1. Introduction
Ovarian cancer is the most lethal gynecological
malignancy that presents at an advanced stage in 75% of patients.
Aggressive surgical debulking and platinum-based adjuvant
chemotherapy are the current standard treatment modalities that
lead to a complete remission in the majority of patients. However,
recurrences are frequently observed in up to 70% of cases (1). As the surgical techniques and systemic
chemotherapeutic agents improve, more patients with prolonged
survival may face secondary and tertiary recurrences. The
management of these recurrences is not as well-established as it is
for the primary disease. Generally, the treatment is tailored to
each patient depending on the location of the recurrence, the
performance of the patient, the disease-free interval, the previous
response to platinum-based agents and the preference of the
surgeon. The treatment usually consists of second-line
chemotherapy, but surgery may be performed on medically fit
patients in certain circumstances. However, the role of
cytoreduction in primary or secondary recurrences remains
controversial. Primary cytoreduction is a radical surgical
procedure in which the aim is to reduce the tumor load to a
non-visible status. Upper abdominal surgeries, including diaphragm
stripping, liver resection, splenectomy and distal pancreatectomy,
are classical procedures that are performed to achieve the goal of
a non-visible tumor (2–4). Surgery following a primary
intervention requires highly-skilled surgeons, a multidisciplinary
approach and tertiary patient care facilities.
When a recurrence is detected during the follow-up
period, a second surgery, termed secondary cytoreduction (SC) may
be performed in a medically fit and selective patient population in
certain circumstances. There are no strict criteria for selecting
candidates for the surgery following primary or secondary
recurrences. In the present review, the role of tertiary
cytoreduction (TC) in secondary ovarian cancer recurrences will be
discussed on the basis of the current literature.
2. Materials and methods
The present review aimed to present the current data
on TC. The publications and data with regard to cytoreduction were
identified using Pubmed, and relevant articles written in English
were selected. There are abundant studies on SC in ovarian cancer.
Using the search terms ‘ovarian cancer’ and ‘secondary
cytoreduction’, 105 articles were identified that were published
between 1989 and 2012. The search terms ‘ovarian cancer’ and
‘tertiary cytoreduction’ yielded 21 articles published between 1983
and 2013, of which eight papers, which were directly associated
with TC, were eligible and included in the present review.
3. Cytoreduction in ovarian cancer
Optimal debulking in primary surgery is
significantly associated with a prolonged survival. If the size of
the tumor is small and/or the growth rate is fast, the tumor cells
are more vulnerable to chemotherapy or radiotherapy. Reducing the
tumor volume prior to chemotherapy synchronizes cellular growth and
increases the bioavailable concentration of chemotherapy in the
tumor cells, hence reducing the chances of drug resistance.
Consequently, maximal cytoreductive surgery increases the effect of
chemotherapy and eventually improves survival (1,5). The
available data indicate a strong inverse correlation between
survival and the residual tumor volume. Currently, a non-visible
tumor is considered to be the optimal tumor volume for maximum
survival (3). At the initial
diagnosis of ovarian cancer, maximal surgical tumoral debulking and
adjuvant chemotherapy are the standards of care. Surgical debulking
in SC is not clearly defined due to a lack of prospective
randomized trials and data that solely consist of retrospective
studies. Berek et al reported the cases of 32 patients who
underwent SC, and defined optimal debulking as a residual tumor of
<1.5 cm (6). Optimally-debulked
patients demonstrated a 20-month survival rate compared with a rate
of 5 months for suboptimally-debulked patients. Chi et al
presented 157 cases of patients with recurrent ovarian cancer who
had undergone SC. The patients with residual tumors of <5 mm had
a median survival of 56 months, whereas those with tumors of >5
mm survived for a median of 27 months (3). Optimal debulking by SC has been shown
to be possible in two-thirds of patients (7). An increased disease-free interval
prior to the secondary surgery and a small residual tumor load
following the secondary surgery are highly-correlated with
prolonged survival. The DESKTOP OVAR trial (the Arbeitsgemeinschaft
Gynaekologische Onkologie Ovarian Committee, Descriptive Evaluation
of pre-operative Selection KriTeria for OPerability in recurrent
OVARian cancer), a multi-institutional retrospective study that
proposed a selection criteria for females undergoing surgery for
SC, attempted to classify and objectively select the appropriate
patients (4). The residual tumor
volume was the most significant prognostic factor that confirmed
the results from the previous retrospective studies (8). The patients without macroscopic tumors
showed longer survival rates than those with visible macroscopic
tumors. The volume of the residual tumor was not significant. By
incorporating the following three parameters of performance status,
the presence of ascites and the outcome of the primary
surgery/initial International Federation of Gynecology and
Obstetrics (FIGO) stage, the recurrent ovarian cancer patients who
had the optimal chance of complete surgical debulking with no
residual tumor were objectively selected. Using the criteria
established in the DESKTOP I trial, a prospective study, the
DESKTOP II trial, confirmed the validity of these three parameters
for selecting the female patients who underwent SC (9).
