence of hepatocellular carcinoma. Hepatol Res.
42:226–232. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Susini C and Buscail L: Rationale for the
use of somatostatin analogs as antitumor agents. Ann Oncol.
17:1733–1742. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Reynaert H, Rombouts K, Vandermonde A, et
al: Expression of somatostatin receptors in normal and cirrhotic
human liver and in hepatocellular carcinoma. Gut. 53:1180–1189.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Bläker M, Schmitz M, Gocht A, et al:
Differential expression of somatostatin receptor subtypes in
hepatocellular carcinomas. J Hepatol. 41:112–118. 2004.PubMed/NCBI
|
|
10
|
Reubi JC, Zimmermann A, Jonas S, et al:
Regulatory peptide receptors in human hepatocellular carcinomas.
Gut. 45:766–774. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Dimitroulopoulos D, Xinopoulos D,
Tsamakidis K, et al: Long acting octreotide in the treatment of
advanced hepatocellular cancer and overexpression of somatostatin
receptors: randomized placebo-controlled trial. World J
Gastroenterol. 13:3164–3170. 2007.
|
|
12
|
Cebon J, Findlay M, Hargreaves C, et al;
Australasian Gastro-Intestinal Trials Group (AGITG) Ag0001H
Investigators. Somatostatin receptor expression, tumour response,
and quality of life in patients with advanced hepatocellular
carcinoma treated with long-acting octreotide. Br J Cancer.
95:853–861. 2006. View Article : Google Scholar
|
|
13
|
Borbath I and Horsmans Y: The results of a
randomized study on the use of long-acting octreotide in
hepatocellular carcinoma. Hepatology. 37:477–478. 2003.PubMed/NCBI
|
|
14
|
Kouroumalis E, Skordilis P, Thermos K,
Vasilaki A, Moschandrea J and Manousos ON: Treatment of
hepatocellular carcinoma with octreotide: a randomised controlled
study. Gut. 42:442–447. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Dimitroulopoulos D, Xinopoulos D,
Tsamakidis K, et al: The role of sandostatin LAR in treating
patients with advanced hepatocellular cancer.
Hepatogastroenterology. 49:1245–1250. 2002.PubMed/NCBI
|
|
16
|
Samonakis DN, Moschandreas J, Arnaoutis T,
et al: Treatment of hepatocellular carcinoma with long acting
somatostatin analogues. Oncol Rep. 9:903–907. 2002.PubMed/NCBI
|
|
17
|
Yuen MF, Poon RT, Lai CL, et al: A
randomized placebo-controlled study of long-acting octreotide for
the treatment of advanced hepatocellular carcinoma. Hepatology.
36:687–691. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Becker G, Allgaier HP, Olschewski M, et
al; HECTOR Study Group. Long-acting octreotide versus placebo for
treatment of advanced HCC: a randomized controlled double-blind
study. Hepatology. 45:9–15. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Barbare JC, Bouché O, Bonnetain F, et al:
Treatment of advanced hepatocellular carcinoma with long-acting
octreotide: a phase III multicentre, randomised, double blind
placebo-controlled study. Eur J Cancer. 45:1788–1797. 2009.
View Article : Google Scholar
|
|
20
|
Edmondson HA and Steiner PE: Primary
carcinoma of the liver: a study of 100 cases among 48,900
necropsies. Cancer. 7:462–503. 1954. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Sobin LH and Compton CC: TNM seventh
edition: what’s new, what’s changed: communication from the
International Union Against Cancer and the American Joint Committee
on Cancer. Cancer. 116:5336–5339. 2010.
|
|
22
|
Fasciani A, Quilici P, Biscaldi E, et al:
Overexpression and functional relevance of somatostatin receptor-1,
-2, and -5 in endometrium and endometriotic lesions. J Clin
Endocrinol Metab. 95:5315–5319. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Raulf F, Pérez J, Hoyer D and Bruns C:
Differential expression of five somatostatin receptor subtypes,
SSTR1–5, in the CNS and peripheral tissue. Digestion. 55(Suppl 3):
46–53. 1994.
|
|
24
|
Pawlikowski M and Melen-Mucha G:
Perspectives of new potential therapeutic applications of
somatostatin analogs. Neuro Endocrinol Lett. 24:21–27.
2003.PubMed/NCBI
|
|
25
|
Xie YM, Yan LN, Wei B, Guo MM and Tang CW:
Correlation of somatostatin receptor expression in human
hepatocellular carcinoma tissue to serum alpha-fetoprotein
concentration. Ai Zheng. 26:688–692. 2007.(In Chinese).
|
|
26
|
Li M, Li W, Kim HJ, Yao Q, Chen C and
Fisher WE: Characterization of somatostatin receptor expression in
human pancreatic cancer using real-time RT-PCR. J Surg Res.
119:130–137. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Jia WD, Xu GL, Wang W, et al: A
somatostatin analogue, octreotide, inhibits the occurrence of
second primary tumors and lung metastasis after resection of
hepatocellular carcinoma in mice. Tohoku J Exp Med. 218:155–160.
2009. View Article : Google Scholar
|
|
28
|
Papotti M, Bongiovanni M, Volante M, et
al: Expression of somatostatin receptor types 1–5 in 81 cases of
gastrointestinal and pancreatic endocrine tumors. A correlative
immunohistochemical and reverse-transcriptase polymerase chain
reaction analysis. Virchows Arch. 440:461–475. 2002.
|
|
29
|
Sestini R, Orlando C, Peri A, et al:
Quantitation of somatostatin receptor type 2 gene expression in
neuroblastoma cell lines and primary tumors using competitive
reverse transcription-polymerase chain reaction. Clin Cancer Res.
2:1757–1765. 1996.
|
|
30
|
Orlando C, Raggi CC, Bianchi S, et al:
Measurement of somatostatin receptor subtype 2 mRNA in breast
cancer and corresponding normal tissue. Endocr Relat Cancer.
11:323–332. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Buscail L, Saint-Laurent N, Chastre E, et
al: Loss of sst2 somatostatin receptor gene expression in human
pancreatic and colorectal cancer. Cancer Research. 56:1823–1827.
1996.PubMed/NCBI
|
|
32
|
Casini Raggi C, Calabrò A, Renzi D, et al:
Quantitative evaluation of somatostatin receptor subtype 2
expression in sporadic colorectal tumor and in the corresponding
normal mucosa. Clin Cancer Res. 8:419–427. 2002.PubMed/NCBI
|
|
33
|
Kumar M, Liu ZR, Thapa L and Qin RY:
Anti-angiogenic effects of somatostatin receptor subtype2 on human
pancreatic cancer xenografts. Carcinogenesis. 25:2075–2081. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Zhou T, Xiao X, Xu B, Li H and Zou Y:
Overexpression of SSTR2 inhibited the growth of SSTR2-positive
tumors via multiple signaling pathways. Acta Oncol. 48:401–410.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Frasca F, Pandini G, Sciacca L, Pezzino V,
Squatrito S, Belfiore A and Vigneri R: The role of insulin
receptors and IGF-I receptors in cancer and other diseases. Arch
Physiol Biochem. 114:23–37. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Guillermet J, Saint-Laurent N, Rochaix P,
et al: Somatostatin receptor subtype 2 sensitizes human pancreatic
cancer cells to death ligand-induced apoptosis. Proc Natl Acad Sci
USA. 100:155–160. 2003. View Article : Google Scholar
|