4. TC
Although recurrence following SC is often
inevitable, there is no established treatment procedure. TC is an
available option for the affected patients. Ideally, cytoreductive
surgery should control the disease, diminish the complaints
associated with the tumor load, increase survival and improve the
quality of life without increasing morbidity. Surgeries for
secondary recurrences are generally performed for palliation
purposes to treat intestinal obstructions and pain, but since there
is no requirement for cytoreduction, this is not classed as TC.
Issues with regard to TC include selecting the appropriate
candidates for the extensive surgery, determining the prognostic
value and identifying the limits of how aggressive the surgery must
be in order to achieve the best outcome.
5. Studies involving TC
The first study to evaluate TC was by Leitao et
al (Memorial Sloan-Kettering Cancer Center, New York, NY, USA)
(10), in which 26 patients with
recurrent ovarian carcinoma were analyzed. Optimal debulking was
defined as a residual tumor of <5 mm. Optimally-debulked
patients had a median disease-specific survival (DSS) rate of 36
months, whereas this rate was 11 months for suboptimally-debulked
patients. The survival rates of the optimally-debulked patients in
this study was comparable to the results of other SC studies. The
patients with longer disease-free intervals (>12 months) prior
to TC survived for an average of 60 months, whereas the survival
time of patients with shorter intervals was 15 months. In the
multivariate analysis, the residual tumor following TC was the only
independent factor associated with survival. Generally, in studies
concerned with SC or TC, platinum resistance is an accepted
exclusion criterion, although this may be a source of selection
bias. In contrast, Leitao et al included 15
platinum-resistant patients (57% of the whole cohort) and 67% of
these patients were successfully debulked. The median survival time
following TC was 25 months. A second study with updated data was
published in 2010 with a total of 77 patients, including the
previously mentioned 26 patients and new patients with fallopian
tube and peritoneal carcinoma (11). Nearly all the cases (92%) were
optimally debulked to leave a residual tumor of <5 mm. Similar
to the previous study, patients with platinum-resistant diseases
(28%) were included. The median DSS, defined as the time between TC
and mortality or last follow-up was 60 months for patients with
optimal cytoreduction (non-visible tumor), 27 months for patients
with a gross tumor of <5 mm and 13 months for patients with a
residual tumor of >5 mm. The patients who had a recurrence
interval of >24 months following SC were more likely to have
optimal cytoreduction (no tumor left) than those with shorter
recurrence-free intervals. The patients with platinum-sensitive
diseases were more likely to have a complete tumor resection
following TC than those with platinum-resistant diseases. Smaller
tumors were more likely to be cytoreduced than larger (>5 cm)
tumors. However, in the multivariate analysis, only the tumors with
a single site of recurrence proved to be associated with a total
resection. The extent of the debulking was the only significant
prognostic factor for survival in the multivariate analysis.
Adjuvant therapy following TC was not associated with an improved
DSS. The post-operative complication rate was 26% and the majority
of these complications were minor events. The study concluded that
only a small group of patients with completely resectable tumors
were considered appropriate candidates for TC (11).
The largest single institution study to evaluate TC
was by Fotopoulou et al and involved 135 patients (12). Similar to the study by Leitao et
al, patients with platinum-resistant diseases(20%) and those
with ascites prior to TC (43%) were included. Patients with
additional symptoms, including a chronic subileus, abscesses and
pain, were also included. A tumor resection resulting in a
non-visible status was achieved in 40% of the patients. Extensive
procedures, including a small bowel resection (64%), large bowel
resection (52%) and extensive peritonectomies (46%), were
performed. The mortality rate within 30 days following the surgery
was relatively high (5.8%). Unlike the findings in SC, the presence
of peritoneal carcinomatosis in TC was not a risk factor associated
with poor survival, regardless of the size of the post-operative
residual tumor. Also, the previously established negative
prognostic factors, including an advanced FIGO stage and the
presence of ascites, did not decrease the survival rate. Therefore,
the study recommended that patients who display these factors
should not be excluded from TC surgery. The study also analyzed the
distribution of the tumor in the upper, middle and lower abdominal
cavity. In TC, the tumor usually involves at least two regions of
the abdominal cavity, suggesting a diffuse involvement rather than
a solitary recurrence site. The presence of a tumor in the middle
abdomen and a diagnosis of peritoneal carcinomatosis in the
multivariate analysis were negative factors associated with tumor
resectability. Following TC, the overall survival rate for patients
with a non-visible tumor was 37 months, whereas for those with a
residual tumor of <1cm, the overall survival rate was 19 months.
In the multivariate analysis, the overall survival rate was
associated with a post-operative residual tumor of <1 cm. These
parameters require challenging, highly-skilled surgical procedures
in order to keep morbidity at a reasonable rate (12).
In a study involving cases from two institutions,
Karam et al evaluated 47 patients who had undergone TC
(13). Patients that were
undergoing palliative procedures, including surgery for bowel
obstructions, were excluded from the study. The surgery was
performed on patients with longer disease-free intervals (e.g. 6
months) and those with a limited number of recurrences.
Pre-operative computerized tomography scans revealed that the
median number of disease sites was four. In approximately
two-thirds of patients, optimal debulking with only a microscopic
residual tumor was achieved, and 81% had a residual tumor of <1
cm. The presence of a macroscopic residual tumor was a poor
prognostic factor. Patients with microscopic tumors following TC
had an average 27-month survival rate, whereas patients with
macroscopic tumors survived for 16 months. In contrast to the study
by Fotopoulou et al, in the multivariate analysis, the
presence of a diffuse disease was a negative predictor of survival,
with optimal debulking having no effect. However, subsequent to the
exclusion of patients with a diffuse disease, a subgroup analysis
was performed in 34 patients. Optimal debulking was a significant
factor in increasing the survival rate in the univariate and
multivariate analyses (37 vs. 16 months). It was concluded that
optimal TC extends the overall survival rate in patients with a
limited range of diseases (13).
Gultekin et al evaluated the characteristics
of 20 patients who had undergone TC (14). Patients with progressive diseases
who were undergoing surgery for palliation were excluded. The tumor
was resected to <2 cm in size in 12 patients, 7 of who had no
visible disease remaining at the end of the TC. During a median
follow-up period of 15 months, 13 patients were alive and three
patients had not experienced any signs of recurrence. A total of
three patients experienced perioperative complications with no
surgery-related mortalities. The median survival was 32 months for
patients with optimal TC compared with 6 months for patients with
suboptimal TC. However, this difference was not statistically
significant and may reflect the effect of the small sample size.
There were no predictors for optimal debulking and no significant
prognostic factors that affected the survival were detected upon
analysis. The morbidity rate of 15% was lower than for other
studies, since the surgery was less radical.
In a study from the Memorial Sloan Kettering Cancer
Center, the value of quaternary cytoreduction (QC) was analyzed
using 15 patients who had undergone surgery with the intent of
surgical cytoreduction (15). A
total of 14 patients had previously undergone optimal SC, which
resulted in a residual tumor of <5 mm, and all patients had also
previously undergone an optimal TC, resulting in 11 with no gross
residual tumors. The median time between the third recurrence and
the TC was 14 months, whereas between the TC and QC, the time
interval was 24 months. Of the total number of patients, 20% were
disease-free at the last follow-up, 25% had the disease but were
alive and 50% had succumbed to the disease. A residual tumor of
>1 cm and the number of recurrence sites (single vs. multiple)
were associated with the survival time. The median DSS was 34
months for patients with a residual tumor of <1 cm and 10 months
for patients with larger residual tumors. Platinum sensitivity did
not affect the survival in QC, therefore it was not necessary to
exclude platinum-resistant patients. QC was associated with certain
complications, including one ileus resolved with conservative
measures, three intra-abdominal abscesses, two of which required
radiological drainage, and one colovesical fistula, which was
managed with an ileal conduit and colostomy. There were no
surgery-related mortalities. It was concluded that patients with
resectable tumors that are ideally in one site may benefit from QC,
provided that the tumor is debulked to a volume of ≤1 cm (15). Similarly, Fotopolou et al
recently published a study with regard to QC in 49 patients,
two-thirds of who exhibited peritoneal carcinomatosis. The survival
time (43 vs. 13 months) increased significantly when no residual
tumor was left. Patients who were administered post-operative
chemotherapy had a significantly improved survival time compared
with those who were not (40 vs. 12 months) (16).
In 2012, Hizli et al published a
retrospective study of 23 patients who had undergone TC (17). A total of 12 patients with
platinum-resistant diseases, diseases that were presumed
unresectable by pre-operative imaging modalities or those with
ascites were excluded. The median disease-free interval prior to SC
was 26 months and the median interval between SC and TC was 21
months. More than one site of recurrence was identified in 82% of
TC patients. Optimal debulking, defined as a residual tumor of
<1 cm, was achieved in 65% of patients. No predictive factor for
optimal debulking was identified and none of the variables were
significant. The median follow-up period was 13 months, during
which all but one patient remained alive. In the univariate
analysis, only the outcome of TC (optimal vs. non-optimal) was
associated with survival and there were no differences between the
age of the patient or the time between progression. There were
three perioperative morbidities, one of which was due to abdominal
dehiscence and there were no surgery-related mortalities. Table I summarizes the data of the
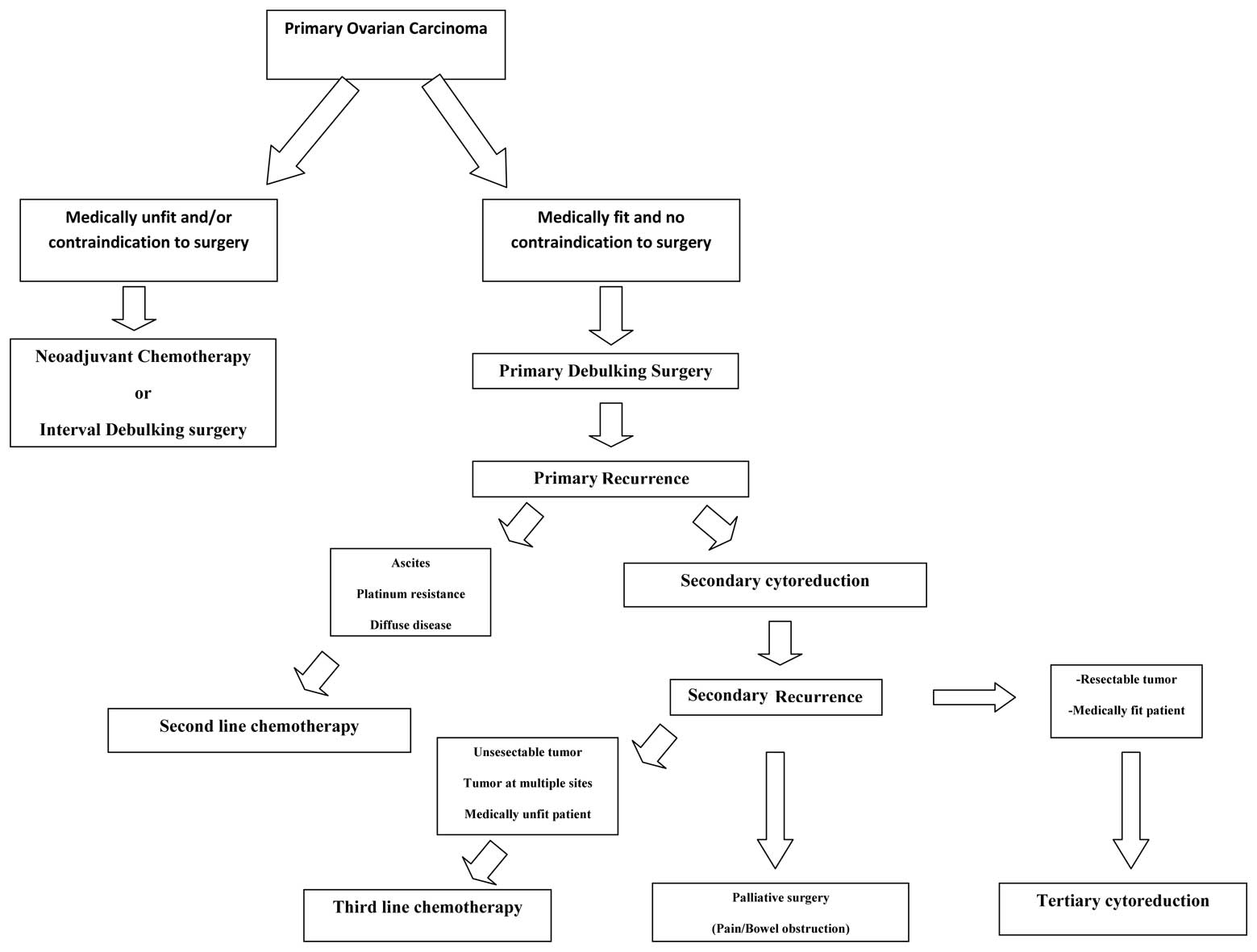
published TC studies and Fig. 1
shows the management options for primary ovarian cancer and primary
and secondary recurrences.
 | Table IRetrospective studies with regard to
TC. |
Table I
Retrospective studies with regard to
TC.
| First author
(ref.) | Year | No. of patients | Percentage of
platinum-sensitive patients | DFSa (months) | DFSb (months) | Major complication
rate (%) | Operative mortality
(%) | Complete tumor
resection rate (%) | Independent factors
associated with survival | Median tumor size
(cm) | Multiple site
recurrence rate (%) |
|---|
| Leitao et
al(10) | 2004 | 26 | 42 | 36 | 10 | 8 | 0 | 53 | Optimal TC and
TFI | 5 | 57 |
| Karam et
al(13) | 2007 | 47 | 0 | 24 | 16 | 14 | 0 | 64 | Presence of diffuse
peritoneal disease | 5 | NA |
| Gultekin et
al(14) | 2008 | 20 | 0 | 32 | 6 | 0 | 0 | 35 | - | 4 | 50 |
| Shih et
al(11) | 2010 | 77 | 28 | 60 | 13 | 13 | 0 | 72 | Extent of
debulking | 5 | 62 |
| Fotopoulou et
al(12) | 2011 | 135 | 19 | 37 | 7 | 20 | 5.8 | 39 | Complete tumor
resection, interval to primary diagnosis >3 years and serous
papillary histology | NA | 85 |
| Hizli et
al(17) | 2012 | 23 | 0 | NA | NA | 4 | 0 | 65 | Complete tumor
resection | 4 | 83 |
| Fotopoulou et
al(18) | 2013 | 406 | 38 | 49c | 12c | 26 | 3.2 | 54 | High-grade histology,
tumor residuals at 2nd and 3rd surgery, interval to second relapse,
ascites, upper abdominal involvement, distant metastases and
non-platinum third-line chemotherapy | NA | NA |
Recently, a retrospective study involving 406
patients from 14 countries used data that were gathered from
ovarian cancer patients who had undergone TC. A number of patients
that were included in this study were also previously reported in
other studies associated with TC. During a median follow-up period
of 14 months, ~50% of the patients succumbed to the disease and
another 49% experienced a new recurrence. The median overall
survival rate was 26 months and the progression-free survival rate
was 11 months. In concordance with other similar studies, the
residual tumor status strongly correlated with the survival rate.
Patients with no residual tumors had a longer overall survival time
compared with patients with visible residual tumors. In contrast to
other studies, a diagnosis of peritoneal carcinomatosis was not
associated with a less favorable outcome. In the multivariate
analysis, peritoneal carcinomatosis was associated with an
incomplete tumor resection, but had no effect on overall survival
(18).
Chemotherapy is generally administered to patients
with secondary recurrences. In prospective trials, non-platinum
agents resulted in complete responses in 3–4% of patients. For
pegylated liposomal doxorubicin, the longest median survival time
in patients with platinum-sensitive tumors was 27 months. For
platinum-resistant patients, the response rates were worse, at less
than one year (19,20). In the study by Leitao et al,
platinum-resistant patients responded similarly to
platinum-sensitive patients and the median survival was longer for
platinum-resistant patients who underwent TC compared with those
who were administered salvage chemotherapy (10). Therefore, platinum-resistant
patients may also be candidates for TC, particularly if the tumor
is resectable. In recent studies, Fotopoulou et al reported
a survival advantage for patients who were administered third-line
chemotherapy following TC over patients without chemotherapy
(18,21).
Perioperative morbidity rates have been shown to
range between 15 and 31% (11–14,17).
In the study by Karam et al, the complication rate was 26%
(13). A total of six patients
experienced pulmonary embolisms and two presented with an
enterocutaneous fistula. A further two patients were diagnosed with
myocardial infarctions, and another two patients with rectovaginal
and vesicovaginal fistulae, respectively. Despite these
morbidities, there were no post-operative mortalities. In contrast,
Fotopoulou et al reported a 5.8% mortality rate at 30 days
post-surgery (12). The potential
candidates for TC are expected to have high morbidity and mortality
risks due to the fact that the majority of patients may have a
diffuse disease, ascites or tumors extending to multiple sites,
including the upper abdominal cavity. The patients should be
informed of the high chances of operative morbidity and mortality
and of the risk of a third operation. These procedures should be
carried out in tertiary centers with multidisciplinary approaches,
including highly-specialized centers with well-trained
gynecological oncologists, gastroenterological surgeons and
anesthesiologists.
There are certain common inherent drawbacks to all
studies involving TC. Firstly, to date, there have been no
randomized controlled trials that compared the various treatment
modalities for recurrent ovarian cancer, since the current studies
represent retrospective data. Secondly, the study groups consist of
small numbers of patients with heterogenous characteristics.
Finally, patient selection is not uniform and is based on the
preferences of the surgeons rather than an objective criteria.
Multicenter randomized studies with larger patient populations and
more objective patient selection criteria are required to clarify
these issues.
6. Conclusion
Conventional negative prognostic factors for SC,
including ascites, an advanced FIGO stage and/or peritoneal
carcinomatosis, do not apply to TC, but the post-operative residual
tumor load is significant in predicting the outcome. Highly
selective patients with completely resectable tumors may have a
reasonable chance for maximal cytoreduction and an improved
survival benefit. The aim should be to reduce the tumor so that no
visible macroscopic residual volume is discernible and to select
patients depending on this criteria. TC appears to have a favorable
outcome and reasonable complication rates. Tertiary surgery is an
available option for patients with recurrence in whom a complete
tumor resection may be achieved.
References
|
1
|
Eisenkop SM, Friedman RL and Wang HJ:
Complete cytoreductive surgery is feasible and maximizes survival
in patients with advanced epithelial ovarian cancer: a prospective
study. Gynecol Oncol. 69:103–108. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Chi DS, Franklin CC, Levine DA, et al:
Improved optimal cytoreduction rates for stages IIIC and IV
epithelial ovarian, fallopian tube, and primary peritoneal cancer:
a change in surgical approach. Gynecol Oncol. 94:650–654. 2004.
View Article : Google Scholar
|
|
3
|
Chi DS, McCaughty K, Diaz JP, et al:
Guidelines and selection criteria for secondary cytoreductive
surgery in patients with recurrent, platinum-sensitive epithelial
ovarian carcinoma. Cancer. 106:1933–1939. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Harter P, du Bois A, Hahmann M, et al;
Arbeitsgemeinschaft Gynaekologische Onkologie Ovarian Committee;
AGO Ovarian Cancer Study Group. Surgery in recurrent ovarian
cancer: the Arbeitsgemeinschaft Gynaekologische Onkologie (AGO)
DESKTOP OVAR trial. Ann Surg Oncol. 13:1702–1710. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Shih KK and Chi DS: Maximal cytoreductive
effort in epithelial ovarian cancer surgery. J Gynecol Oncol.
21:75–80. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Berek JS, Hacker NF, Lagasse LD, Nieberg
RK and Elashoff RM: Survival of patients following secondary
cytoreductive surgery in ovarian cancer. Obstet Gynecol.
61:189–193. 1983.PubMed/NCBI
|
|
7
|
Munkarah AR and Coleman RL: Critical
evaluation of secondary cytoreduction in recurrent ovarian cancer.
Gynecol Oncol. 95:273–280. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Eisenkop SM, Friedman RL and Spirtos NM:
The role of secondary cytoreductive surgery in the treatment of
patients with recurrent epithelial ovarian carcinoma. Cancer.
88:144–153. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Harter P, Sehouli J, Reuss A, et al:
Prospective validation study of a predictive score for operability
of recurrent ovarian cancer: the Multicenter Intergroup Study
DESKTOP II. A project of the AGO Kommission OVAR, AGO Study Group,
NOGGO, AGO-Austria, and MITO. Int J Gynecol Cancer. 21:289–295.
2011. View Article : Google Scholar
|
|
10
|
Leitao MM Jr, Kardos S, Barakat RR and Chi
DS: Tertiary cytoreduction in patients with recurrent ovarian
carcinoma. Gynecol Oncol. 95:181–188. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Shih KK, Chi DS, Barakat RR and Leitao MM
Jr: Tertiary cytoreduction in patients with recurrent epithelial
ovarian, fallopian tube, or primary peritoneal cancer: an updated
series. Gynecol Oncol. 117:330–335. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Fotopoulou C, Richter R, Braicu IE, et al:
Clinical outcome of tertiary surgical cytoreduction in patients
with recurrent epithelial ovarian cancer. Ann Surg Oncol. 18:49–57.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Karam AK, Santillan A, Bristow RE, et al:
Tertiary cytoreductive surgery in recurrent ovarian cancer:
selection criteria and survival outcome. Gynecol Oncol.
104:377–380. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Gultekin M, Velipaşaoǧlu M, Aksan G, et
al: A third evaluation of tertiary cytoreduction. J Surg Oncol.
98:530–534. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Shih KK, Chi DS, Barakat RR and Leitao MM
Jr: Beyond tertiary cytoreduction in patients with recurrent
epithelial ovarian, fallopian tube, or primary peritoneal cancer.
Gynecol Oncol. 116:364–369. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Fotopoulou C, Savvatis K, Kosian P, et al:
Quaternary cytoreductive surgery in ovarian cancer: does surgical
effort still matter? Br J Cancer. 108:32–38. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Hizli D, Boran N, Yilmaz S, et al: Best
predictors of survival outcome after tertiary cytoreduction in
patients with recurrent platinum-sensitive epithelial ovarian
cancer. Eur J Obstet Gynecol Reprod Biol. 163:71–75. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Fotopoulou C, Zang R, Gultekin M, et al:
Value of tertiary cytoreductive surgery in epithelial ovarian
cancer: an international multicenter evaluation. Ann Surg Oncol.
20:1348–1354. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Gordon AN, Fleagle JT, Guthrie D, Parkin
DE, Gore ME and Lacave AJ: Recurrent epithelial ovarian carcinoma:
a randomized phase III study of pegylated liposomal doxorubicin
versus topotecan. J Clin Oncol. 19:3312–3322. 2001.PubMed/NCBI
|
|
20
|
Mutch DG, Orlando M, Goss T, Teneriello
MG, et al: Randomized phase III trial of gemcitabine compared with
pegylated liposomal doxorubicin in patients with platinum-resistant
ovarian cancer. J Clin Oncol. 25:2811–2818. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Fotopoulou C, Richter R, Braicu IE, et al:
Clinical outcome of tertiary surgical cytoreduction in patients
with recurrent epithelial ovarian cancer. Ann Surg Oncol. 18:49–57.
2011. View Article : Google Scholar : PubMed/NCBI
|